Inhibitory Effect of Selaginellins from Selaginella tamariscina (Beauv.) Spring against Cytochrome P450 and Uridine 5′-Diphosphoglucuronosyltransferase Isoforms on Human Liver Microsomes
Abstract
:1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. Reagents
3.2. Microsomal Incubation
3.2.1. Inhibitory Effects of Selaginellins on P450 Activity
3.2.2. Inhibitory Effects of Selaginellins on UGT Activity
3.3. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shin, D.I.; Kim, J. Flavonoid constituents of Selaginella tamariscina. Korean J. Pharmacogn. 1991, 87, 207–210. [Google Scholar]
- Nguyen, P.H.; Ji, D.J.; Han, Y.R.; Choi, J.S.; Rhyu, D.Y.; Min, B.S.; Woo, M.H. Selaginellin and biflavonoids as protein tyrosine phosphatase 1b inhibitors from Selaginella tamariscina and their glucose uptake stimulatory effects. Bioorg. Med. Chem. 2015, 23, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-K.; Noel, J.P. Chemodiversity in selaginella: A reference system for parallel and convergent metabolic evolution in terrestrial plants. Front. Plant Sci. 2013, 4, 119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-P.; Liang, Y.-M.; Wei, X.-C.; Cheng, D.-L. A new unusual natural pigment from selaginella sinensis and its noticeable physicochemical properties. J. Org. Chem. 2007, 72, 3921–3924. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Lin, C.-W.; Hsieh, Y.-S.; Cheng, H.-L.; Lue, K.-H.; Yang, S.-F.; Lu, K.-H. Selaginella tamariscina (Beauv.) possesses antimetastatic effects on human osteosarcoma cells by decreasing MMP-2 and MMP-9 secretions via p38 and akt signaling pathways. Food Chem. Toxicol. 2013, 59, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Chu, S.C.; Liu, S.J.; Chen, Y.C.; Chang, Y.Z.; Hsieh, Y.S. Antimetastatic activities of Selaginella tamariscina (Beauv.) on lung cancer cells in vitro and in vivo. J. Ethnopharmacol. 2007, 110, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.G.; Jing, Y.; Zhang, H.M.; Ma, E.L.; Guan, J.; Xue, F.N.; Liu, H.X.; Sun, X.Y. Isolation and cytotoxic activity of selaginellin derivatives and biflavonoids from Selaginella tamariscina. Planta Medica 2012, 78, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.-H.; Zhao, B.-T.; Ali, M.Y.; Choi, J.-S.; Rhyu, D.-Y.; Min, B.-S.; Woo, M.-H. Insulin-mimetic selaginellins from Selaginella tamariscina with protein tyrosine phosphatase 1B (PTP1B) inhibitory activity. J. Nat. Prod. 2015, 78, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.K.; Zhang, L.; Wang, W.W.; Wu, Y.Y.; Zhang, Q.B.; Feng, W.S. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) spring in rats induced by high fat diet and low dose stz. J. Ethnopharmacol. 2011, 137, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.-J.; Tan, N.-H.; Wu, Y.-P.; Yang, J.; Wang, Q. Structure determination of selaginellins g and h from selaginella pulvinata by nmr spectroscopy. Magn. Reson. Chem. 2010, 48, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.-J.; Tan, N.-H.; Oberer, L.; Wagner, T.; Wu, Y.-P.; Zeng, G.-Z.; Yan, H.; Wang, Q. Antimicrobial selaginellin derivatives from selaginella pulvinata. Bioorg. Med. Chem. Lett. 2010, 20, 2456–2460. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shao, Y.; Li, K.; Xia, W. Bioactive selaginellins from Selaginella tamariscina (Beauv.) spring. Beilstein J. Org. Chem. 2012, 8, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.K.; Wang, W.W.; Zhang, L.; Su, C.F.; Wu, Y.Y.; Ke, Y.Y.; Hou, Q.W.; Liu, Z.Y.; Gao, A.S.; Feng, W.S. Antihyperlipidaemic and antioxidant effect of the total flavonoids in Selaginella tamariscina (Beauv.) spring in diabetic mice. J. Pharm. Pharmacol. 2013, 65, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-F.; Xu, Y.-Y.; Xu, K.-P.; Wu, W.-H.; Tan, G.-S.; Li, Y.-J.; Hu, C.