Biomimetic-Functionalized, Tannic Acid-Templated Mesoporous Silica as a New Support for Immobilization of NHase
Abstract
:1. Introduction
2. Results and Discussion
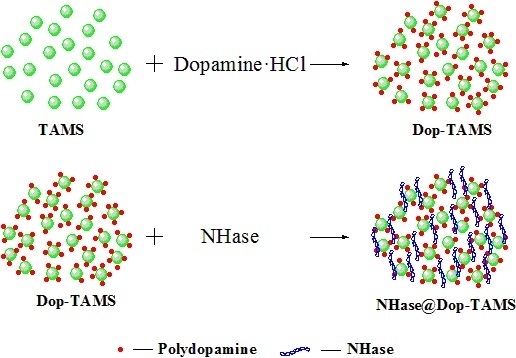
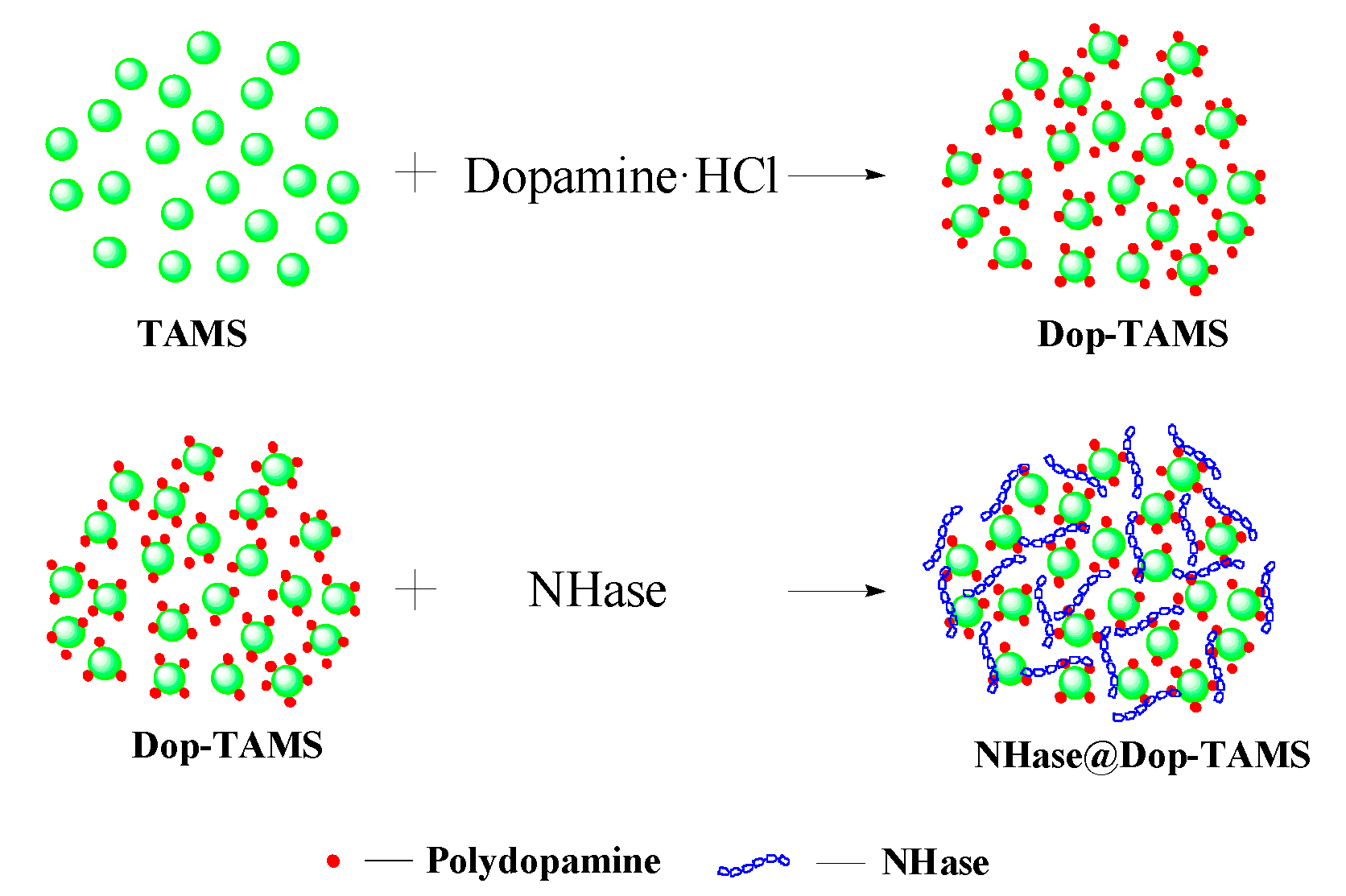
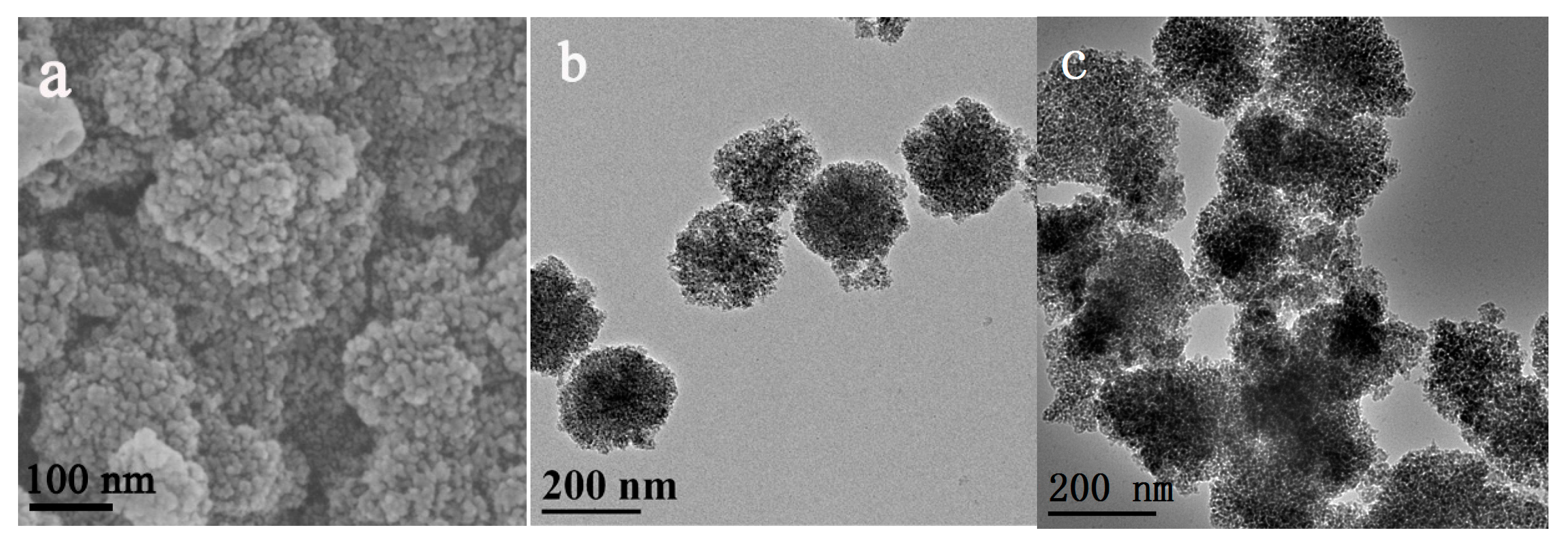
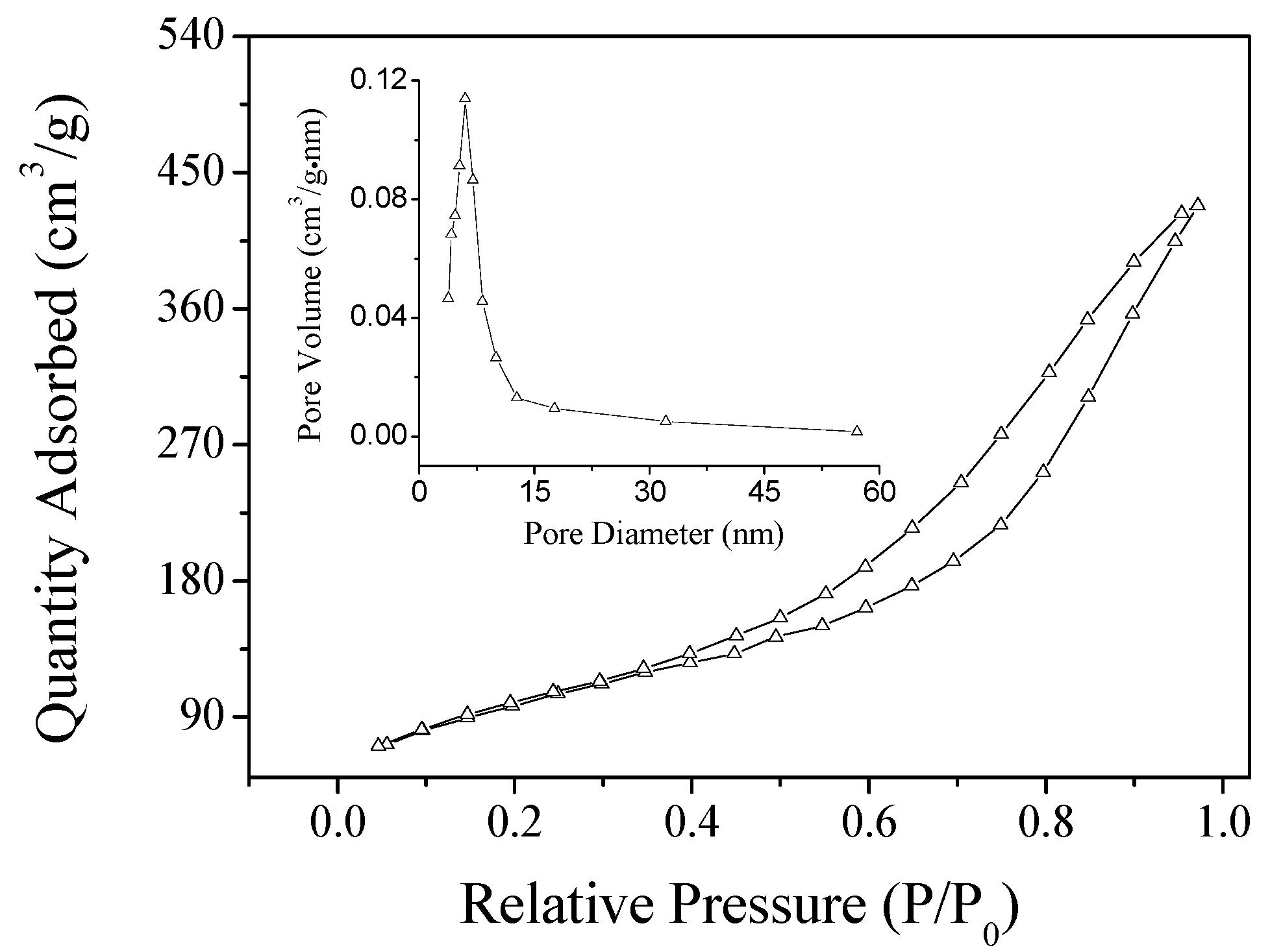
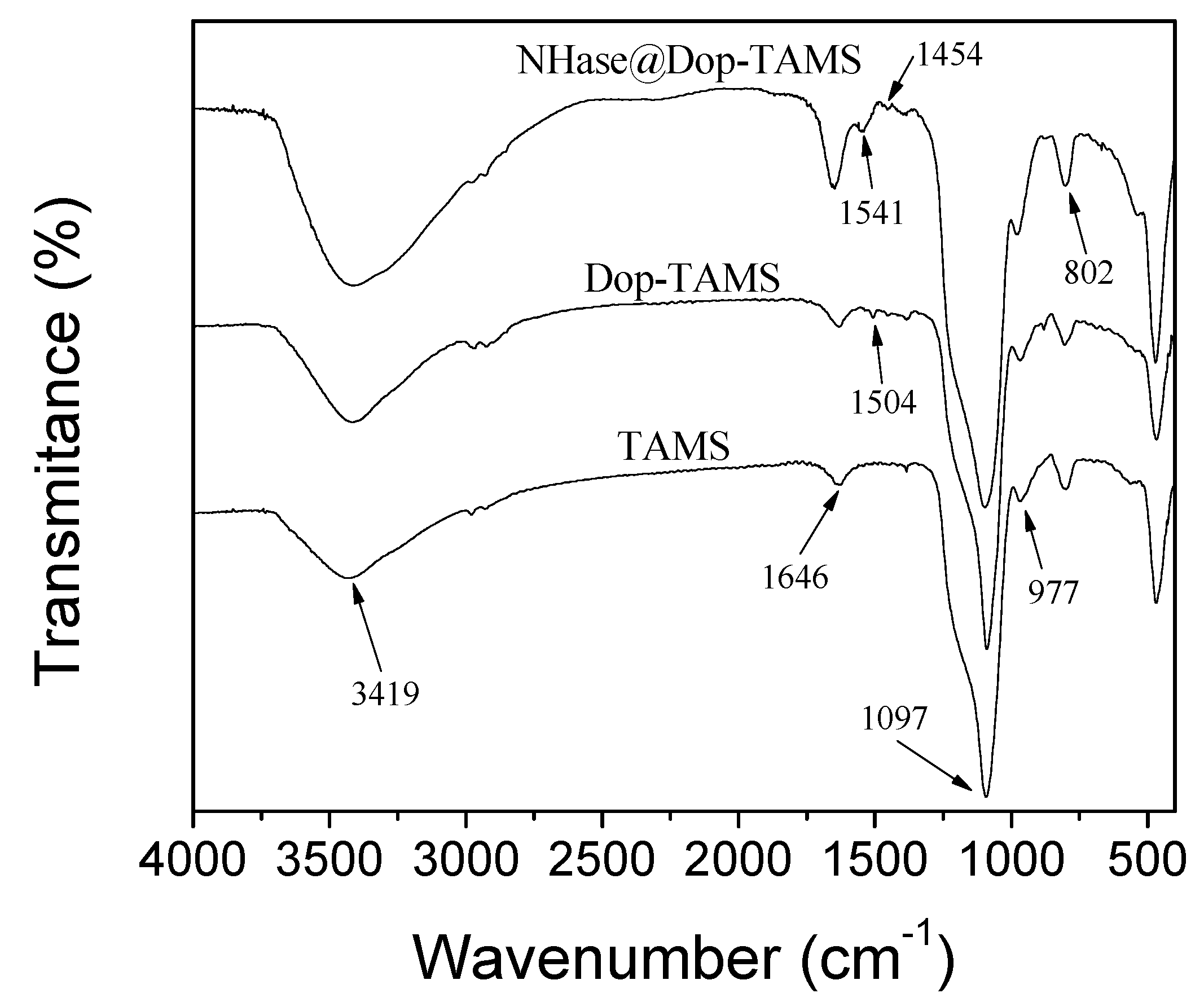
2.1. Characterization of the Dop-TAMS and NHase@Dop-TAMS
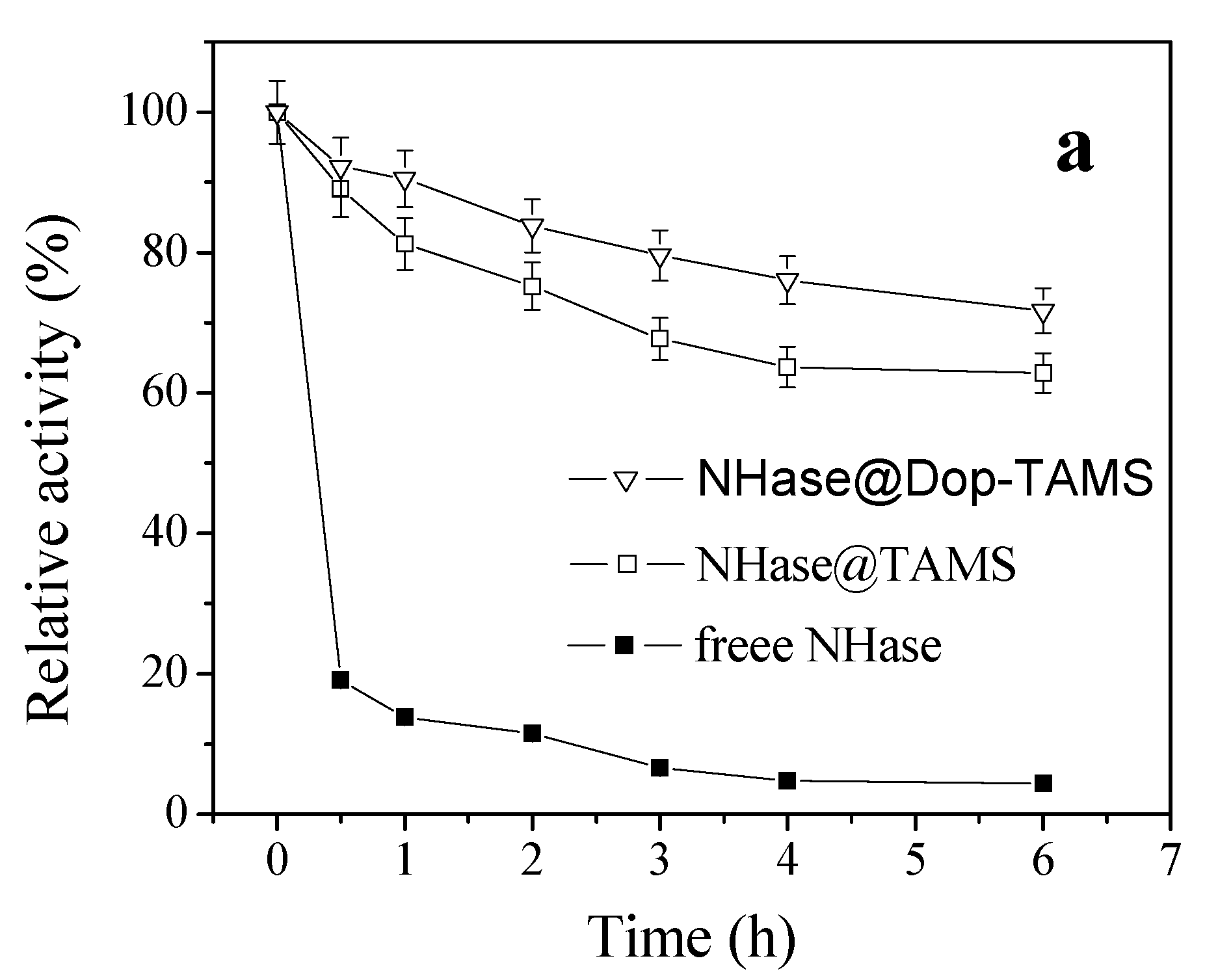
2.2. Thermal Stability of Free and Immobilized NHase
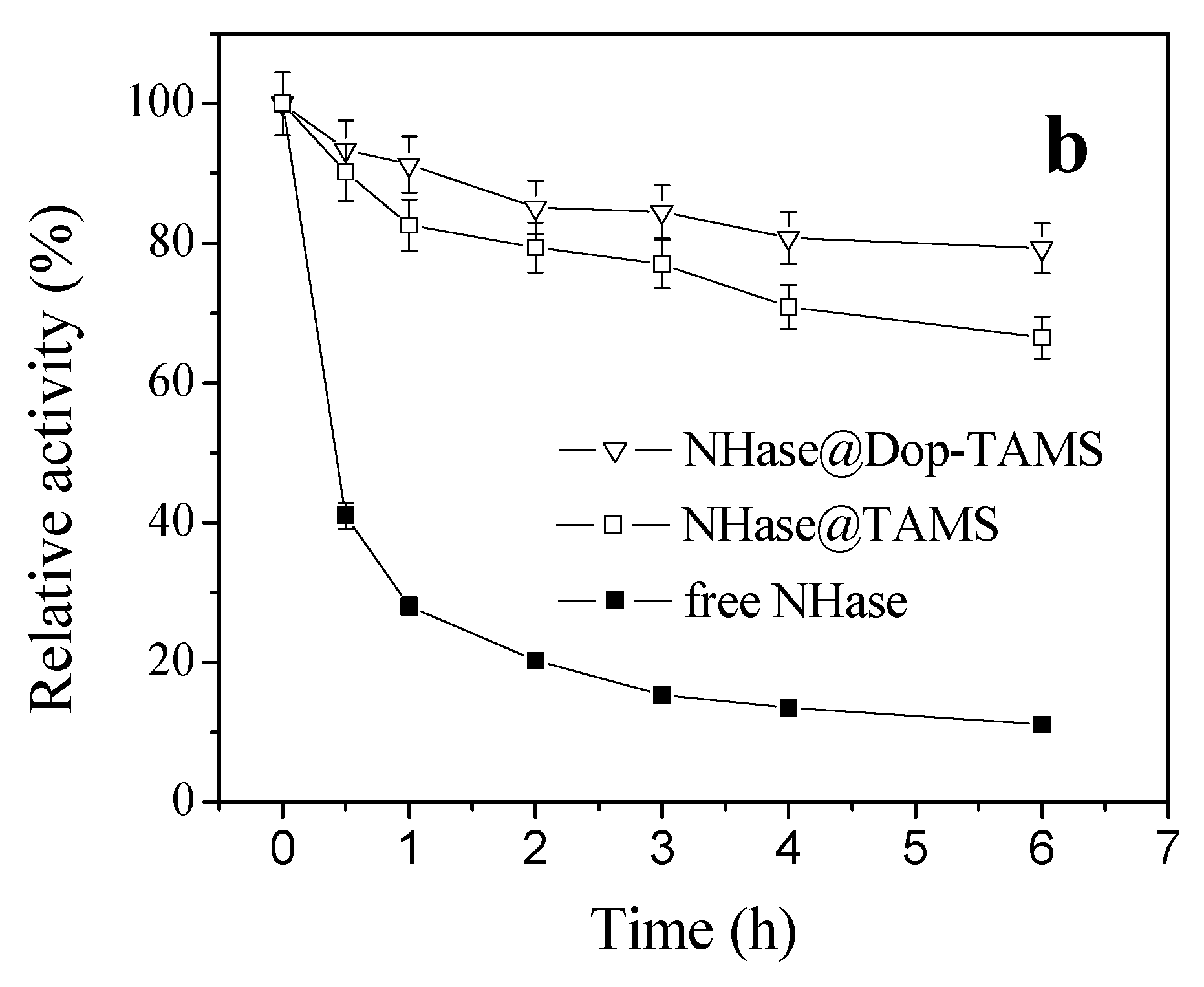
2.3. pH Stability of Free and Immobilized NHase
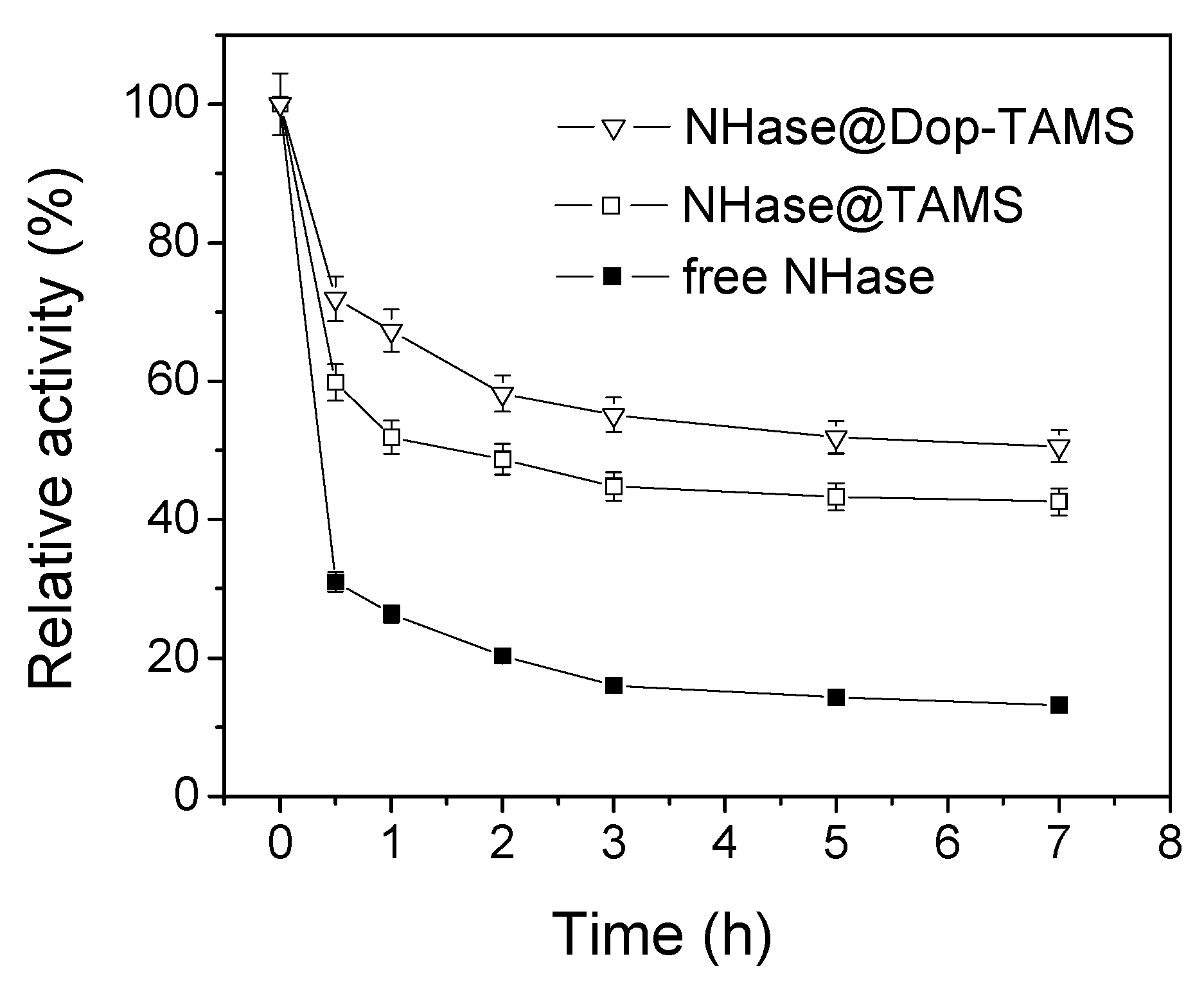
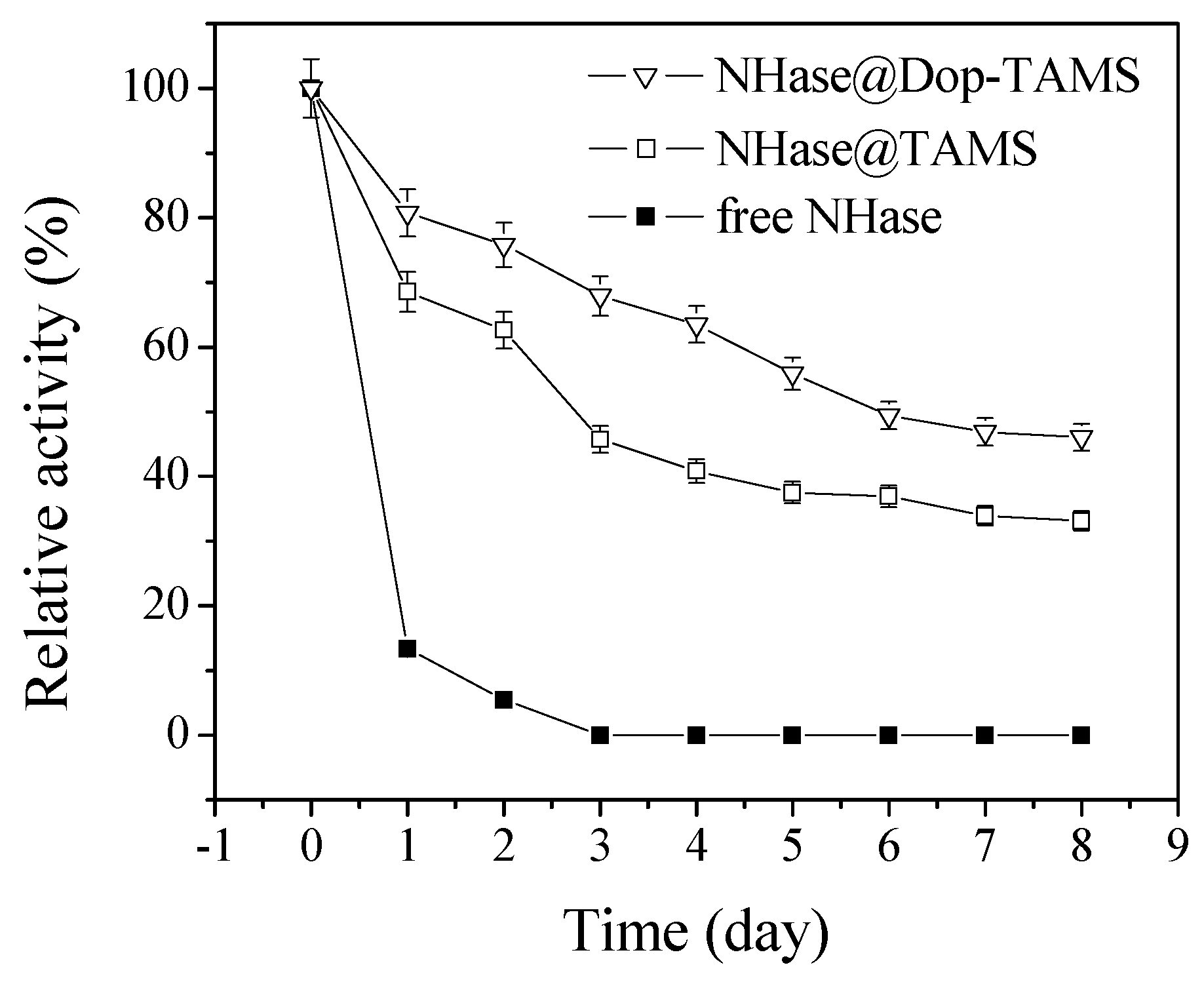
2.4. Stability of Free and Immobilized NHase in Shaking Conditions
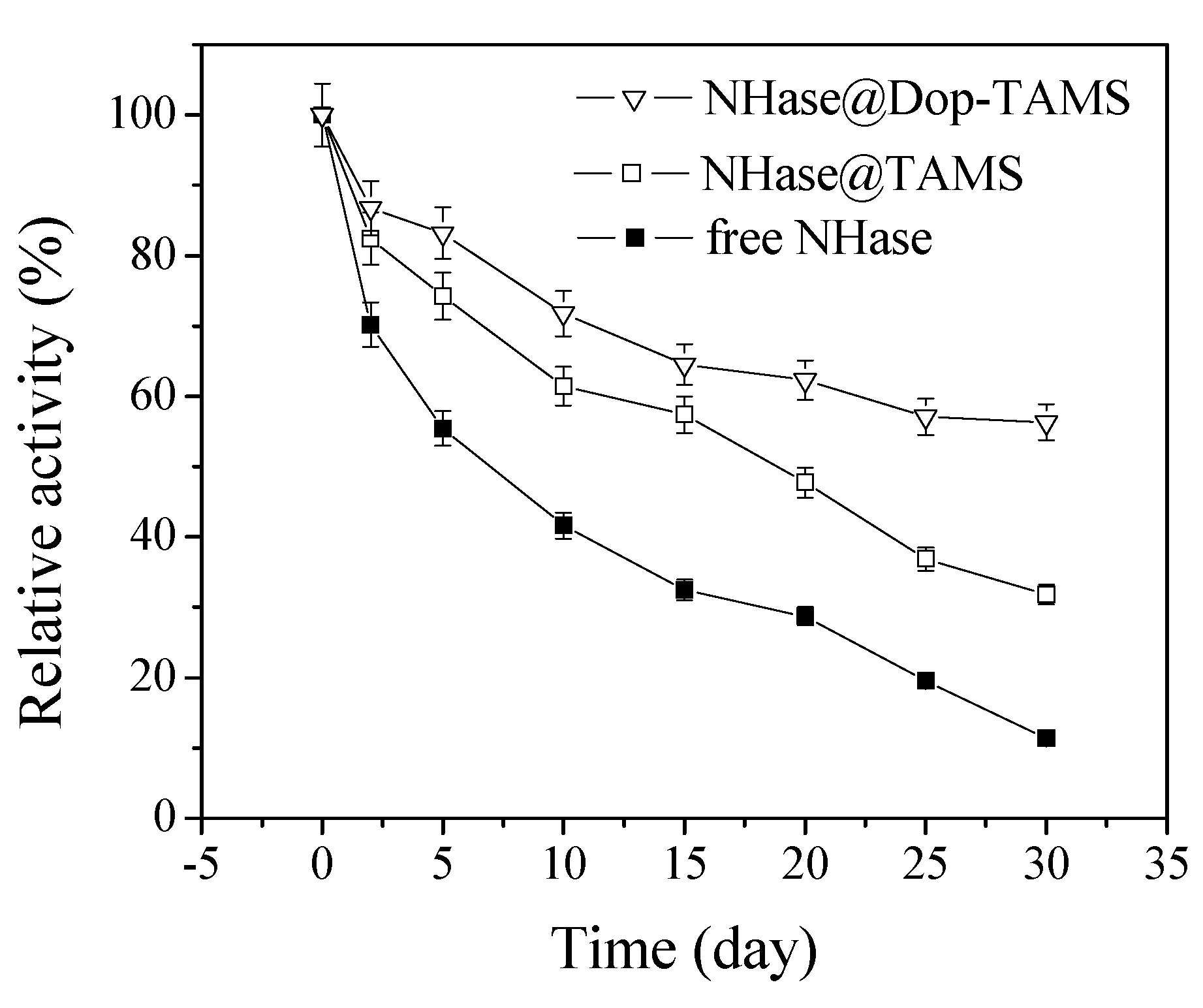
2.5. Storage Stability of Free and Immobilized NHase
2.6. Kinetics of Free and Immobilized NHase
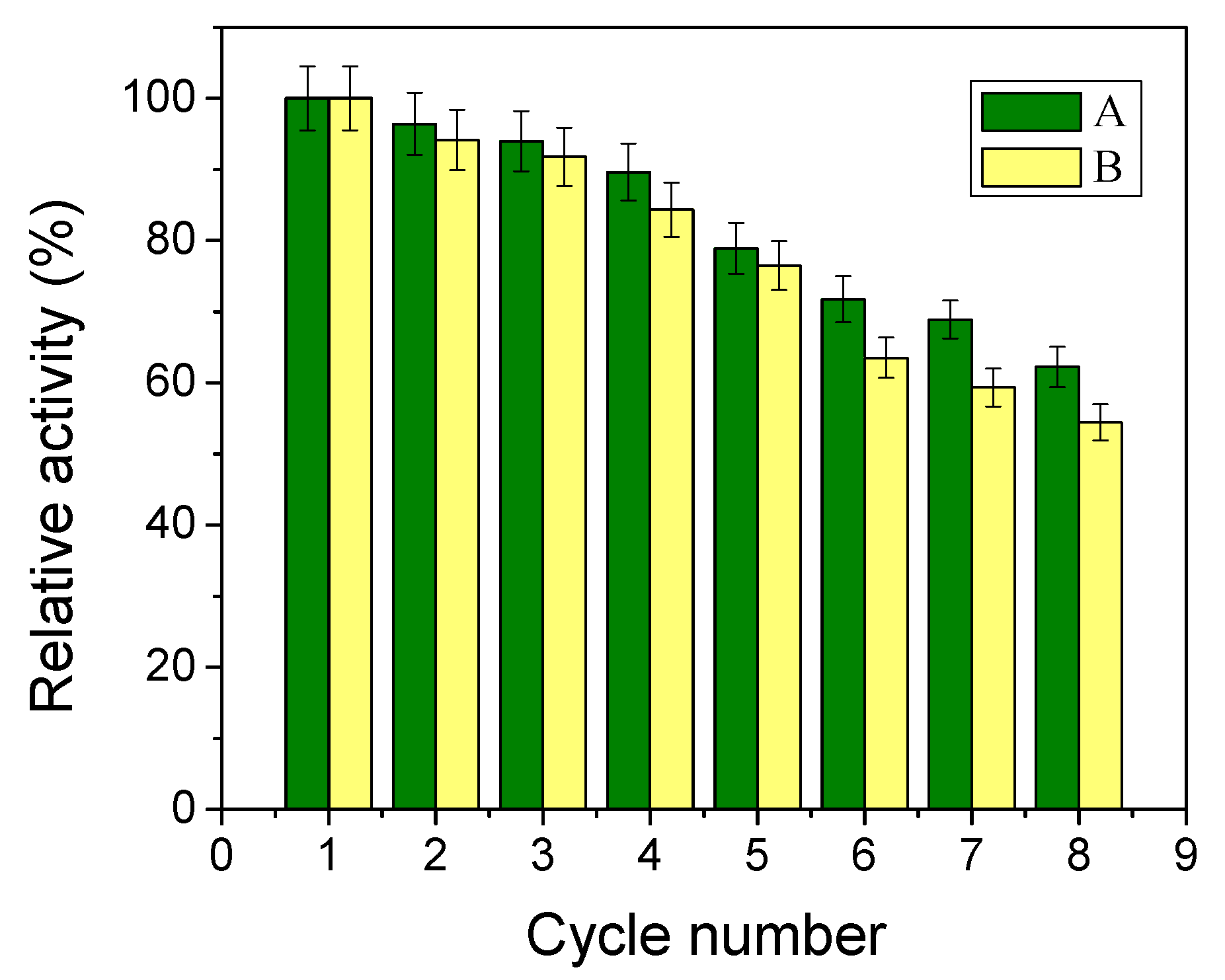
2.7. Reusability of Immobilised NHase
3. Materials and Methods
3.1. Materials
3.2. Preparation of Dopamine-Functionalized TAMS (Dop-TAMS)
3.3. Immobilization of NHase in Dop-TAMS
3.4. Enzymatic Activity Assay
3.5. Thermal and pH Stability
3.6. Stability of Free and Immobilized NHase in Shaking and Storage Conditions
3.7. Determination of Kinetic Parameters
