Anti-Inflammatory Phenolic Metabolites from the Edible Fungus Phellinus baumii in LPS-Stimulated RAW264.7 Cells
Abstract
:1. Introduction
2. Results & Discussion
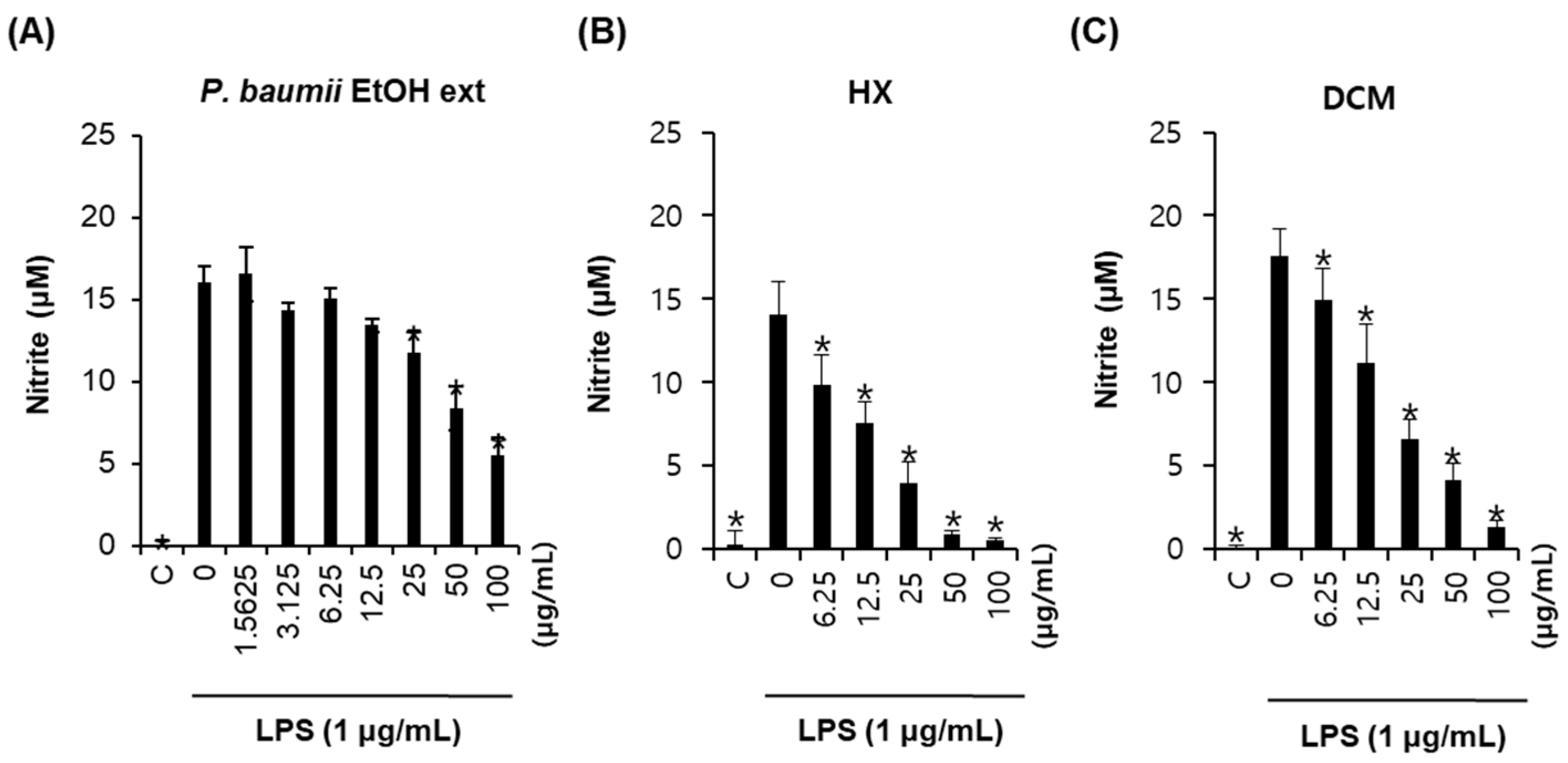
2.1. Bioactivity-Guided Fractionation for Anti-Inflammatory Effects
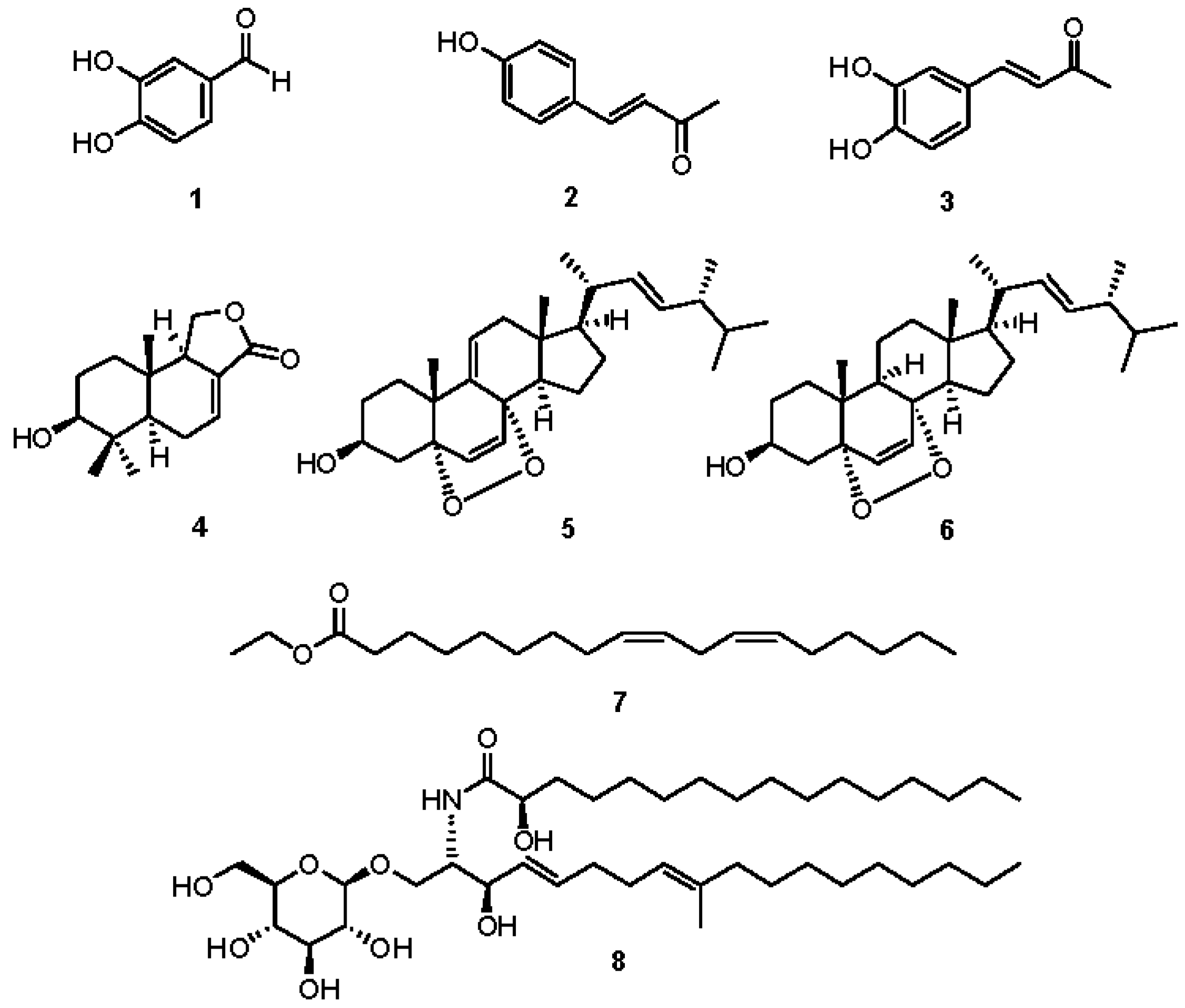
2.2. Chemical Investigation and Identification of the Active Compounds
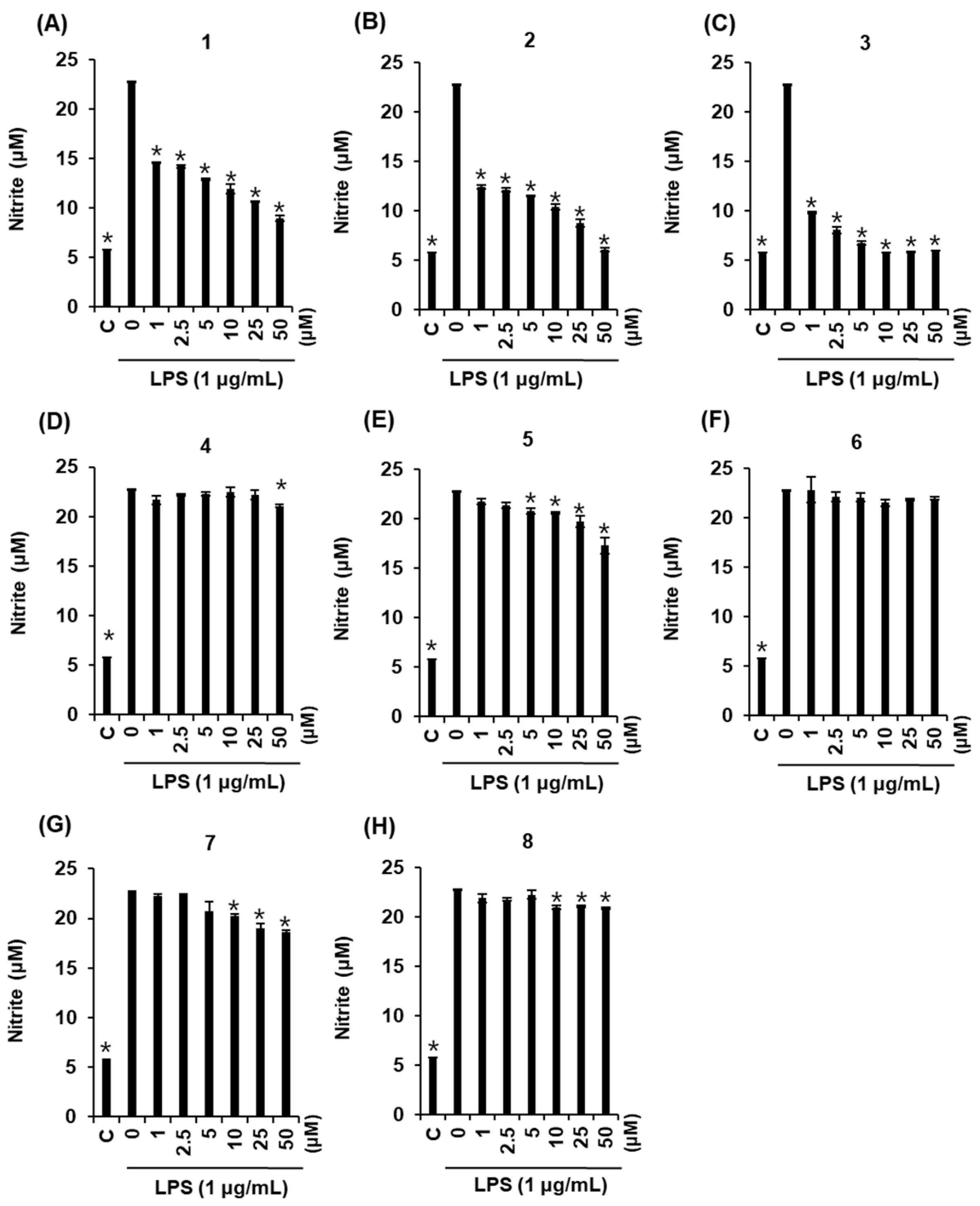
2.3. Effects of Compounds 1–8 on NO Production
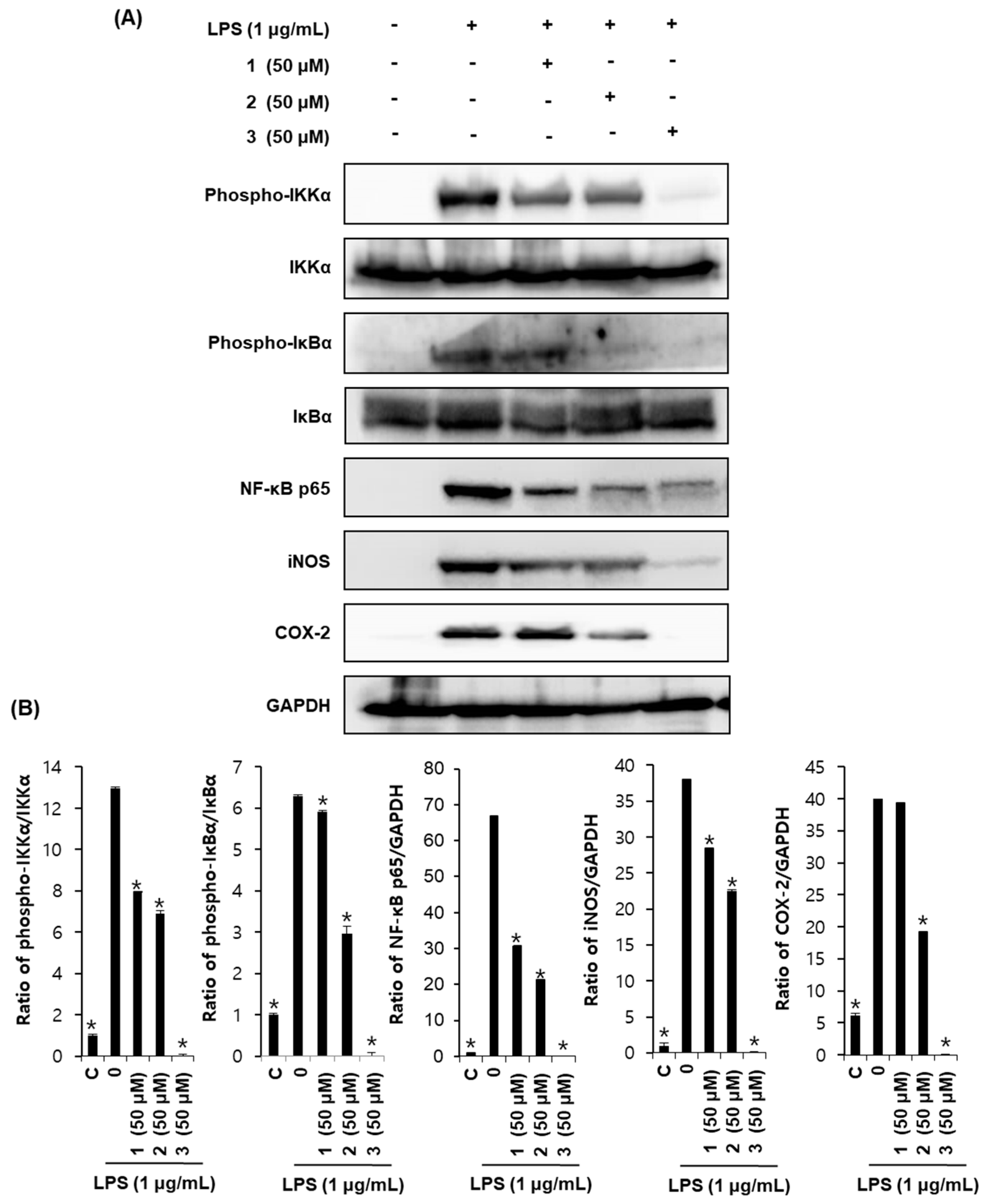
2.4. Effects of Compounds 1–3 on Proteins Associated with Inhibition of NF-κB in LPS-Stimulated RAW264.7 Mouse Macrophages
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungus Material
3.3. Extraction, Fractionation, and Purification Methods
3.4. Cell Culture and MTT Cell Viability Assay
3.5. Measurement of Nitric Oxide Production
3.6. Western Blotting Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, Y.; Lee, S.; Kim, J.Y.; Shin, J.H.; Kwon, O.A. Phellinus baumii—Based supplement containing Salvia miltiorrhiza Bunge improves atherothrombotic profiles through endothelial nitric oxide synthase and cyclooxygenase pathways in vitro and in vivo. J. Funct. Foods 2016, 24, 231–243. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Langner, E.; Kaczor, J.; Kandefer-Szerszen, M.; Sanecka, B.; Mazurkiewicz, W.; Rzeski, W. Anticancer effects of fraction isolated from fruiting bodies of Chaga medicinal mushroom, Inonotus obliquus (Pers.:Fr.) Pilát (Aphyllophoromycetideae): In vitro studies. Int. J. Med. Mushrooms 2011, 13, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.R.; Lee, I.K.; Ly, S.Y.; Yang, K.J.; Gang, G.T.; Kim, Y.H.; Hwang, J.H.; Yun, B.S.; Lee, C.H. A