Therapeutic Mechanisms of Vernonia amygdalina Delile in the Treatment of Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Vernonia amygdalina Delile Preparation
2.3. Tissue/Cell Culture
2.4. Cell Treatment and Determination Cell Viability
2.5. Measurement of Malondialdehyde (MDA) Level by Lipid Peroxidation Assay
2.6. Evaluation of DNA Damage by Comet Assay
2.7. Evaluation of Apoptosis and/or Necrosis by Annexin V FITC/PI Assay
2.8. Statistical Analysis
3. Results
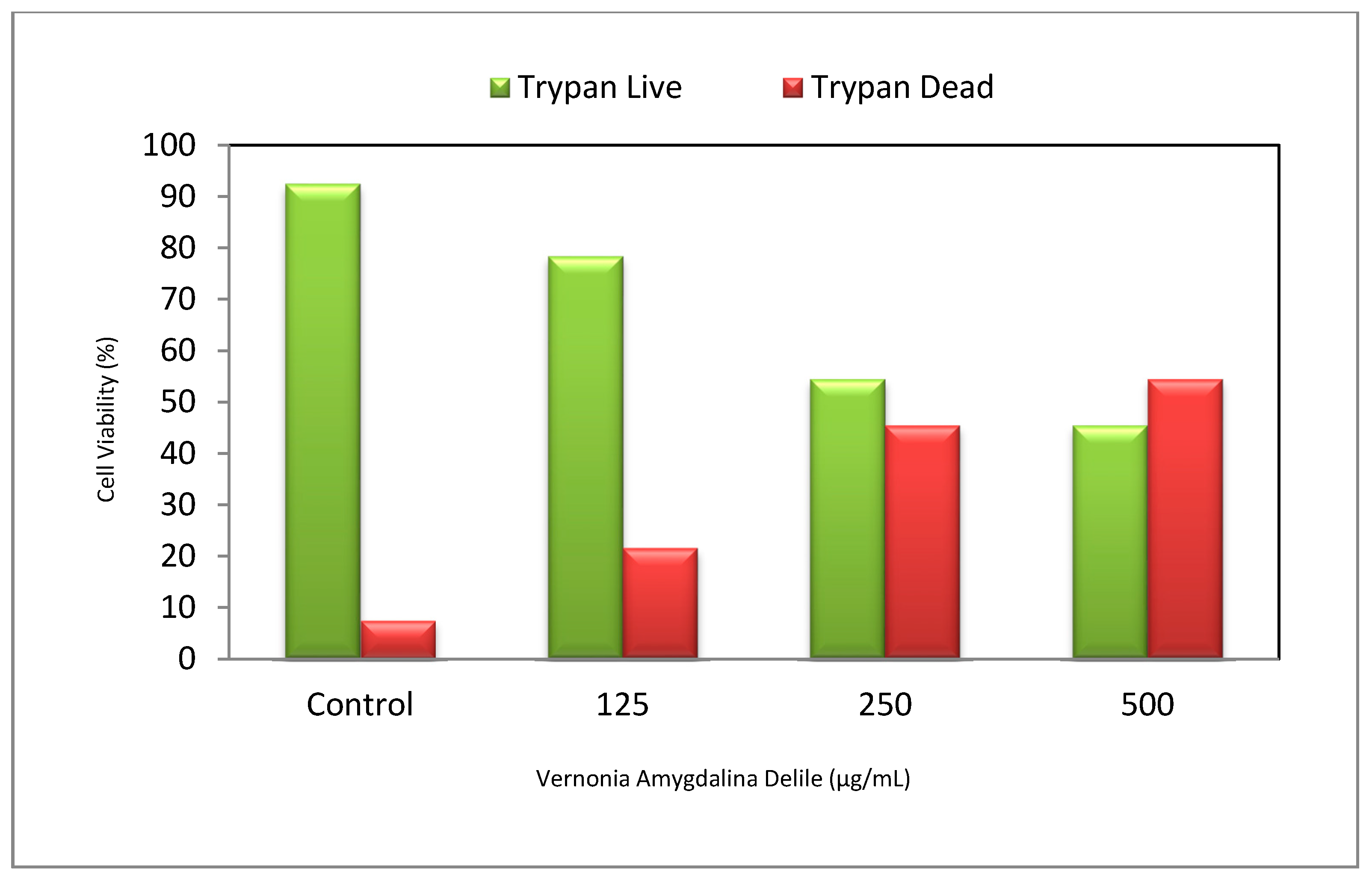
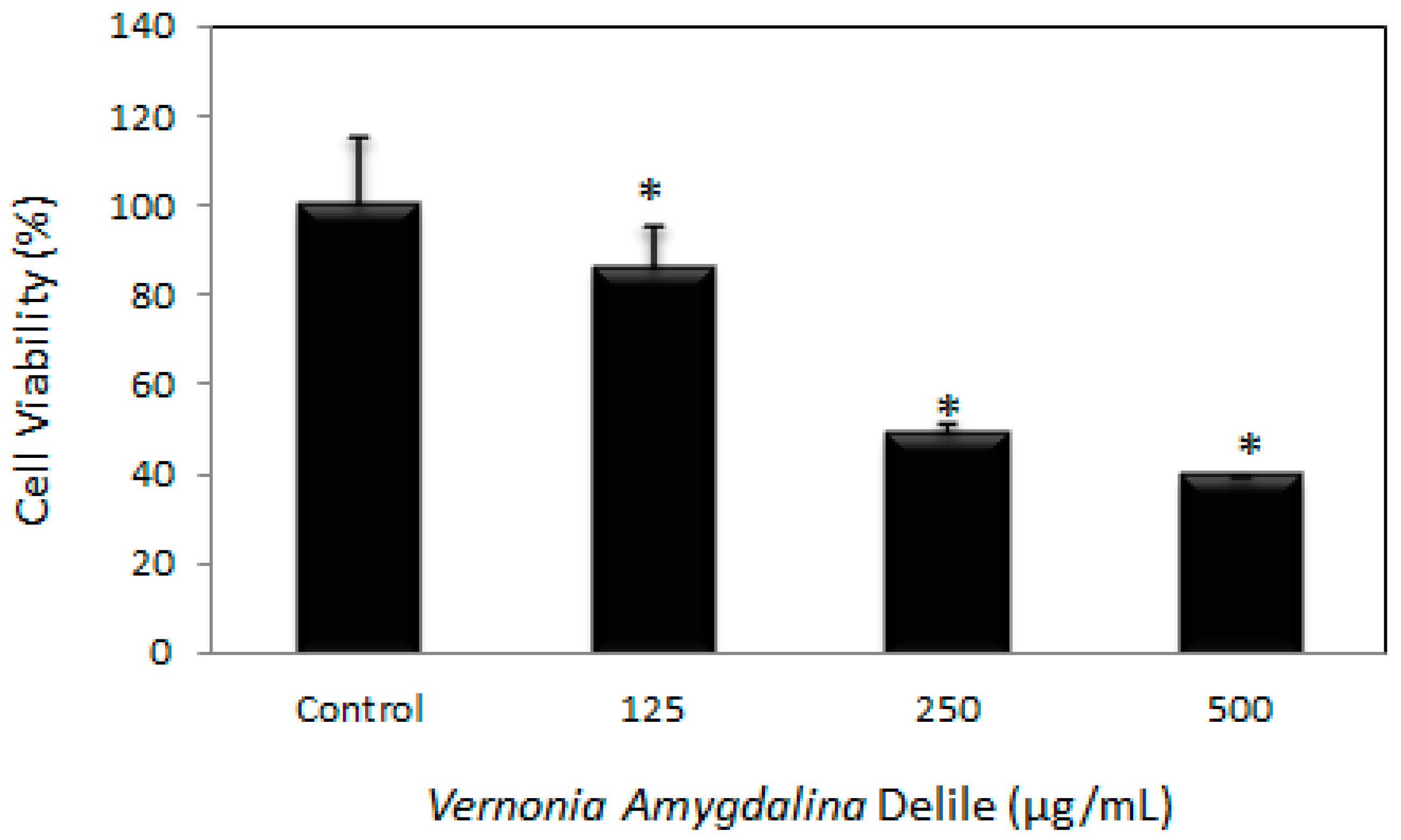
3.1. Cytotoxic Efficacy of Vernonia amygdalina Delile on PC-3 Human Prostate Carcinoma Cells
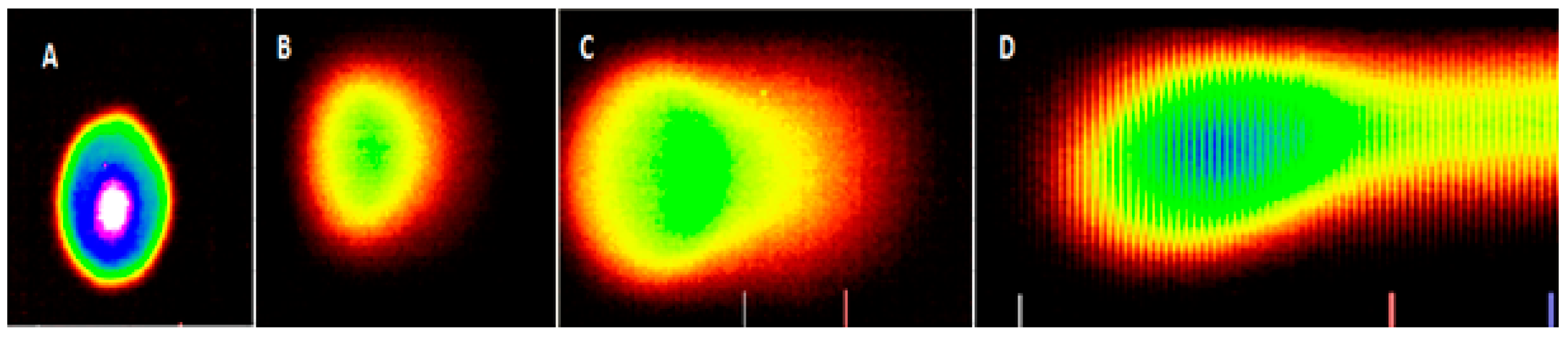
3.2. Morphological Effects of Vernonia amygdalina Delile on PC-3 Human Prostate Carcinoma Cells
3.3. Vernonia amygdalina Delile Modulated Oxidative Stress on PC-3 Human Prostate Carcinoma Cells
3.4. Genotoxic Effects of Vernonia amygdalina Delile on PC-3 Human Prostate Carcinoma Cells
3.5. Apoptotic and Necrotic Effects of Vernonia amygdalina Delile on PC-3 Human Prostate Carcinoma Cells
4. Discussion
4.1. Cytotoxic Efficacy of Vernonia amygdalina Delile on PC-3 Human Prostate Carcinoma Cells
4.2. Vernonia amygdalina Delile-Modulated Oxidative Stress on PC-3 Human Prostate Carcinoma Cells
4.3. Genotoxic Effects of Vernonia amygdalina Delile on PC-3 Human Prostate Carcinoma Cells
4.4. Apoptotic and Necrotic Effects of Vernonia amygdalina Delile on PC-3 Human Prostate Carcinoma Cells
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.H.; Vessella, R.L. Mechanisms, Hypotheses and Questions Regarding Prostate Cancer Micrometastases to Bone. Cancer Metastasis Rev. 1998, 4, 331–336. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Roudier, M.P.; True, L.D.; Higano, C.S.; Vesselle, H.; Ellis, W.; Lange, P.; Vessella, R.L. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum. Pathol. 