3.8. Reusability
3.9. Characterizations
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hartmann, M.; Kostrov, X. Immobilization of enzymes on porous silicas-benefits and challenges. Chem. Soc. Rev. 2013, 42, 6277–6289. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, A.Y.; Maksimova, Y.G.; Kuznetsova, M.V.; Olontsev, V.F.; Demakov, V.A. Immobilization of rhodococcus ruber strain gt1, possessing nitrile hydratase activity, on carbon supports. Appl. Biochem. Microbiol. 2007, 43, 173–177. [Google Scholar] [CrossRef]
- Gao, J.; Shi, L.; Jiang, Y.; Zhou, L.; He, Y. Formation of lipase Candida sp. 99–125 CLEAs in mesoporous silica: Characterization and catalytic properties. Catal. Sci. Technol. 2013, 3, 3353–3359. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Q.; Jiang, Y.; Gao, J.; Liu, Z.; Zhou, L.; Zhang, Y. Formation of nitrile hydratase cross-linked Enzyme aggregates in mesoporous onion-like silica: Preparation and catalytic properties. Ind. Eng. Chem. Res. 2015, 54, 83–90. [Google Scholar] [CrossRef]
- Kabaivanova, L.V.; Chernev, G.E.; Miranda Salvado, I.M.; Fernandes, M.H.V. Silica-carrageenan hybrids used for cell immobilization realizing high-temperature degradation of nitrile substrates. Cent. Eur. J. Chem. 2011, 9, 232–239. [Google Scholar] [CrossRef]