Phellinus baumii extract reduces obesity in high-fat diet-fed mice and absorption of triglyceride in lipid-loaded mice. J. Med. Food 2011, 14, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Kim, S.H.; Chen, C.Y. A medicinal mushroom: Phellinus linteus. Curr. Med. Chem. 2008, 15, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.S.; Kim, J.C.; Bae, J.S.; Rhee, M.H.; Jang, K.H.; Song, J.C.; Kwon, O.D.; Park, S.C. Extracts of Phellinus gilvus and Phellinus baumii inhibit pulmonary inflammation induced by lipopolysaccharide in rats. Biotechnol. Lett. 2004, 26, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Zeng, P.; Li, X.T.; Shi, L.G. Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. Int. J. Biol. Macromol. 2016, 91, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Cho, S.D.; Hu, H.; Kim, S.H.; Lee, S.K.; Lee, Y.S.; Kang, K.S. The roles of ERK1/2 and p38 MAP kinases in the preventive mechanisms of mushroom Phellinus linteus against the inhibition of gap junctional intercellular communication by hydrogen peroxide. Carcinogenesis 2002, 23, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.; Janardhanan, K. Cytotoxic and antitumor activities of a polypore macrofungus, Phellinus rimosus (Berk) Pilat. J. Ethnopharmacol. 2003, 84, 157–162. [Google Scholar] [CrossRef]
- Wu, C.S.; Lin, Z.M.; Wang, L.N.; Guo, D.X.; Wang, S.Q.; Liu, Y.Q.; Yuan, H.Q.; Lou, H.X. Phenolic compounds with NF-κB inhibitory effects from the fungus Phellinus baumii. Bioorg. Med. Chem. Lett. 2011, 21, 3261–3267. [Google Scholar] [CrossRef] [PubMed]