2003, 34, 646–653. [Google Scholar] [CrossRef]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 1, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Kaelin, W.G., Jr. Using cancer genetics to guide the selection of anticancer drug targets. Curr. Opin. Pharmacol. 2002, 2, 366–373. [Google Scholar] [CrossRef]
- McGarvey, T.W.; Meng, R.D.; Johnsona, O.; El-Deiry, W.; Malkowicz, S.B. Growth inhibitory effect of p21 and p53 containing adenoviruses on transitional cell carcinoma cell lines in vitro and in vivo. Urol. Oncol. 2001, 6, 155–162. [Google Scholar] [CrossRef]
- Surh, Y.J. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem. Toxicol. 2002, 40, 1091–1097. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Oomen, D.; Lemmens, V.; Oenema, A.; Benetou, V.; Trichopoulou, A.; Coebergh, J.W.; Barendregt, J.; de Vries, E. Increased consumption of fruit and vegetable and future cancer incidence in selected European countries. Eur. J. Cancer 2010, 46, 2563–2580. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113, 102–110. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Traditional, Complementary and Alternative Medicines and Therapies, Working Group OPS/OMS; WHO Regional Office for the Americas/Pan American Health Organization: Washington, DC, USA, 1999. [Google Scholar]
- World Health Organization. WHO Traditional Medicine Strategy 2002–2005; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Fouladbakhsh, J.M.; Stommel, M. Gender, symptom experience, and use of complementary and alternative medicine practices among cancer survivors in the, U.S. cancer population. Oncol. Nurs. Forum 2010, 37, E7–E15. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Farrar, J.T.; Xie, S.X.; Bowman, M.A.; Armstrong, K. Use of complementary and alternative medicine and prayer among a national sample of cancer survivors compared to other populations without cancer. Complement. Ther. Med. 2007, 15, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.L.; Ganz, P.A.; Bernstein, L. Complementary and alternative therapies among very long-term breast cancer survivors. Breast Cancer Res. Treat. 2009, 116, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.M.; Davis, R.B.; Ettner, S.L.; Appel, S.; Wilkey, S.; Van Rompay, M.; Kessler, R.C. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA 1998, 280, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.; Ige, T.; Ude, N.A.; Omotoso, O.D.; Alege, G.O.; Balogun, J.O.; Asuquo, E.I.; Taiga, A. Aqueous extract of bitter leaf Vernonia amygdalina Delile (Asterales: Asteraceae) ameliorates testosterone-induced benign prostatic hyperplasia (BPH) in Wistar rats. Braz. J. Biol. Sci. 2017, 4, 45–52. [Google Scholar] [CrossRef]
- Izevbigie, E.B. Discovery of water soluble anticancer agents (Edotides) from vegetables found in Benin City, Nigeria. Exp. Biol. Med. 2003, 228, 293–298. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Izevbigie, E.B.; Tchounwou, P.B. Preclinical assessment of Vernonia amygdalina leaf extracts as DNA damaging anti-cancer agent in the management of breast cancer. Int. J. Environ. Res. Public Health 2008, 5, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Izevbigie, E.B.; Tchounwou, P.B. Vernonia amygdalina-Induced Growth Arrest and Apoptosis of Breast Cancer (MCF-7) Cells. 2013. Pharmacol. Pharm. 2013, 4, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Akah, P.A.; Okafor, C.I. Hyperglycemic effect of Vernonia amygdalina Del. on experimental rabbit. Plant Med. Res. 1992, 1, 6–10. [Google Scholar]
- Ebong, P.E.; Atangwho, I.J.; Eyong, E.U.; Egbung, G.E. The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica (A. Juss) (Neem) and Vernonia amygdalina (Del.) (African bitter leaf). Am. J. Biochem. Biotechnol. 2008, 4, 239–244. [Google Scholar] [CrossRef]