- Lane, S.M.; Kuang, Z.; Yom, J.; Arifuzzaman, S.; Genzer, J.; Farmer, B.; Naik, R.; Vaia, R.A. Poly(2-hydroxyethyl methacrylate) for enzyme immobilization: Impact on activity and stability of horseradish peroxidase. Biomacromolecule 2011, 12, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Magner, E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Ince, A.; Bayramoglu, G.; Karagoz, B.; Altintas, B.; Bicak, N.; Arica, M.Y. Method for fabrication of polyaniline coated polymer microspheres and its application for cellulase. Chem. Eng. J. 2012, 189, 404–412. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, B.; Zhang, M.; Jun, S.-H.; Shim, J.; Lee, J.; Kim, J.; Gu, M.B. Immobilization and stabilization of subtilisin Carlsberg in magnetically separable mesoporous silica for the transesterification in an organic solvent. Green Chem. 2012, 14, 1884–1887. [Google Scholar] [CrossRef]
- Bai, Y.X.; Li, Y.F.; Yang, Y.; Yi, L.X. Covalent immobilization of triacylglycerol lipase onto functionalized novel mesoporous silica supports. Biotechnology 2006, 125, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Hung, B.Y.; Kuthati, Y.; Kankala, R.K.; Kankala, S.; Deng, J.P.; Liu, C.L.; Lee, C.H. Utilization of enzyme-immobilized mesoporous silica nanocontainers (IBN-4) in prodrug-activated cancer theranostics. Nanomaterials 2015, 5, 2169–2191. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Tian, L.; Zhao, P.; Zhang, B.; Ali, N.; Khan, M.; Zhang, Q. Immobilization of lipase on mesoporous silica nanoparticles with hierarchical fibrous pore. J. Mol. Catal. B-Enzym. 2016, 134, 129–135. [Google Scholar] [CrossRef]
- Gao, J.; Kong, W.; Zhou, L.; He, Y.; Ma, L.; Wang, Y.; Yin, L.; Jiang, Y. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization. Chem. Eng. J. 2017, 309, 70–79. [Google Scholar] [CrossRef]
- Li, J.; Li, L.S.; Xu, L. Hierarchically macro/mesoporous silica spheres for catalase immobilization and catalysis. Mater. Lett. 2017, 193, 67–69. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D.Y. Controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zharov, I. Large pore mesoporous silica nanoparticles by templating with a nonsurfactant molecule, tannic acid. Chem. Mater. 2014, 26, 2030–2037. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, D.; Ding, T.; Shih, W.H.; Liu, X.; Cheng, S.Z.; Fu, Q. A non-surfactant templating route to mesoporous silica materials. Adv. Mater. 1998, 10, 313–316. [Google Scholar] [CrossRef]
- Pang, J.B.; Qiu, K.Y.; Xu, J.; Wei, Y.; Chen, J. Synthesis of mesoporous silica materials via nonsurfactant urea-templated sol-gel reactions. J. Inorg. Organomet. Polym. 2000, 10, 39–49. [Google Scholar] [CrossRef]