- Yayeh, T.; Oh, W.J.; Park, S.C.; Kim, T.H.; Cho, J.Y.; Park, H.J.; Lee, I.K.; Kim, S.K.; Hong, S.B.; Yun, B.S.; et al. Phellinus baumii ethyl acetate extract inhibits lipopolysaccharide-induced iNOS, COX-2, and proinflammatory cytokine expression in RAW264.7 cells. J. Nat. Med. 2012, 66, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Han, M.S.; Lee, M.S.; Kim, Y.S.; Yun, B.S. Styrylpyrones from the medicinal fungus Phellinus baumii and their antioxidant properties. Bioorg. Med. Chem. Lett. 2010, 20, 5459–5461. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, S.M.; Endale, M.; Oh, W.J.; Park, S.C.; Kim, T.H.; Lee, I.K.; Cho, J.Y.; Park, H.J.; Kim, S.K.; Yun, B.S.; et al. Antiplatelet activity of Phellinus baummii methanol extract is mediated by cyclic AMP elevation and inhibition of collagen-activated integrin-αIIbβ3 and MAP kinase. Phytother. Res. 2011, 25, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Kim, S.W.; Lim, J.M.; Joo, J.H.; Kim, H.O.; Kim, H.M.; Yun, J.W. Hypoglycemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocin-induced diabetic rats. Life Sci. 2005, 76, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Lee, C.W.; Jeon, Y.J.; Hong, N.D.; Yoo, I.D.; Yang, K.H.; Kim, H.M. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology 1999, 41, 157–164. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, I.K.; Choi, H.J.; Yun, B.S. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015, 25, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.S.; Lee, S.R.; Kim, C.S.; Moon, E.; Kim, S.Y.; Choi, S.U.; Kang, K.S.; Lee, K.R.; Kim, K.H. A new monoacylglycerol from the fruiting bodies of Gymnopilus spectabilis. J. Chem. Res. 2016, 40, 156–159. [Google Scholar] [CrossRef]
