Antimicrobial Effects of Violacein against Planktonic Cells and Biofilms of Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. Structure Characterization
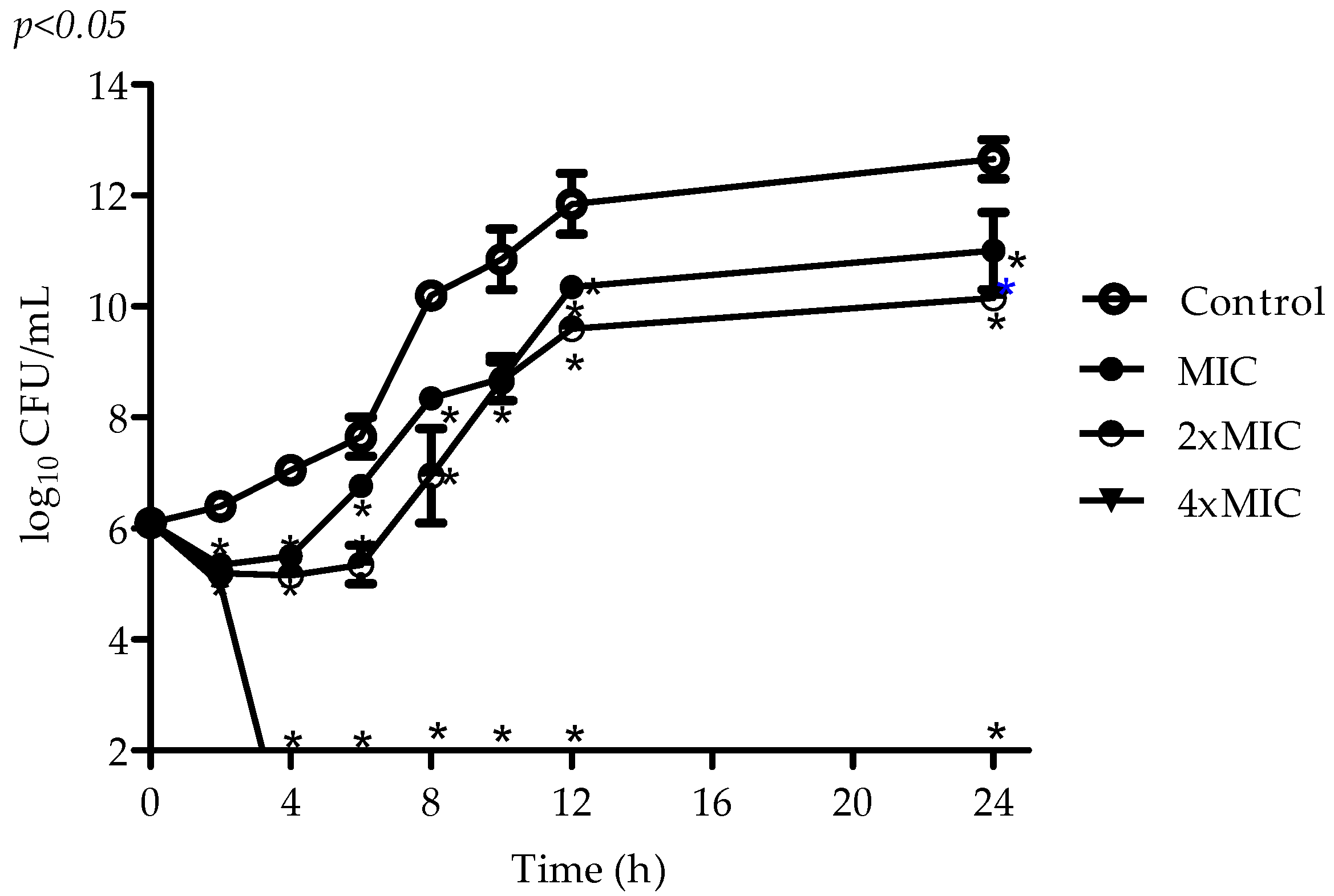
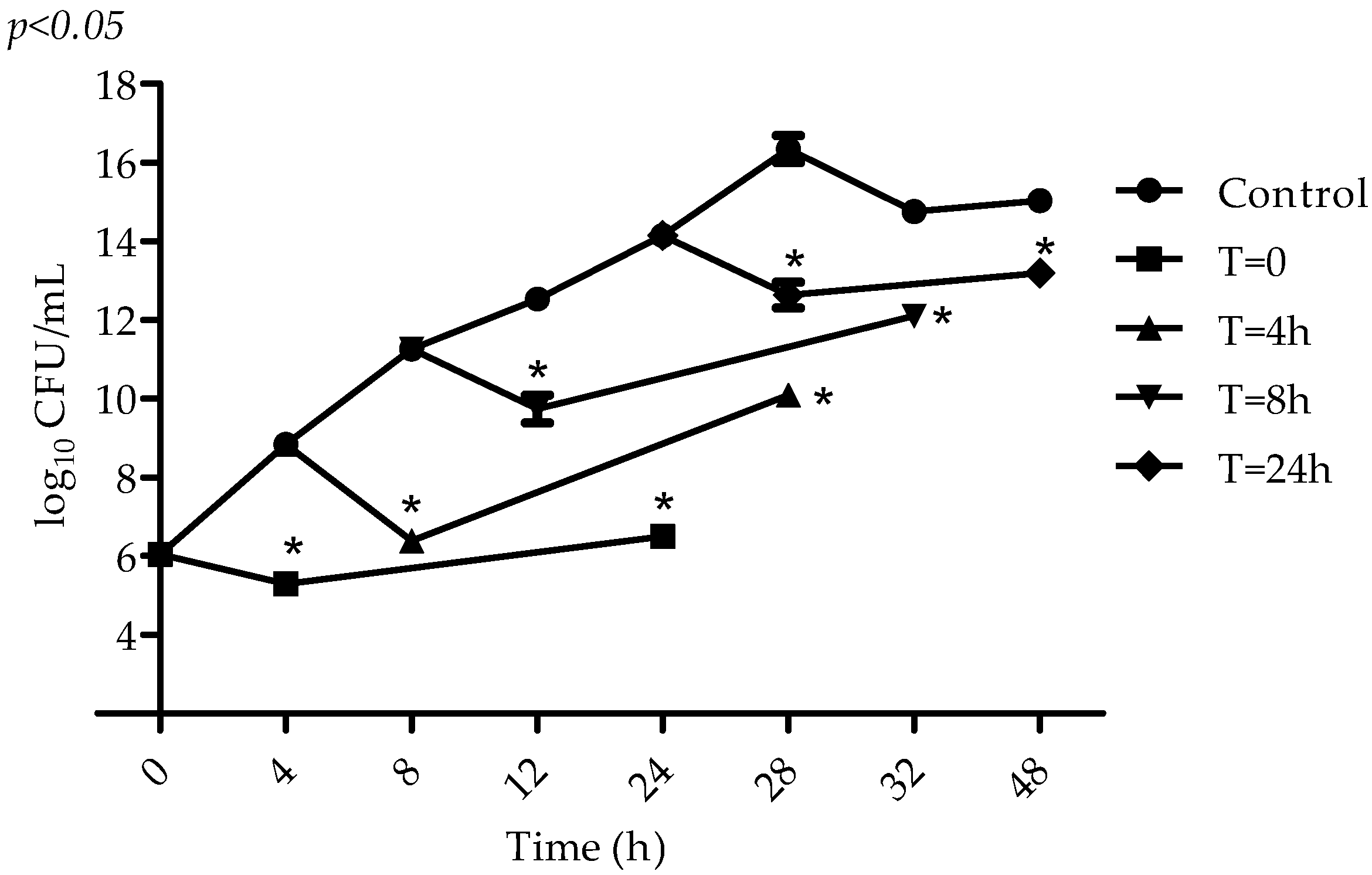
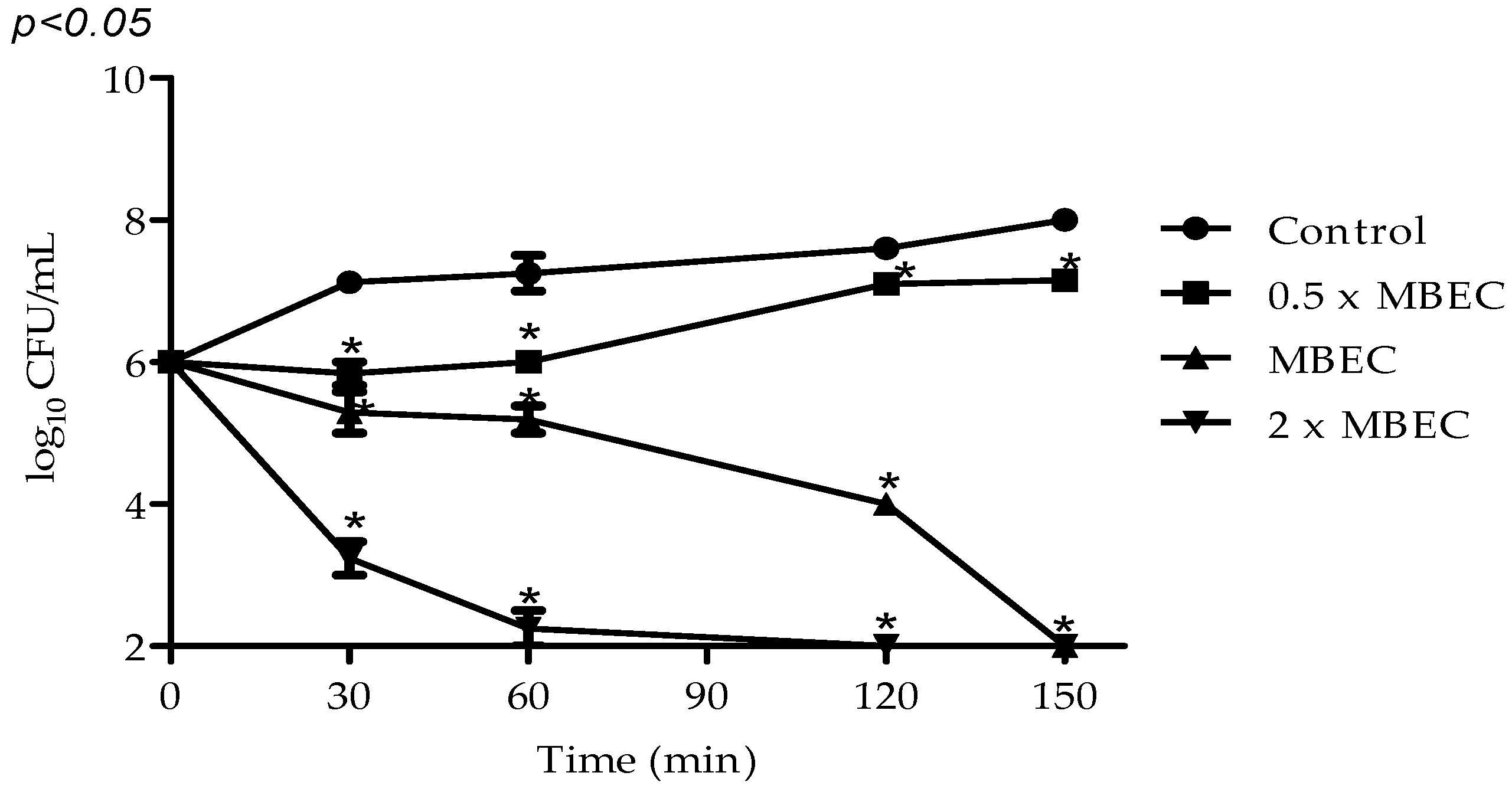
2.2. Antimicrobial Activity of VIO
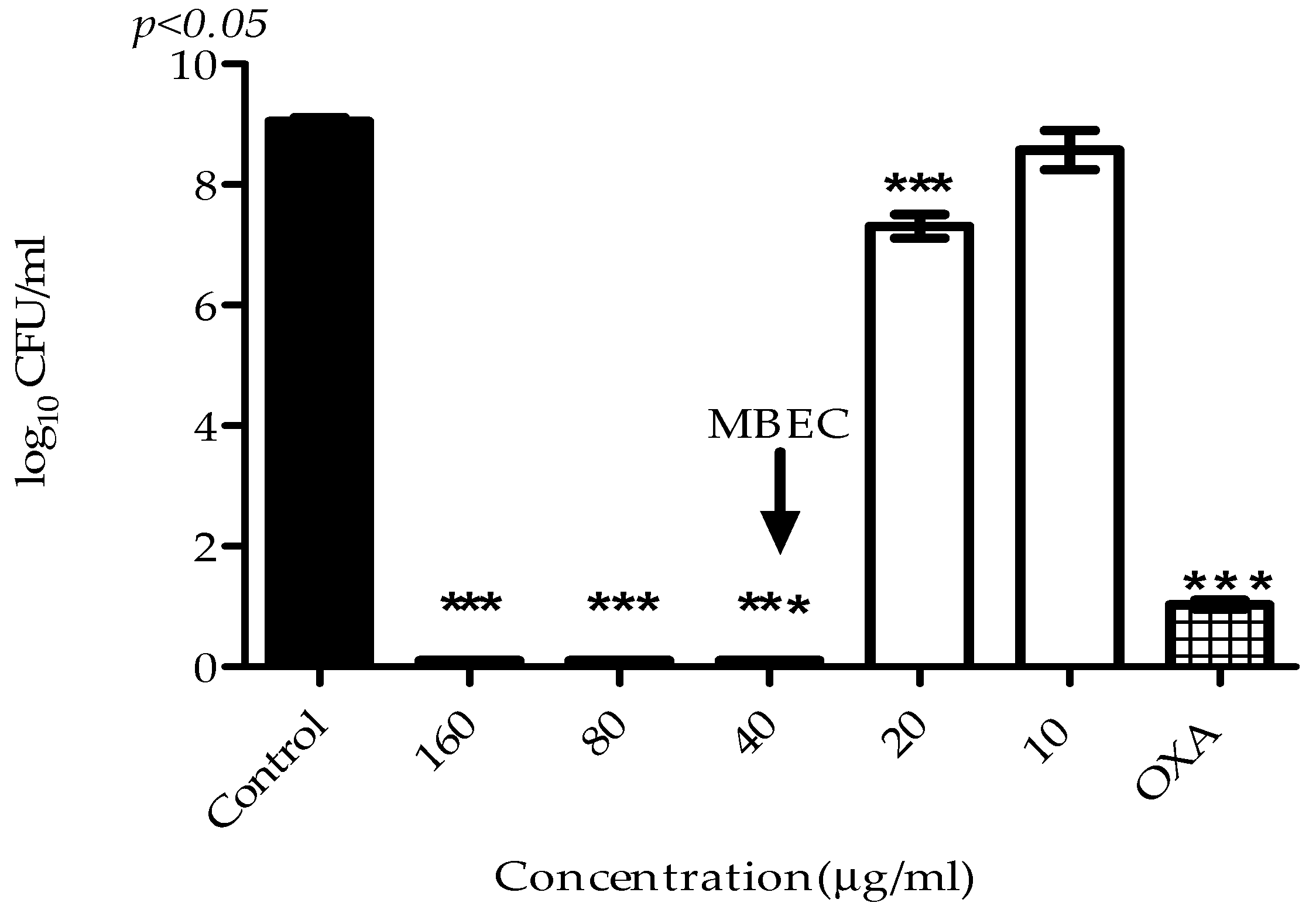
2.3. Antibiofilm Activity
3. Discussion
4. Materials and Methods
4.1. Violacein
4.2. Bacterial Strains
4.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.4. Time–Kill Assay
4.5. Determination of VIO Effect on S. aureus at Different Stages of Microbial Metabolism
4.6. Determination of Minimum Biofilm Inhibitory Concentration (MBIC)
4.7. Determination of Minimum Biofilm Eradication Concentration (MBEC)
4.8. Biofilm Time–Kill Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lister, J.L.; HorswilL, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [PubMed]
- Mccallum, N.; Berger-Bächi, B.; Senn, M.M. Regulation of antibiotic resistance in Staphylococcus aureus. Int. J. Med. Microbiol. 2010, 300, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, X.; Li, W.; Meng, R.; Liu, Z.; Liu, M.; Guo, N.; Yu, L. Inhibitory effect of totarol on exotoxin proteins hemolysin and enterotoxins secreted by Staphylococcus aureus. World J. Microbiol. Biotechnol. 