-P. Inhibitory effect of selaginellin on high glucose-induced apoptosis in differentiated pc12 cells: Role of nadph oxidase and lox-1. Eur. J. Pharmacol. 2012, 694, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Chang, J. Medicinal herbs: Drugs or dietary supplements? Biochem. Pharmacol. 2000, 59, 211–219. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Zhou, Z.-W.; Li, C.-G.; Chen, X.; Yu, X.; Xue, C.C.; Herington, A. Identification of drugs that interact with herbs in drug development. Drug Discov. Today 2007, 12, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St john’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Spence, J.D.; Munoz, C.; Arnold, J.M. Interaction of citrus juices with felodipine and nifedipine. Lancet 1991, 337, 268–269. [Google Scholar] [CrossRef]
- Mai, I.; Bauer, S.; Perloff, E.S.; Johne, A.; Uehleke, B.; Frank, B.; Budde, K.; Roots, I. Hyperforin content determines the magnitude of the st john’s wort-cyclosporine drug interaction. Clin. Pharmacol. Ther. 2004, 76, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Goosen, T.C.; Cillie, D.; Bailey, D.G.; Yu, C.; He, K.; Hollenberg, P.F.; Woster, P.M.; Cohen, L.; Williams, J.A.; Rheeders, M.; et al. Bergamottin contribution to the grapefruit juice-felodipine interaction and disposition in humans. Clin. Pharmacol. Ther. 2004, 76, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Wienkers, L.C.; Heath, T.G. Predicting in vivo drug interactions from in vitro drug discovery data. Nat. Rev. Drug Discov. 2005, 4, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Dresser, G.K. Interactions between grapefruit juice and cardiovascular drugs. Am. J. Cardiovasc. Drugs 2004, 4, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Zhao, Z.Q.; Qin, Z.S.; Wu, K.; Xia, T.F.; Pang, L.Q. Herb-drug interaction between irinotecan and psoralidin-containing herbs. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Krippendorff, B.F.; Lienau, P.; Reichel, A.; Huisinga, W. Optimizing classification of drug-drug interaction potential for cyp450 isoenzyme inhibition assays in early drug discovery. J. Biomol. Screen. 2007, 12, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Backman, J.T.; Filppula, A.M.; Niemi, M.; Neuvonen, P.J. Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol. Rev. 2016, 68, 168–241. [Google Scholar] [CrossRef] [PubMed]
- Sahi, J.; Black, C.B.; Hamilton, G.A.; Zheng, X.; Jolley, S.; Rose, K.A.; Gilbert, D.; LeCluyse, E.L.; Sinz, M.W. Comparative effects of thiazolidinediones on in vitro p450 enzyme induction and inhibition. Drug Metab. Dispos. 2003, 31, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H.; Cha, I.J.; Park, J.S.; Shon, J.H.; Liu, K.H.; Shin, J.G. High-throughput screening of inhibitory potential of nine cytochrome p450 enzymes in vitro using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Walsky, R.L.; Gaman, E.A.; Obach, R.S. Examination of 209 drugs for inhibition of cytochrome P450 2C8. J. Clin. Pharmacol. 2005, 45, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome p450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.; Ensom, M.H.; Chang, T.K. Udp-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol. Ther. 2005, 106, 97–132. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Hyland, R.; Jones, B.C.; Smith, D.A.; Hurst, S.; Goosen, T.C.; Peterkin, V.; Koup, J.R.; Ball, S.E. Drug-drug interactions for udp-glucuronosyltransferase substrates: A pharmacokinetic explanation for typically observed low exposure (auci/auc) ratios. Drug Metab. Dispos. 2004, 32, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- India University School of Medicine. Department of Medicine: Clinical Pharmacology: Main Table—Drug Interactions: P450 Drug Interaction Table: Substrates. Available online: http://medicine/iupui.edu/clinpharm/ddis/main-table/ (accessed on 20 September 2017).