- Lee, S.R.; Jung, K.; Noh, H.J.; Park, Y.J.; Lee, H.L.; Lee, K.R.; Kang, K.S.; Kim, K.H. A new cerebroside from the fruiting bodies of Hericium erinaceus and its applicability to cancer treatment. Bioorg. Med. Chem. Lett. 2015, 25, 5712–5715. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Yang, H.H.; Kim, G.S.; Lee, S.E.; Lee, D.Y.; Choi, J.H.; Kim, S.Y.; Lee, E.S.; Ji, S.H.; Kang, K.S.; et al. Chemical constituents of Hericium erinaceum associated with the inhibitory activity against cellular senescence in human umbilical vascular endothelial cells. J. Enzyme Inhib. Med. Chem. 2015, 30, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Moon, E.; Choi, S.U.; Kim, S.Y.; Lee, K.R.; Kim, K.H. Cytotoxic and anti-inflammatory disulfide compounds from the fruiting bodies of Boletus pseudocalopus. J. Antibiot. 2015, 68, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, S.U.; Noh, H.J.; Zee, O.; Lee, K.R. Cytotoxic ergosterol derivatives from the mushroom Naematoloma fasciculare. Nat. Prod. Sci. 2014, 20, 76–79. [Google Scholar]
- Lee, S.R.; Lee, S.; Moon, E.; Park, H.J.; Park, H.B.; Kim, K.H. Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells. Bioorg. Chem. 2017, 70, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Aminuddin, A.; Susanti, D.; Aminudin, N.I.; On, S.; Ahmad, F.; Hamidon, H. Cytotoxic, anti-inflammatory and adipogenic effects of inophyllum D, calanone, isocordato-oblongic acid, and morelloflavone on cell lines. Nat. Prod. Sci. 2016, 22, 122–128. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Kang, K.S.; Chun, K.H.; Hwang, G.S. Protective effect of Korean Red Ginseng against glucocorticoid-induced osteoporosis in vitro and in vivo. J. Ginseng Res. 2015, 39, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhong, Y.; Li, G. Tubeimoside-1 suppresses breast cancer metastasis through downregulation of CXCR4 chemokine receptor expression. BMB Rep. 2016, 49, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Sohn, J.H.; Kim, Y.C.; Oh, H. Viridicatol from marine-derived fungal strain Penicillium sp. SF-5295 exerts anti-inflammatory effects through inhibiting NF-κB signaling pathway on lipopolysaccharide-induced RAW264.7 and BV2 cells. Nat. Prod. Sci. 2015, 21, 240–247. [Google Scholar] [CrossRef]
- Yoon, H.R.; Paik, Y.S. Antioxidative and prolyl endopeptidase inhibitory activities of the phenolic constituents isolated from Phellinus linteus. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 652–656. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Sharma, L.; Nayak, M.K. Demand-based thiolate anion generation under virtually neutral conditions: Influence of steric and electronic factors on chemo- and regioselective cleavage of aryl alkyl ethers. J. Org. Chem. 2002, 67, 6406–6414. [Google Scholar] [CrossRef] [PubMed]
- Ayer, W.A.; Trifonov, L.S. Drimane sesquiterpene lactones from Peniophora polygonia. J. Nat. Prod. 1992, 55, 1454–1461. [Google Scholar] [CrossRef]
- Chen, Y.K.; Kuo, Y.H.; Chiang, B.H.; Lo, J.M.; Sheen, L.Y. Cytotoxic activities of 9,11-dehydroergosterol peroxide and ergosterol peroxide from the fermentation mycelia of Ganoderma lucidum cultivated in the medium containing leguminous plants on Hep 3B cells. J. Agric. Food Chem. 2009, 57, 5713–5719. