Concentration Effect on Quenching of Chlorophyll a Fluorescence by All-Trans-β-Carotene in Photosynthesis
Abstract
:1. Introduction
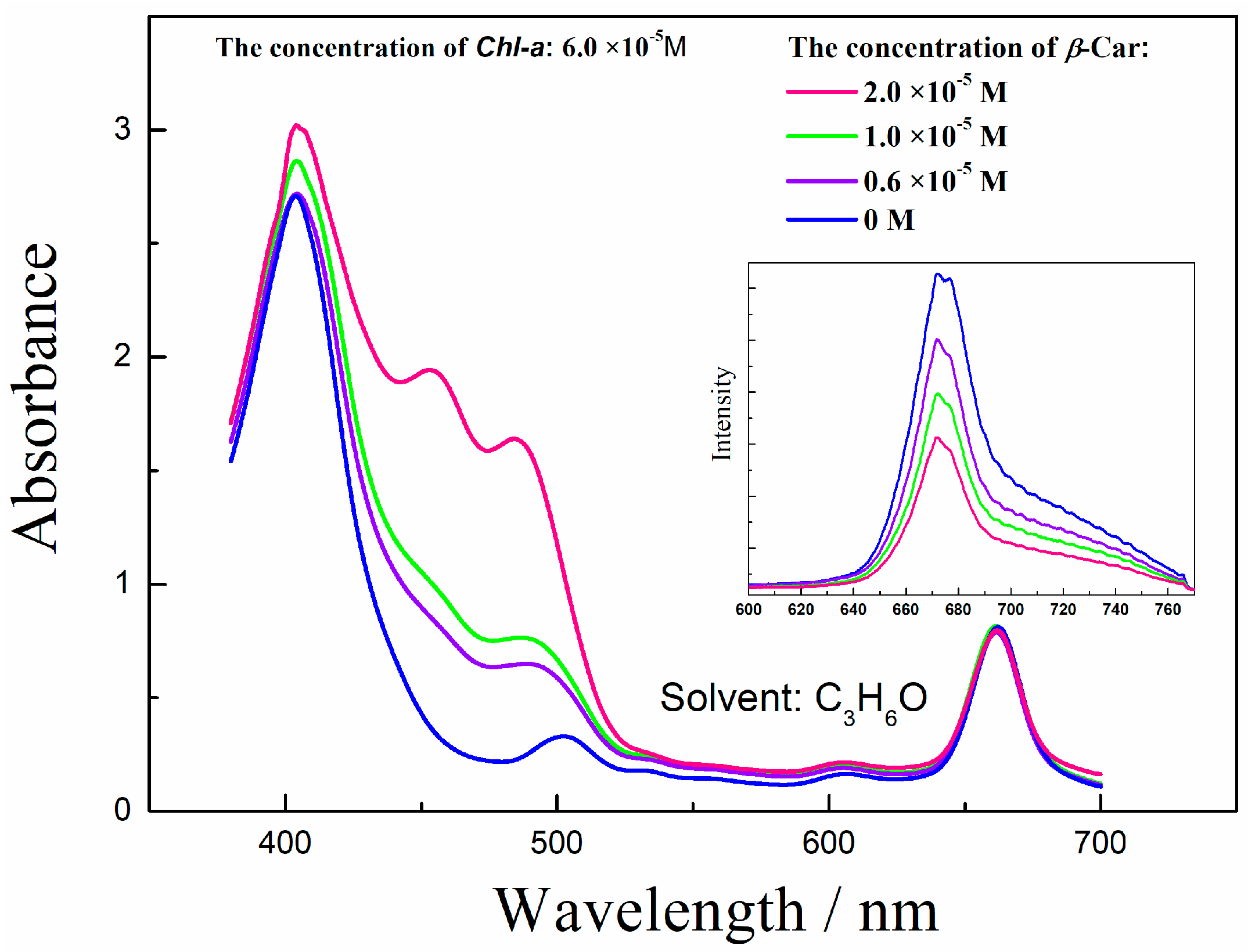
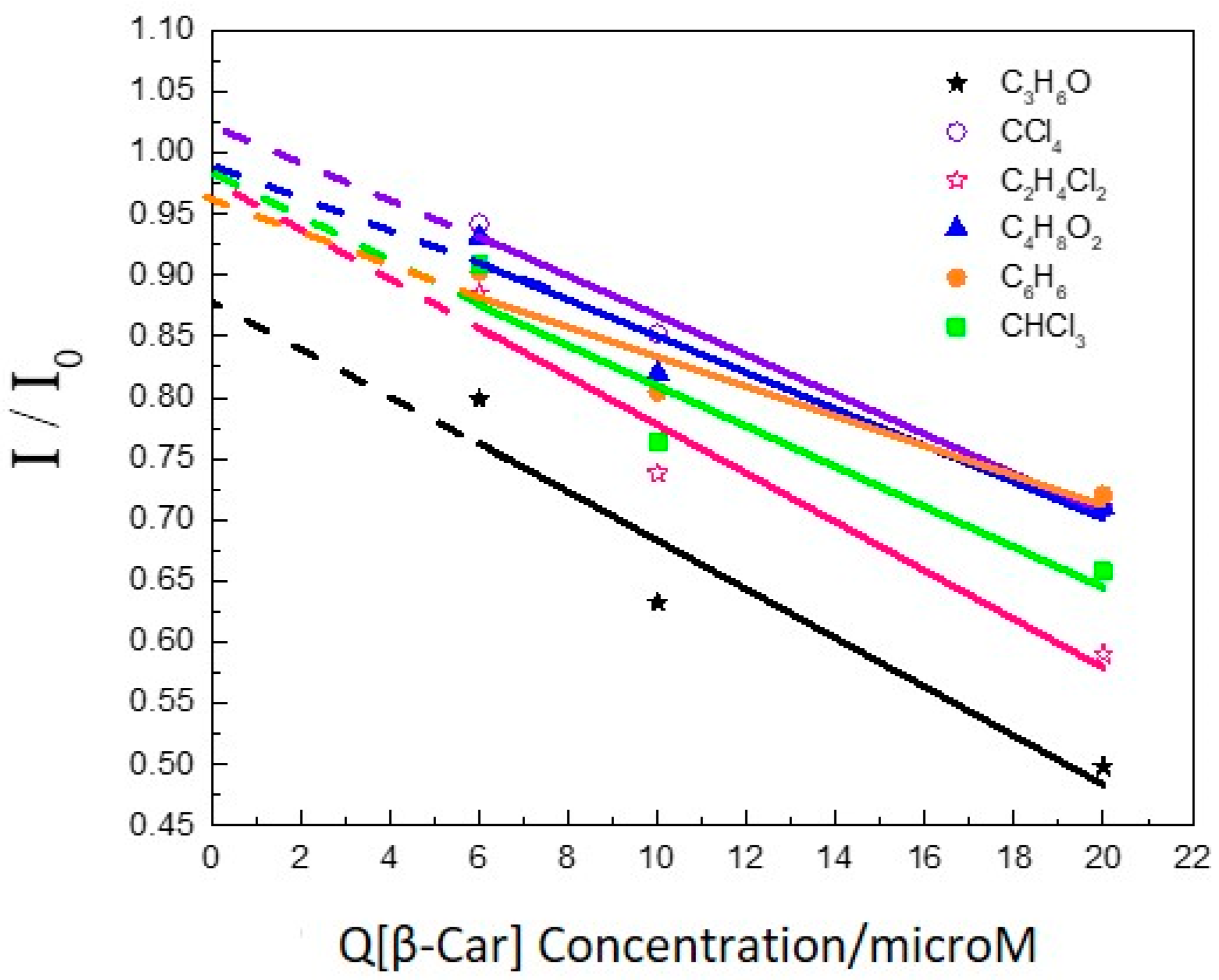
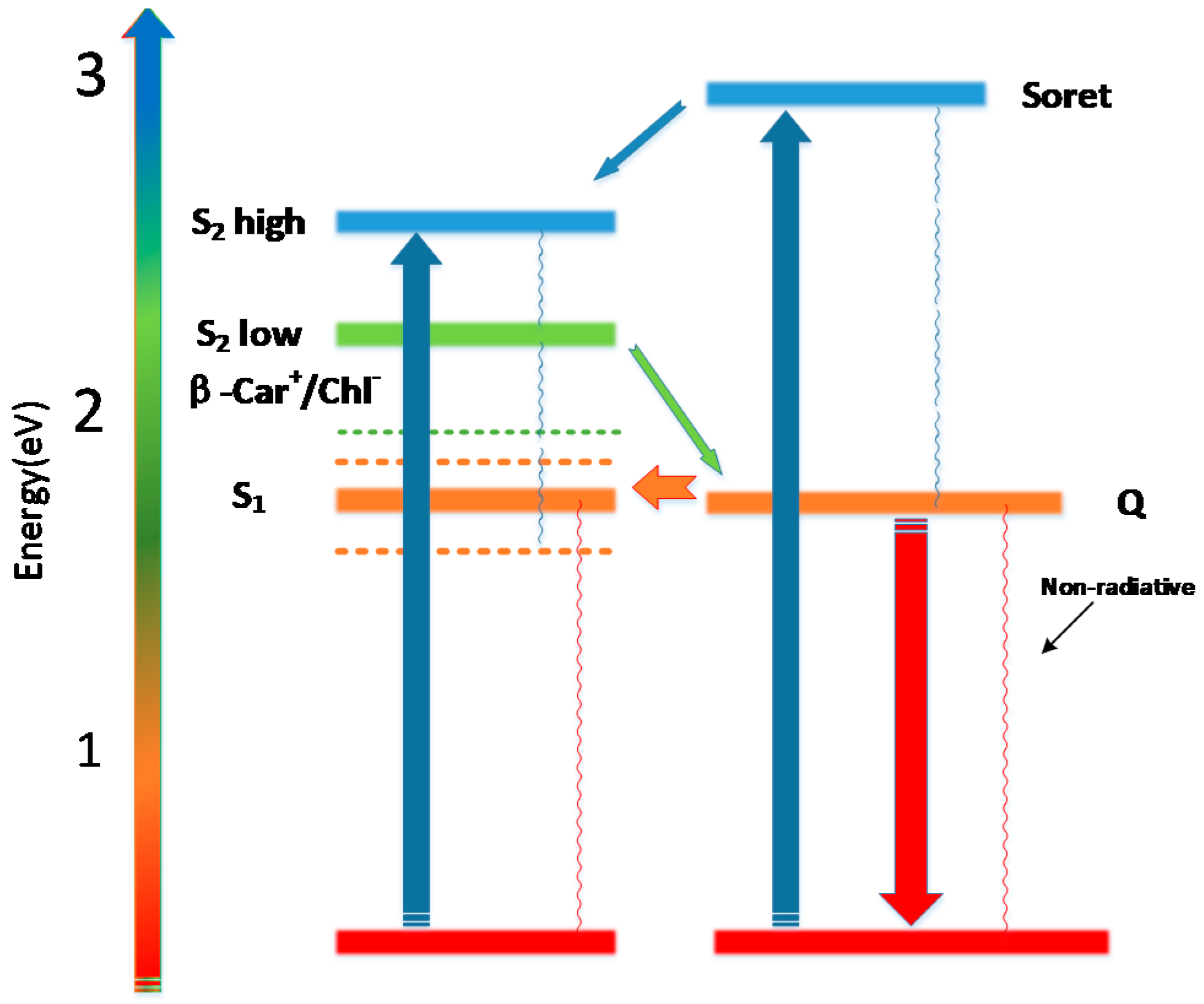
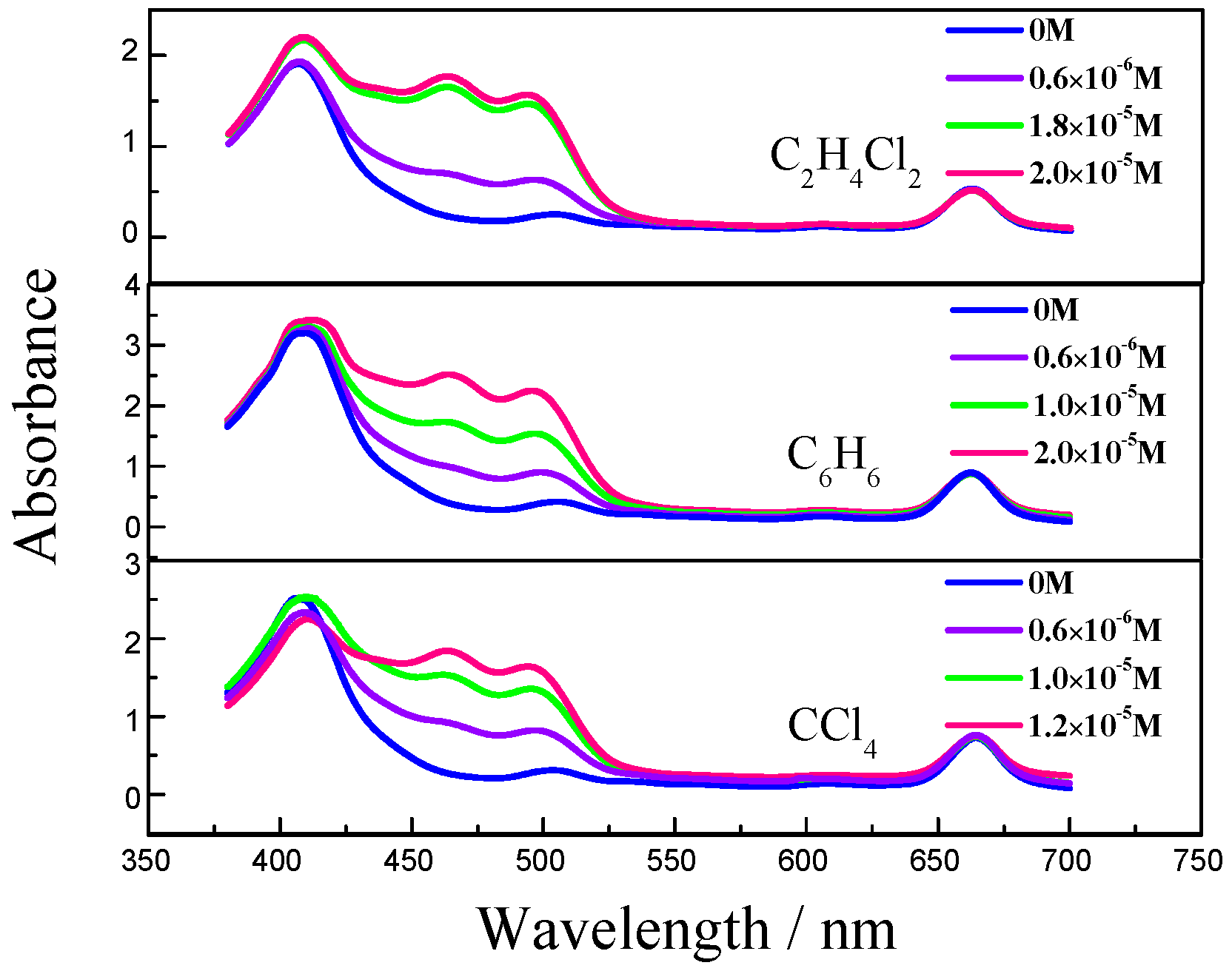
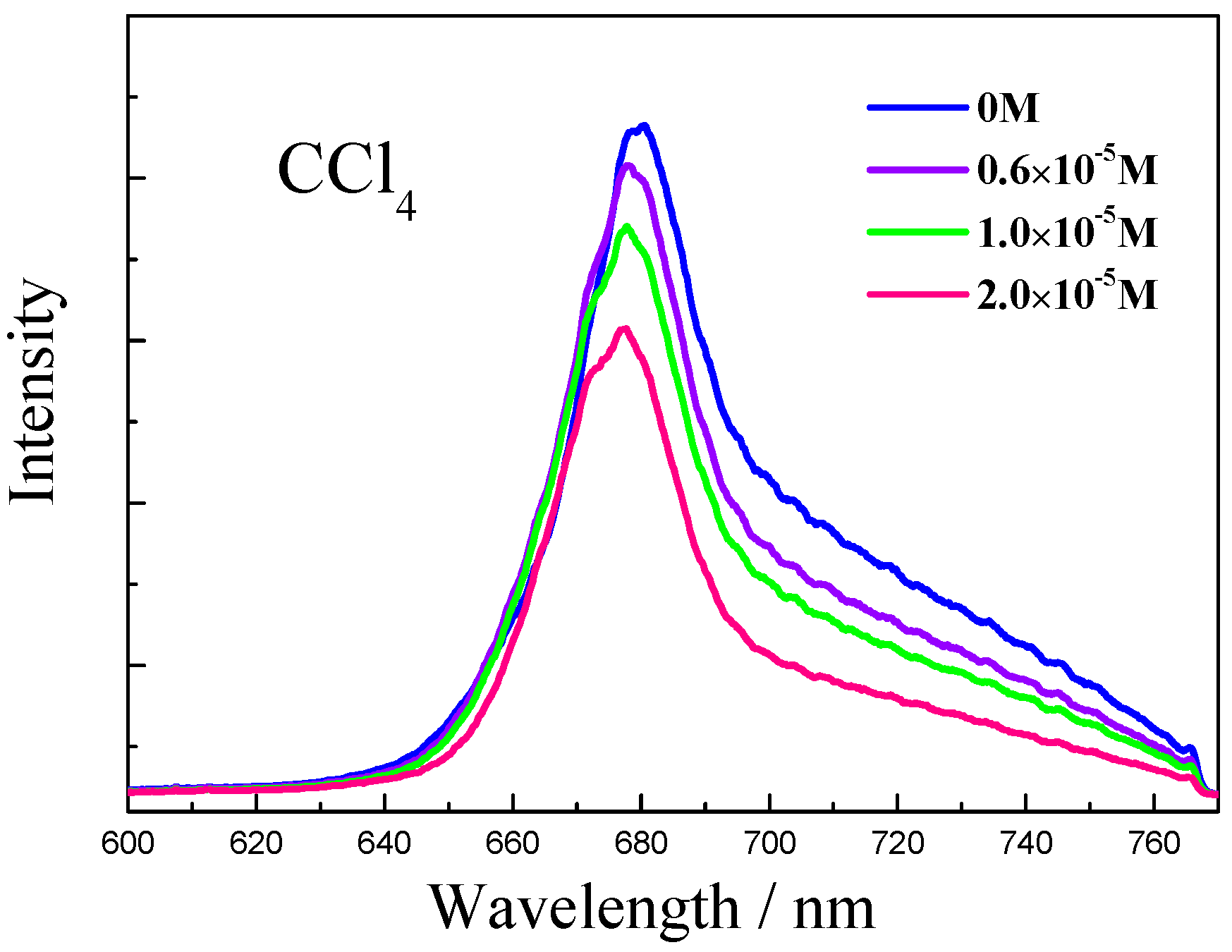
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qin, X.; Suga, M.; Kuang, T.; Shen, J.R. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 2015, 348, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Ballottari, M.; Mozzo, M.; Girardon, J.; Hienerwadel, R.; Bassi, R. Chlorophyll triplet quenching and photoprotection in the higher plant monomeric antenna protein Lhcb5. J. Phys. Chem. B 2013, 117, 11337–11348. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Chen, W.J.; Schlau-Cohen, G.S. Single-molecule fluorescence spectroscopy of photosynthetic systems. Chem. Rev. 2017, 117, 860–898. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, L.; Fiedor, J.; Pilch, M. Effects of molecular symmetry on the electronic transitions in carotenoids. J. Phys. Chem. Lett. 2016, 7, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Frank, H.A.; Cogdell, R.J. Carotenoids in photosynthesis. Photochem. Photobiol. 1996, 63, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ballottari, M.; Truong, T.B.; De Re, E.; Erickson, E.; Stella, G.R.; Fleming, G.R.; Niyogi, K.K. Identification of pH-sensing sites in the light harvesting complex stress-related 3 protein essential for triggering non-photochemical quenching in Chlamydomonas reinhardtii. J. Biol. Chem. 2016, 291, 7334–7346. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.H.; Gruenke, N.L.; Oliver, T.A.; Ballottari, M.; Bassi, R.; Fleming, G.R. Observation of electronic excitation transfer through light harvesting complex II using two-dimensional electronic-vibrational spectroscopy. J. Phys. Chem. Lett. 2016, 7, 4197–4206. [Google Scholar] [CrossRef] [PubMed]
- Gall, A.; Pascal, A.A.; Robert, B. Vibrational techniques applied to photosynthesis: Resonance Raman and fluorescence line-narrowing. BBA-Bioenerg. 2015, 1847, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.K.; Avenson, T.J.; Ballottari, M.; Cheng, Y.C.; Niyogi, K.K.; Bassi, R.; Fleming, G.R. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 2008, 320, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Gong, N.; Li, Z.; Sun, C.; Men, Z. The chlorophyll a fluorescence modulated by all-trans-β-carotene in the process of photosystem II. Int. J. Mol. Sci. 2016, 17, 978. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinto, M.M.; Sansiaume, E.; Hashimoto, H.; Robert, B. Electronic absorption and ground state structure of carotenoid molecules. J. Phys. Chem. B 2013, 117, 11015–11021. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, S.; Bressan, M.; Carbonera, D.; Agostini, A.; Dall’Osto, L. Differential roles of carotenes and xanthophylls in photosystem I photoprotection. Biochemistry 2016, 55, 3636–3649. [Google Scholar] [CrossRef] [PubMed]