- Yang, S. The Divine Farmer’s Material Medica: A Translation of the Shen Nong Ben Cao Jing, 1st ed.; Blue Poppy Press: Boulder, CO, USA, 1998. [Google Scholar]
- Joo, J.; Lee, B.; Lee, T.; Liu, K.H. Screening of six ugt enzyme activities in human liver microsomes using liquid chromatography/triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Wu, Z.; Sung, S.H.; Lee, T.; Song, K.S.; Lee, M.Y.; Liu, K.H. Potential of decursin to inhibit the human cytochrome p450 2J2 isoform. Food Chem. Toxicol. 2014, 70, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kang, W.; Shon, J.; Park Ki, H.; Song, K.-S.; Liu, K.-H. Potential of 4′-(p-toluenesulfonylamide)-4-hydroxychalcone to inhibit the human cytochrome p450 2j2 isoform. Appl. Biol. Chem. 2014, 57, 31–34. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
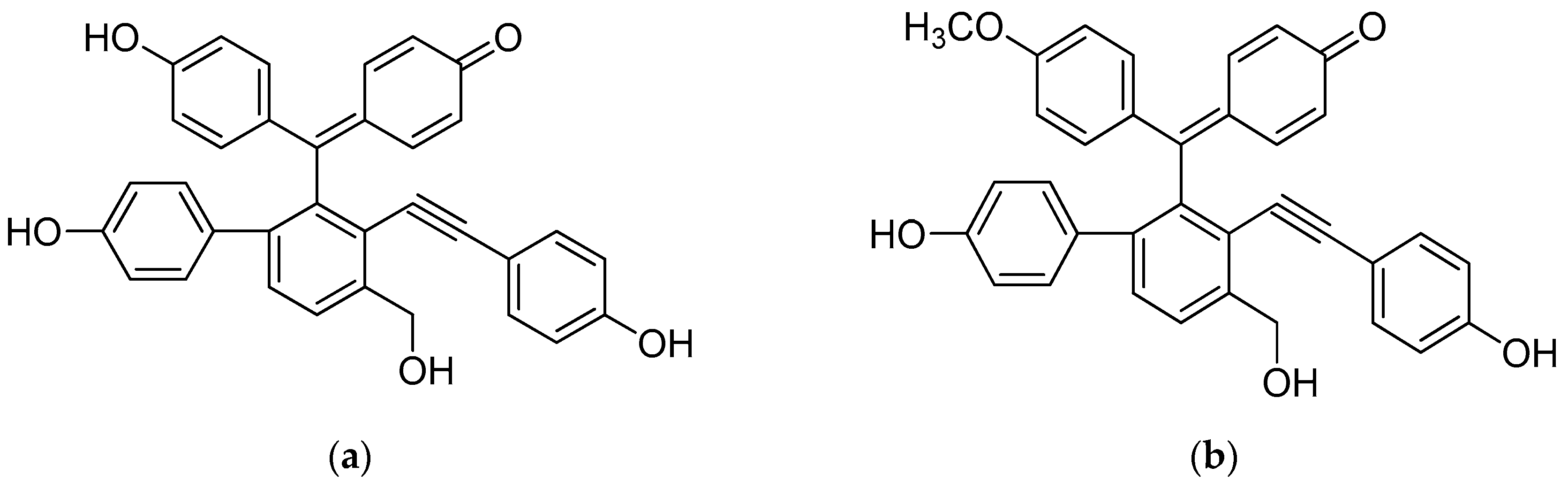

| Compound | IC50 (μM) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P450 Isoforms | UGT Isoforms | |||||||||||||||
| 1A2 | 2A6 | 2B6 | 2C8 | 2C9 | 2C19 | 2D6 | 2E1 | 2J2 | 3A | 1A1 | 1A3 | 1A4 | 1A6 | 1A9 | 2B7 | |
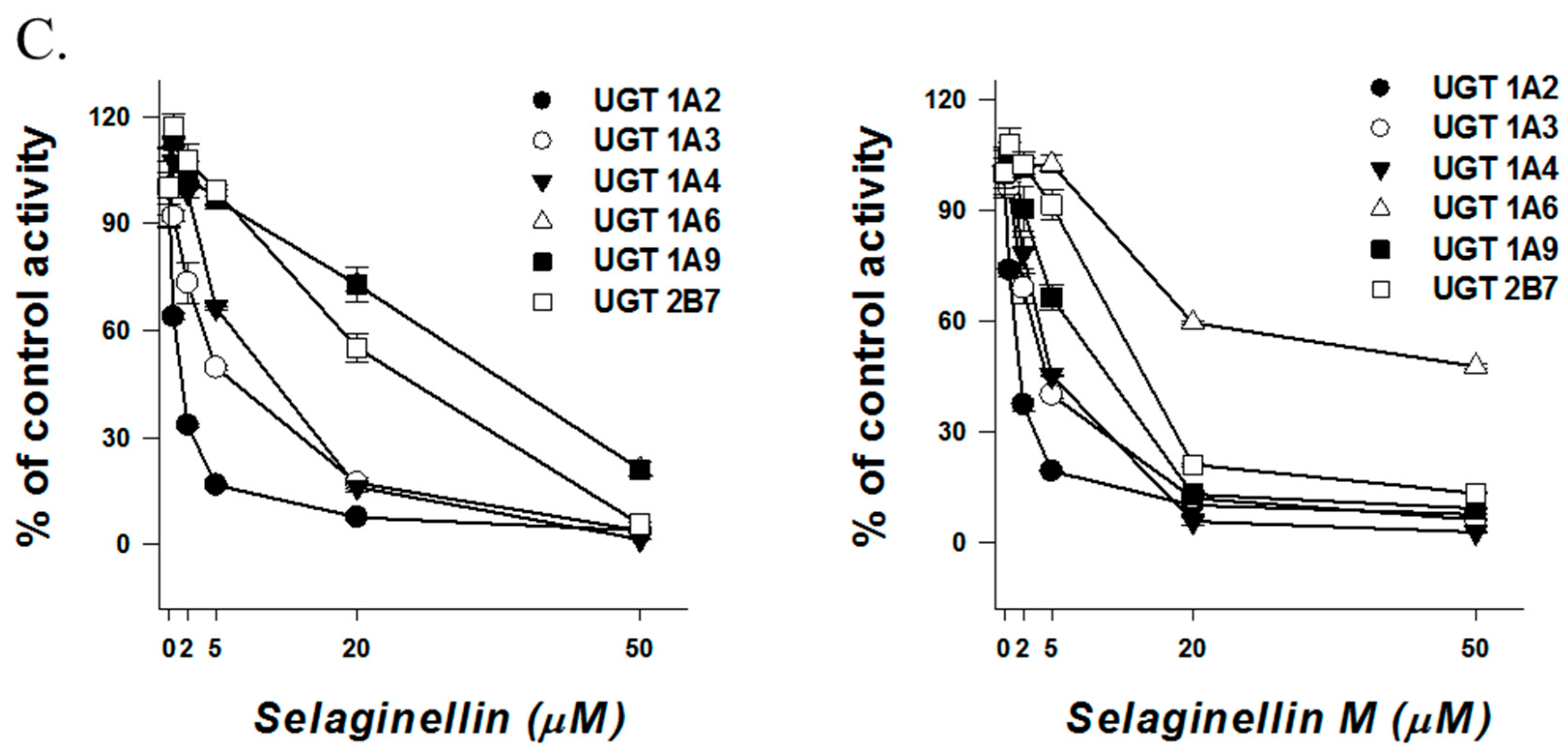
| Selaginellin | 36.4 | >50 | 10.7 | 0.5 | 1.2 | 10.0 | 5.8 | 38.5 | 0.8 | 11.7 | 1.0 | 4.7 | 6.6 | 25.3 | 8.7 | 15.6 |