- Dugo, E.B.; Yedjou, C.G.; Stevens, J.J.; Tchounwou, P.B. Therapeutic Potential of Arsenic Trioxide (ATO) in Treatment of Hepatocellular Carcinoma: Role of Oxidative Stress in ATO-Induced Apoptosis. Ann. Clin. Pathol. 2017, 5, 1101. [Google Scholar]
- Collins, A.R.; Dusinská, M.; Horská, A. Detection of alkylation damage in human lymphocyte DNA with the comet assay. Acta Biochim. Pol. 2001, 48, 611–614. [Google Scholar] [PubMed]
- Collins, A.R. Comet Assay for DNA damage and repair: Principles, applications and limitations. Mol. Biotechnol. 2001, 26, 249–261. [Google Scholar] [CrossRef]
- Hao, X.N.; Chan, S.W.; Chen, S.L. Detection of Puerarin and Danshensu in traditional Chinese medicinal preparation containing Pueraria lobata and Salvia Miltiorrhiza by high-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 2779–2787. [Google Scholar] [CrossRef]
- Hong, J.Y.; Nam, J.W.; Seo, E.K.; Lee, S.K. Daphnane diterpene esters with anti-proliferative activities against human lung cancer cells from Daphne genkwa. Chem. Pharm. Bull. 2010, 58, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Toyang, N.J.; Ateh, E.N.; Davis, H.; Tane, P.; Sondengam, L.B.; Bryant, J.; Verpoorte, R. In vivo antiprostate tumor potential of Vernonia guineensis Benth. (Asteraceae) tuber extract (VGDE) and the cytotoxicity of its major compound pentaisovaleryl sucrose. J. Ethnopharmacol. 2013, 150, 724–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Rizwani, G.H.; Guo, H.; Ahmed, M.; Ahmed, M.; Hassan, S.Z.; Hassan, A.; Chen, Z.S.; Xu, R.H. Annona squamosa Linn: Cytotoxic activity found in leaf extract against human tumor cell lines. Pak. J. Pharm. Sci. 2014, 27, 1559–1563. [Google Scholar] [PubMed]
- Xiao-Dong, S.; Xing, -E.L.; Dong-Sheng, H. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol. Med. Rep. 2012, 6, 1267–1270. [Google Scholar]