2015, 31, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Scherr, T.D.; Hanke, M.L.; Huang, O.; James, D.B.; Horswill, A.R.; Bayles, K.W.; Fey, P.D.; Torres, V.J.; Kielian, T. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin ab and alpha-toxin. mBio 2015, 6, e01021-15. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fairfull-Smith, K.E.; Hancock, R.E. Effect of nitroxides on swarming motility and biofilm formation, multicellular behaviors in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4877–4881. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228, PMC2777538. [Google Scholar] [PubMed]
- Beenken, K.E.; Dunman, P.M.; Mcaleese, F.; Macapagal, D.; Murphy, E.; Projan, S.J.; Blevins, J.S.; Smeltzer, M.S. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 2004, 186, 4665–4684. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T. Violacein and related tryptophan metabolites produced by Chromobacterium violaceum: Biosynthetic mechanism and pathway for construction of violacein core. Appl. Microbiol. Biotechnol. 2011, 91, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Yoon, K.H.; Lee, J.I.; Mitchell, R.J. Violacein: Properties and Production of a Versatile Bacterial Pigment. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Justo, G.Z.; Ferreira, C.V.; Melo, P.S.; Cordi, L.; Martins, D. Violacein: Properties and biological activities. Biotechnol. Appl. Biochem. 2007, 48, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Anju, S.; Kumar, N.S.; Krishnakumar, B.; Kumar, B.S.D. Synergistic combination of violacein and azoles that leads to enhanced killing of major human pathogenic dermatophytic fungi Trichophyton rubrum. Front. Cell. Infect. Microbiol. 2015, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Cazoto, L.L.; Martins, D.; Ribeiro, M.G.; Duran, N.; Nakazato, G. Antibacterial activity of violacein against Staphylococcus aureus isolated from bovine mastitis. J. Antibiot. 2011, 64, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Ravi, V.; Sivasubramanian, A. Synergistic antimicrobial profiling of violacein with commercial antibiotics against pathogenic micro-organisms. Pharm. Biol. 2014, 52, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Costa, F.; Brocchi, M.; Duran, N. Evaluation of the antibacterial activity of poly-(d, l-lactide-co-glycolide) nanoparticles containing violacein. J. Nanopart. Res. 2011, 13, 355–363. [Google Scholar] [CrossRef]