| Selaginellin M | >50 | >50 | 11.3 | 0.9 | 3.9 | 16.1 | 6.8 | >50 | 2.7 | >50 | 1.3 | 3.5 | 3.9 | 36.5 | 6.5 | 10.4 |
| Enzyme | Substrate | Concentration (μM) | Metabolite | Transition (m/z) | Collision Energy (eV) | Polarity * |
|---|---|---|---|---|---|---|
| CYP1A2 | Phenacetin | 100 | Acetaminophen | 152 > 110 | 25 | ESI+ |
| CYP2A6 | Coumarin | 5.0 | Hydroxycoumarin | 163 > 107 | 17 | ESI+ |
| CYP2B6 | Bupropion | 50 | Hydroxybupropion | 256 > 238 | 20 | ESI+ |
| CYP2C8 | Amodiaquine | 1.0 | N-Desethylamodiaquine | 328 > 283 | 17 | ESI+ |
| CYP2C9 | Tolbutamide | 100 | Hydroxytolbutamide | 287 > 89 | 42 | ESI+ |
| CYP2C19 | Omeprazole | 20 | Hydroxyomeprazole | 362 > 214 | 10 | ESI+ |
| CYP2D6 | Dextromethorphan | 5.0 | Dextrorphan | 258 > 157 | 35 | ESI+ |
| CYP2E1 | Chlorzoxazone | 50 | Hydroxychlorzoxazone | 184 > 120 | 15 | ESI− |
| CYP2J2 | Astemizole | 1.0 | O-Desmethyl astemizole | 445 > 204 | 35 | ESI+ |
| CYP3A | Midazolam | 5.0 | Hydroxymidazolam | 342 > 203 | 25 | ESI+ |
| UGT1A1 | SN-38 | 0.5 | SN-38-glucuronide | 569 > 393 | 30 | ESI+ |
| UGT1A3 | Chenodeoxycholic acid | 2.0 | Chenodeoxycholic acid glucuronide | 567 > 391 | 35 | ESI− |
| UGT1A4 | Trifluoperazine | 0.5 | Trifluoperazine glucuronide | 584 > 408 | 25 | ESI+ |