- Yi, L.; Chen, Y.X.; Lin, P.C.; Schröder, H.; Niemeyer, C.M.; Wu, Y.W.; Goody, R.S.; Triola, G.; Waldmann, H. Direct immobilization of oxyamine-modified proteins from cell lysates. Chem. Commun. 2012, 48, 10829–10831. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Rezai-Zadeh, K.; Koyama, N.; Arendash, G.W.; Yamaguchi, H.; Kakuda, N.; Horikoshi-Sakuraba, Y.; Tan, J.; Town, T. Tannic acid is a natural beta-secretase inhibitor that prevents cognitive impairment and mitigates alzheimer-like pathology in transgenic mice. J. Biol. Chem. 2012, 287, 6912–6927. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, W.; Zhou, L.; Ma, L.; He, Y.; Gao, J. Improved performance of lipase immobilized on tannic acid-templated mesoporous silica nanoparticles. Appl. Biochem. Biotechnol. 2016, 179, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Lou, L.L.; Yan, B.; Zhang, C.; Wu, S.; Liu, S. Immobilization of papain by carboxyl-modified SBA-15: Rechecking the carboxyl after excluding the contribution of H2SO4 treatment. Microporous. Microporous. Mater. 2011, 143, 341–347. [Google Scholar] [CrossRef]