- Ahmad, W.A.; Yusof, N.Z.; Nordin, N.; Zakaria, Z.A.; Rezali, M.F. Production and Characterization of Violacein by Locally Isolated Chromobacterium violaceum Grown in Agricultural Wastes. Appl. Biochem. Biotechnol. 2012, 167, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Menck, C.F. Chromobacterium violaceum: A review of pharmacological and industiral perspectives. Crit. Rev. Microbiol. 2001, 27, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Antonisamy, P.; Kannan, P.; Aravinthan, A.; Duraipandiyan, V.; Valan Arasu, M.; Ignacimuthu, S.; Abdullah AL-Dhabi, N.; Kim, J.H. Gastroprotective activity of violacein isolated from Chromobacterium violaceum on indomethacin-induced gastric lesions in rats: Investigation of potential of mechanisms of action. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Verinaud, L.; Lopes, S.C.P.; Prado, I.C.N.; Zanucoli, F.; Da Costa, T.A.; Di Gangi, R.; Issayama, L.K.; Carvalho, A.C.; Bonfanti, A.P.; Niederauer, G.F. Violacein treatment modulates acute and chronic inflammation through the suppression of cytokine production and induction of regulatory t cells. PLoS ONE 2015, 10, e0125409. [Google Scholar] [CrossRef] [PubMed]