- Tsabang, N.; Yedjou, C.G.; Tsambang, L.W.D.; Tchinda, A.T.; Donfagsiteli, N.; Agbor, G.A.; Tchounwou, P.B.; Nkongmeneck, B.A. Treatment of diabetes and/or hypertension using medicinal plants in Cameroon. Med. Aromat Plants 2015, Suppl. 2, 3. [Google Scholar]
- Mukherjee, A.K.; Basu, S.; Sarkar, N.; Ghosh, A.C. Advances in cancer therapy with plant based natural products. Curr. Med. Chem. 2001, 8, 1467–1486. [Google Scholar] [CrossRef] [PubMed]
- Gratus, C.; Wilson, S.; Greenfield, S.M.; Damery, S.L.; Warmington, S.A.; Grieve, R.; Steven, N.; Jones, J.; Greenfield, S. The use of herbal medicines by people with cancer in the UK: A systematic review of the literature. QJM-Int. J. Med. 2009, 102, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Miar, S.; Zadehzare, M.M.; Akbari, H. Evaluation of Effectiveness of Herbal Medication in Cancer Care: A Review Study. Iran. J. Cancer Prev. 2012, 5, 144–156. [Google Scholar] [PubMed]
- Wheat, J.; Currie, G. Herbal medicine for cancer patients: An evidence based review. Int. J. Altern. Med. 2008, 5, 28–30. [Google Scholar]
- Joel, L.S. The Dual Roles of Nutrients as Antioxidants and Pro-Oxidants: Their Effects of Tumor Cell Growth. In Proceedings of the “Prooxidant Effects of Antioxidant Vitamins” Experimental Biology Meeting, Atlanta, GA, USA, 13 April 1995; Herbert, V., Ed.; American Society of Nutrition: Bethesda, MD, USA, 1995; pp. 1221–1226. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [PubMed]
- Ahsan, H.; Hadi, S.M. Strand Scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett. 1998, 124, 23–30. [Google Scholar] [CrossRef]