| UGT1A6 | N-Acetylserotonin | 1.0 | N-Acetylserotonin glucuronide | 395 > 219 | 15 | ESI+ |
| UGT1A9 | Mycophenolic acid | 0.2 | Mycophenolic acid glucuronide | 495 > 319 | 20 | ESI− |
| UGT2B7 | Naloxone | 1.0 | Naloxone glucuronide | 504 > 310 | 30 | ESI+ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, J.-K.; Nguyen, P.-H.; Kim, W.C.; Phuc, N.M.; Liu, K.-H. Inhibitory Effect of Selaginellins from Selaginella tamariscina (Beauv.) Spring against Cytochrome P450 and Uridine 5′-Diphosphoglucuronosyltransferase Isoforms on Human Liver Microsomes. Molecules 2017, 22, 1590. https://doi.org/10.3390/molecules22101590
Heo J-K, Nguyen P-H, Kim WC, Phuc NM, Liu K-H. Inhibitory Effect of Selaginellins from Selaginella tamariscina (Beauv.) Spring against Cytochrome P450 and Uridine 5′-Diphosphoglucuronosyltransferase Isoforms on Human Liver Microsomes. Molecules. 2017; 22(10):1590. https://doi.org/10.3390/molecules22101590
Chicago/Turabian StyleHeo, Jae-Kyung, Phi-Hung Nguyen, Won Cheol Kim, Nguyen Minh Phuc, and Kwang-Hyeon Liu. 2017. "Inhibitory Effect of Selaginellins from Selaginella tamariscina (Beauv.) Spring against Cytochrome P450 and Uridine 5′-Diphosphoglucuronosyltransferase Isoforms on Human Liver Microsomes" Molecules 22, no. 10: 1590. https://doi.org/10.3390/molecules22101590