- Lu, S.; An, Z.; He, J.; Li, B. Hierarchically-structured immobilized enzyme displaying the multi-functions of bio-membranes. J. Mater. Chem. 2012, 22, 3882–3888. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Rivera, J.G.; He, L.; Kulkarni, H.; Lee, D.K.; Messersmith, P.B. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.V.; Yadav, G.D. PVA/chitosan-glutaraldehyde cross-linked nitrile hydratase as reusable biocatalyst for conversion of nitriles to amides. J. Mol. Catal. B-Enzym. 2014, 101, 115–121. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, W.; Zheng, J.; Frisco, S.; Song, P.; Li, X. The shape-stabilized phase change materials composed of polyethylene glycol and various mesoporous matrices (AC, SBA-15 and MCM-41). Sol. Energy Mater. Sol. Cells 2011, 95, 3550–3556. [Google Scholar] [CrossRef]
- Meng, M.J.; Meng, X.G.; Liu, Y.; Liu, Z.C.; Han, J.; Wang, Y.; Luo, M.; Chen, R.; Ni, L.; Yan, Y.S. An ion-imprinted functionalized SBA-15 adsorbent synthesized by surface imprinting technique via reversible addition-fragmentation chain transfer polymerization for selective removal of Ce(III) from aqueous solution. J. Hazard. Mater. 2014, 278, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, J.K.; Wen, X.F.; Wu, W.F. Efficient removal of cadmium using facile functionalized of mesoporous silica via a biomimetic coating. Rsc. Adv. 2016, 6, 18340–18347. [Google Scholar] [CrossRef]