- Rahman, A.; Shahabuddin, M.; Hadi, S.M.; Parish, J. Complexes involving quercetin, DNA and Cu(II). Carcinogenesis 1990, 11, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Farhan, A.S.; Ahmad, A.; Khan, N.U.; Hadi, S.M. Oxidative DNA damage by capsaicin and dihydrocapsaicin in the presence of Cu(II). Cancer Lett. 2001, 169, 139–146. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Leonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Lee, H.; Yoo, Y.S.; Hah, D.Y.; Kim, C.H.; Kim, E.; Kim, J.S. Evaluation of oxidative DNA damage using an alkaline single cell gel electrophoresis (SCGE) comet assay, and the protective effects of N-acetylcysteine amide on zearalenone-induced cytotoxicity in chang liver cells. Toxicol. Res. 2013, 29, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ventura, L.; Giovannini, A.; Savio, M.; Donà, M.; Macovei, A.; Buttafava, A.; Balestrazzi, A. Single cell gel electrophoresis (comet) assay with plants: Research on DNA repair and ecogenotoxicity testing. Chemosphere 2013, 92, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Tchounwou, P.B. In Vitro genotoxic effect of arsenic trioxide to human leukemia (HL-60) cells using the comet assay. Mol. Cell. Biochem. 2007, 301, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Sutton, L.; Tchounwou, P.B. Genotoxic mechanisms of arsenic trioxide effect in human Jurkat T-lymphoma cells. Met. Ions Biol. Med. 2008, 10, 495–499. [Google Scholar] [PubMed]
- Hanahan, D.; Robert, A.W. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010, 76, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Ready for a comeback of natural products in oncology. Biochem. Pharmacol. 2009, 77, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Taraphdar, A.K.; Roy, M.; Bhattacharya, R.K. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. Curr. Sci. 2001, 80, 1387–1396. [Google Scholar]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Pal, S.K.; Shukla, Y. Herbal medicine: Current status and the future. Asian Pac. J. Cancer Prev. 2003, 4, 281–288. [Google Scholar] [PubMed]