- Beddard, G.S.; Porter, G. Concentration quenching in chlorophyll. Nature 1976, 260, 366–367. [Google Scholar] [CrossRef]
- Gazdaru, D.M.; Iorga, B. Characterization of the quenching of chlorophyll a fluorescence by β-carotene using the non-linear analysis. Photosynthetica 2001, 39, 607–609. [Google Scholar] [CrossRef]
- Noguchi, T.; Hayashi, H.; Tasumi, M.; Atkinson, G.H. Solvent effects on the ag carbon-carbon double bond stretching mode in the 21Ag-excited state of beta-carotene and two derivatives: Picosecond time-resolved resonance Raman spectroscopy. J. Phys. Chem. 1991, 95, 3167–3172. [Google Scholar] [CrossRef]
- Connelly, J.P.; Müller, M.G.; Bassi, R.; Croce, R.; Holzwarth, A.R. Femtosecond transient absorption study of carotenoid to chorophyll energy transfer in the light-harvesting complex II of photosystem II. Biochemistry 1997, 36, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Dashdorj, N.; Zhang, H.; Kim, H.; Yan, J.; Cramer, W.A.; Savikhin, S. The single chlorophyll a molecule in the cytochrome b6f complex: Unusual optical properties protect the complex against single oxygen. Biophys. J. 2005, 88, 4178–4187. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.A.; Hiller, R.G.; Sharples, F.P.; Gosztola, D.; Wasielewski, M.; Frank, H.A. Singlet and triplet energy transfer in the peridinin-chlorophyll a-protein from Amphidinium carterae. J. Phys. Chem. A 1999, 103, 2267–2273. [Google Scholar] [CrossRef]
- Wellman, S.M.; Jockusch, R.A. Tuning the intrinsic photophysical properties of chlorophyll a. Chem. A Eur. J. 2017, 23, 7728–7736. [Google Scholar] [CrossRef] [PubMed]
- Bechaieb, R.; Akacha, A.B.; Gérard, H. Quantum chemistry insight into Mg-substitution in chlorophyll by toxic heavy metals: Cd, Hg and Pb. Chem. Phys. Lett. 2016, 663, 27–32. [Google Scholar] [CrossRef]
- Götze, J.P.; Kröner, D.; Banerjee, S.; Karasulu, B.; Thiel, W. Carotenoids as a shortcut for chlorophyll Soret-to-Q band energy flow. ChemPhysChem 2014, 15, 3392–3401. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds all-trans-β-Carotene and Chlorophyll-a are available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Gong, N.; Li, Z.; Sun, C.; Men, Z. Concentration Effect on Quenching of Chlorophyll a Fluorescence by All-Trans-β-Carotene in Photosynthesis. Molecules 2017, 22, 1585. https://doi.org/10.3390/molecules22101585
Chen C, Gong N, Li Z, Sun C, Men Z. Concentration Effect on Quenching of Chlorophyll a Fluorescence by All-Trans-β-Carotene in Photosynthesis. Molecules. 2017; 22(10):1585. https://doi.org/10.3390/molecules22101585
Chicago/Turabian StyleChen, Chen, Nan Gong, Zuowei Li, Chenglin Sun, and Zhiwei Men. 2017. "Concentration Effect on Quenching of Chlorophyll a Fluorescence by All-Trans-β-Carotene in Photosynthesis" Molecules 22, no. 10: 1585. https://doi.org/10.3390/molecules22101585