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Lu, W.; Xiong, X.; Wu, Z.; Chen, W. Anti-inflammatory constituents from Bidens frondosa. Molecules 2015, 20, 18496–18510. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Lee, D.; Lee, H.J.; Noh, H.J.; Jung, K.; Kang, K.S.; Kim, K.H. Renoprotective chemical constituents from an edible mushroom, Pleurotus cornucopiae in cisplatin-induced nephrotoxicity. Bioorg. Chem. 2017, 71, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S. The NF-κB and IκB proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–681. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Zandi, E.; Karin, M. Bridging the gap: Composition, regulation, and physiological function of the IκB kinase complex. Mol. Cell. Biol. 1999, 19, 4547–4551. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, D.; Jang, T.S.; Kang, K.S.; Nam, J.-W.; Lee, H.-J.; Kim, K.H. Anti-Inflammatory Phenolic Metabolites from the Edible Fungus Phellinus baumii in LPS-Stimulated RAW264.7 Cells. Molecules 2017, 22, 1583. https://doi.org/10.3390/molecules22101583
Lee S, Lee D, Jang TS, Kang KS, Nam J-W, Lee H-J, Kim KH. Anti-Inflammatory Phenolic Metabolites from the Edible Fungus Phellinus baumii in LPS-Stimulated RAW264.7 Cells. Molecules. 2017; 22(10):1583. https://doi.org/10.3390/molecules22101583
Chicago/Turabian StyleLee, Seulah, Dahae Lee, Tae Su Jang, Ki Sung Kang, Joo-Won Nam, Hae-Jeung Lee, and Ki Hyun Kim. 2017. "Anti-Inflammatory Phenolic Metabolites from the Edible Fungus Phellinus baumii in LPS-Stimulated RAW264.7 Cells" Molecules 22, no. 10: 1583. https://doi.org/10.3390/molecules22101583