- Savage, V.J.; Chopra, I.; O’neill, A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Haussler, S.; Fuqua, C. Biofilms 2012: New discoveries and significant wrinkles in a dynamic field. J. Bacteriol. 2013, 195, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Rettori, D.; Durán, N. Production, extraction and purificationof violacein: An antibiotic pigment producedby Chromobacterium violaceum. World J. Microbiol. Biotechnol. 1988, 14, 685–688. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; CLSI Document M100-S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Shanholtzer, C.J.; Peterson, L.R.; Mohn, M.L.; Moody, J.A.; Gerding, D.N. Mbcs for Staphylococcus aureus as determined by macrodilution and microdilution techniques. Antimicrob. Agents Chemother. 1984, 26, 214–219, PMCID:284123. [Google Scholar] [CrossRef] [PubMed]
- Olajuyigbe, O.O.; Afolayan, A.J. In vitro antibacterial and time–kill assessment of crude methanolic stem bark extract of acacia mearnsii de wild against bacteria in shigellosis. Molecules 2012, 17, 2103–2118. [Google Scholar] [CrossRef] [PubMed]
- Muroi, H.; Kubo, I. Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin resistant Staphylococcus aureus. J. Appl. Bacteriol. 1996, 80, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of Staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Kwieciński, J.; Eick, S.; Wójcik, K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int. J. Antimicrob. Agents 2009, 33, 343–347. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