- Yang, S.; Zhao, Q.; Xiang, H.; Liu, M.; Zhang, Q.; Xue, W.; Song, B.; Yang, S. Antiproliferative activity and apoptosis-inducing mechanism of constituents from Toona sinensis on human cancer cells. Cancer Cell Int. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, A.; Inagaki, Y.; Gao, J.; Li, J.; Kokudo, N.; Li, X.K.; Tang, W. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci. Trends 2010, 4, 297–307. [Google Scholar] [PubMed]
- Moragoda, L.; Jaszewski, R.; Majumdar, A.P. Curcumin induces modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001, 21, 873–878. [Google Scholar] [PubMed]
- Bush, J.; Cheung, K.J.J.; Li, G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp. Cell Res. 2001, 271, 305–314. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


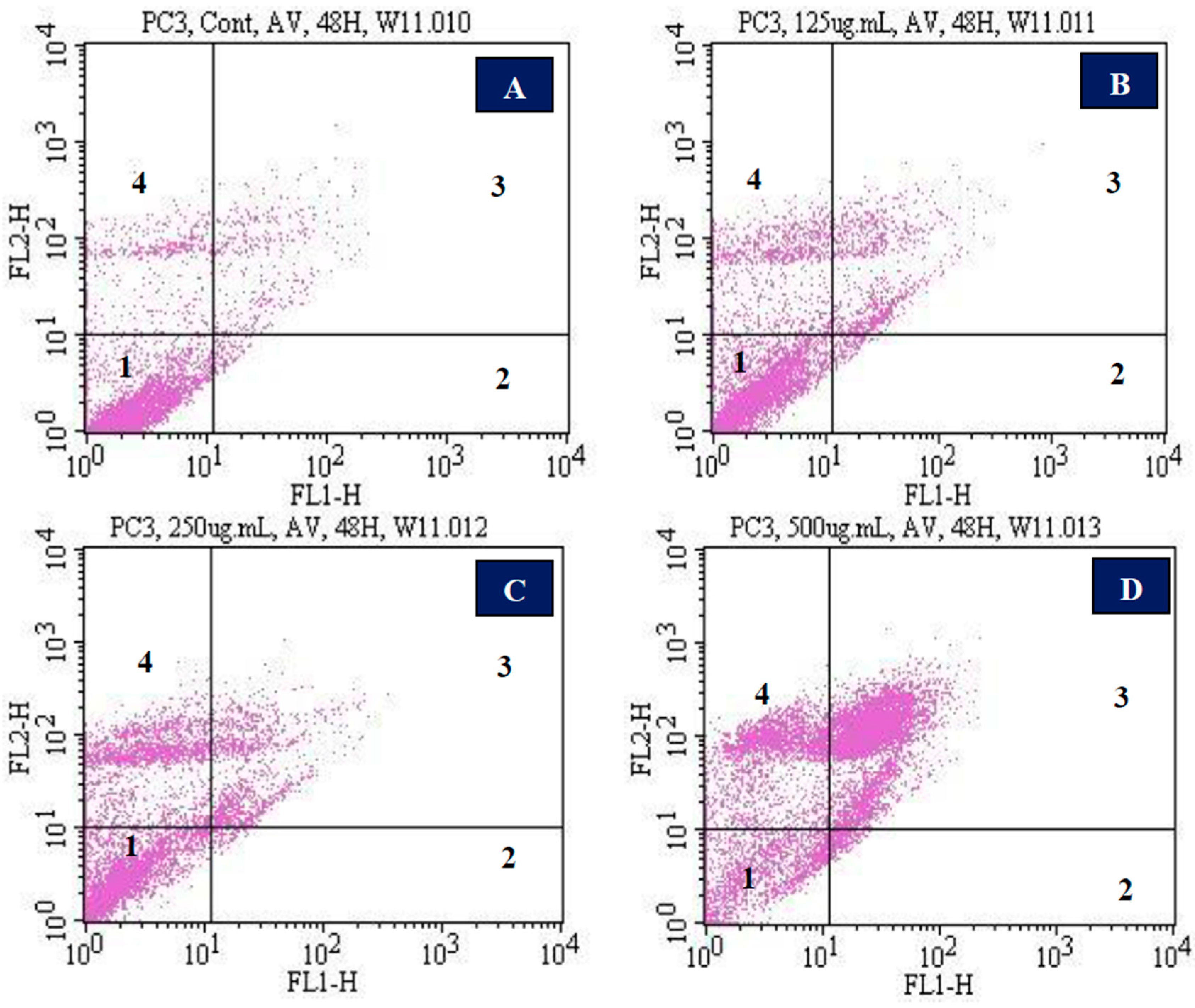
| Percentage of Cells and Corresponding Responses in Annexin V/PI Assay | ||
|---|---|---|
| Doses (µg/mL VAD) | Annexin V and PI Negative or Viable Cells (Mean ± SD) % | Annexin V and PI Positive Cells (Mean ± SD) % |
| Control | 90.9 ± 0.212 | 9.1 ± 0.212 |
| 125 | 81.2 ± 1.0 * | 18.8 ± 1.0 * |
| 250 | 70.5 ± 2.9 * | 29.5 ± 2.9 * |
| 500 | 17.9 ± 0.8 * | 82.1 ± 0.8 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, W.; Tchounwou, P.B.; Yedjou, C.G. Therapeutic Mechanisms of Vernonia amygdalina Delile in the Treatment of Prostate Cancer. Molecules 2017, 22, 1594. https://doi.org/10.3390/molecules22101594
Johnson W, Tchounwou PB, Yedjou CG. Therapeutic Mechanisms of Vernonia amygdalina Delile in the Treatment of Prostate Cancer. Molecules. 2017; 22(10):1594. https://doi.org/10.3390/molecules22101594
Chicago/Turabian StyleJohnson, William, Paul B. Tchounwou, and Clement G. Yedjou. 2017. "Therapeutic Mechanisms of Vernonia amygdalina Delile in the Treatment of Prostate Cancer" Molecules 22, no. 10: 1594. https://doi.org/10.3390/molecules22101594