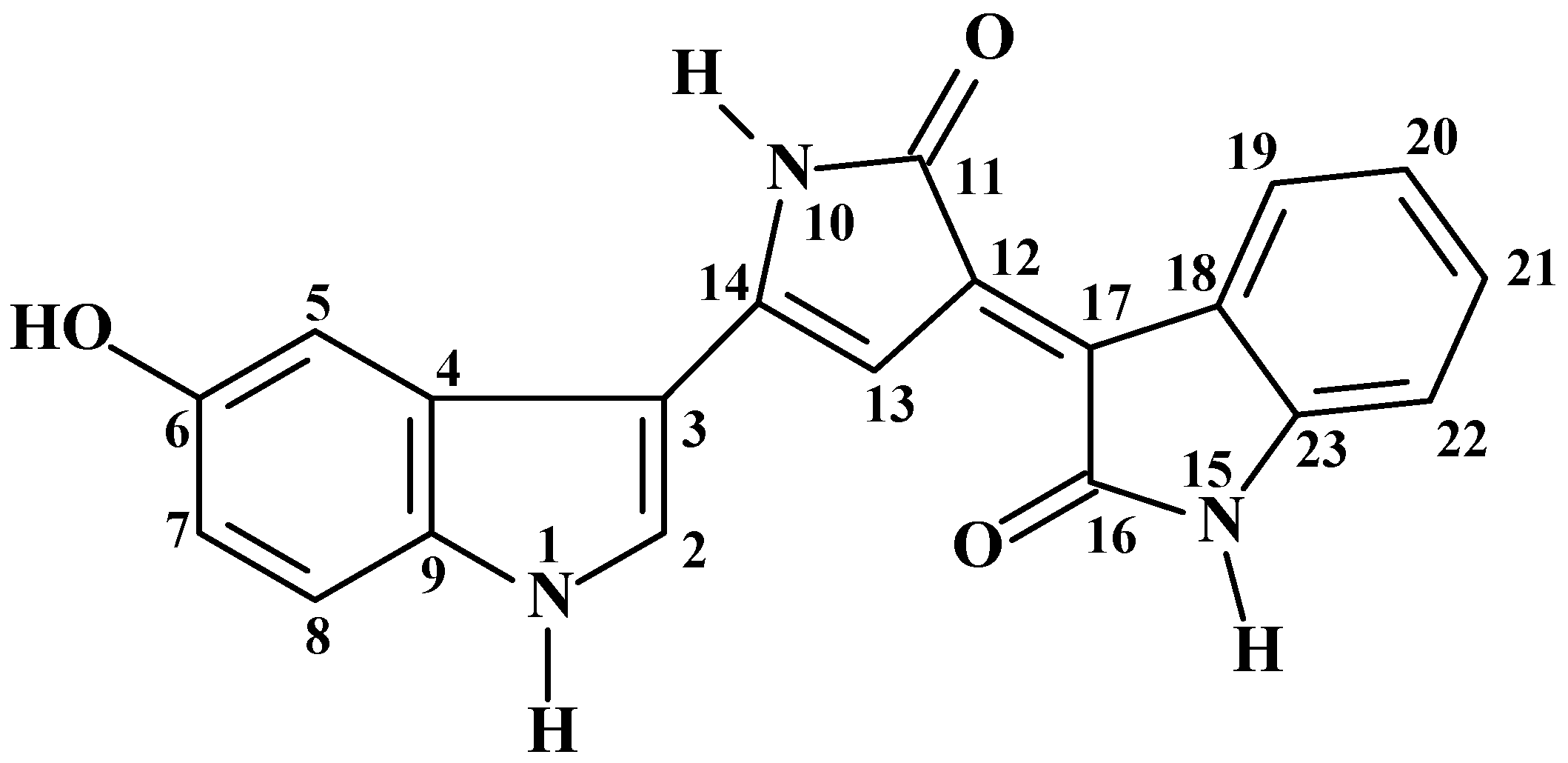
| HSQC | HMBC | |||
|---|---|---|---|---|
| δC | δH (Mult, J/Hz) | 2JCH | 3JCH | |
| 2 | 129.7 | 8.06 (d 3.1) | ||
| 3 | 105.8 | — | H-2 | NH-1, H-13, H-5 |
| 4 | 125.6 | — | NH-1, H-2, H-8 | |
| 5 | 104.6 | 7.23 d (2.0) | OH, H-7 | |
| 6 | 152.9 | — | OH, H-5, H-7 | H-8 |
| 7 | 113.1 | 6.79 dd (8.7, 2.0) | OH, H-5 | |
| 8 | 113.4 | 7.35 d (8.7) | ||
| 9 | 131.6 | — | NH-1 | H-2, H-5, H-7 |
| 11 | 171.7 | — | NH-10 | H-13 |
| 12 | 137.0 | — | H-13 | NH-10 |
| 13 | 97.0 | 7.55 d (2.0) | NH-10 | |
| 14 | 147.7 | — | NH-10, H-13 | |
| 16 | 170.3 | — | NH-15 | |
| 17 | 118.7 | — | NH-15, H-19 | |
| 18 | 122.4 | — | NH-15, H-20, H-22 | |
| 19 | 126.4 | 8.93 d (8.0) | H-20 | H-21 |
| 20 | 120.9 | 6.95 td (8.0, 1.0) | H-22 | |
| 21 | 129.4 | 7.20 td (8.0, 1.0) | H-20 | H-19 |
| 22 | 109.1 | 6.83 d (8.0) | H-20 | |
| 23 | 141.8 | — | NH-15 | H-19, H-21 |
| NH-1 | 11.88 (s) | |||
| NH-10 | 10.72 (s) | |||
| NH-15 | 10.60 (s) | |||
| OH | 9.33 (s) | |||
| Microbial Strain | VIO μg/mL | ||
|---|---|---|---|
| MIC | MBC | MBIC | |
| S. aureus ATCC 6538P (OSSA) | 1.25 | 5 | 1.25 |
| S. aureus ATCC 14458 (OSSA) | 2.5 | 10 | 2.5 |
| S. aureus ATCC 3359 (ORSA) | 20 | 40 | 20 |
| S. aureus CCBH 5330 (ORSA) | 5 | 20 | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, A.H.M.; Moreira, A.C.D.; De Carvalho, R.M.; Sales, G.W.P.; Nogueira, P.C.N.; Grangeiro, T.B.; Medeiros, S.C.; Silveira, E.R.; Nogueira, N.A.P. Antimicrobial Effects of Violacein against Planktonic Cells and Biofilms of Staphylococcus aureus. Molecules 2017, 22, 1534. https://doi.org/10.3390/molecules22101534
Batista AHM, Moreira ACD, De Carvalho RM, Sales GWP, Nogueira PCN, Grangeiro TB, Medeiros SC, Silveira ER, Nogueira NAP. Antimicrobial Effects of Violacein against Planktonic Cells and Biofilms of Staphylococcus aureus. Molecules. 2017; 22(10):1534. https://doi.org/10.3390/molecules22101534
Chicago/Turabian StyleBatista, Andressa H. M., Anne C. D. Moreira, Rafael M. De Carvalho, Gleilton W. P. Sales, Patrícia C. N. Nogueira, Thalles B. Grangeiro, Suelen C. Medeiros, Edilberto R. Silveira, and Nádia A. P. Nogueira. 2017. "Antimicrobial Effects of Violacein against Planktonic Cells and Biofilms of Staphylococcus aureus" Molecules 22, no. 10: 1534. https://doi.org/10.3390/molecules22101534