- Sureshkumar, M.; Lee, C.-K. Polydopamine coated magnetic-chitin (MCT) particles as a new matrix for enzyme immobilization. Carbohyd. Polym. 2011, 84, 775–780. [Google Scholar] [CrossRef]
- Gao, J.; Lei, H.; Han, Z.; Shi, Q.; Chen, Y.; Jiang, Y. Dopamine functionalized tannic-acid-templated mesoporous silica nanoparticles as a new sorbent for the efficient removal of Cu2+ from aqueous solution. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Y.; Wang, X.; Li, Q.; Wang, J.; Yan, Y. Improving catalytic performance of Burkholderia cepacia lipase immobilized on macroporous resin NKA. J. Mol. Catal. B-Enzym. 2011, 71, 45–50. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Liu, J.; Hahn, M.; Qiao, S.; Middelberg, A.P.J.; He, L. Encapsulation of lipase in mesoporous silica yolk-shell spheres with enhanced stability. Rsc. Adv. 2013, 3, 22008–22013. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, Y.; Lu, J.; Han, Z.; Deng, J.; Chen, Y. Dopamine-functionalized mesoporous onion-like silica as a new matrix for immobilization of lipase Candida sp. 99–125. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Su, E.; Wang, W.; Wei, D. Efficient asymmetric synthesis of d-N-formyl-phenylglycine via cross-linked nitrilase aggregate catalyzed dynamic kinetic resolution. Catal. Commun. 2014, 51, 19–23. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Gao, J.; Wu, W.; Dong, L.; Yang, Z. Facile immobilization of nitrile hydratase in SBA-15 via a biomimetic coating. J. Porous Mater. 2017, 24, 787–793. [Google Scholar] [CrossRef]
- Lee, J.; Na, H.B.; Kim, B.C.; Lee, J.H.; Lee, B.; Kwak, J.H.; Hwang, Y.; Park, J.-G.; Gu, M.B.; Kim, J.; et al. Magnetically-separable and highly-stable enzyme system based on crosslinked enzyme aggregates shipped in magnetite-coated mesoporoussilica. J. Mater. Chem. 2009, 19, 7864–7870. [Google Scholar] [CrossRef]
- Kim, M.I.; Kim, J.; Lee, J.; Jia, H.; Na, H.B.; Youn, J.K.; Kwak, J.H.; Dohnalkova, A.; Grate, J.W.; Wang, P.; et al. Crosslinked enzyme aggregates in hierarchically ordered mesoporous silica: A simple and effective method for enzyme stabilization. Biotechnol. Bioeng. 2007, 96, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Paradiso, M.; Wallacher, D.; Brandt, A.; Hartmann, M. Formation of cross-linked chloroperoxidase aggregates in the pores of mesocellular foams: Characterization by SANS and catalyticproperties. ChemSusChem 2009, 2, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Ozyilmaz, G.; Tukel, S.S.; Alptekin, O. Activity and storage stability of immobilized glucose oxidase onto magnesium silicate. J. Mol. Catal. B-Enzym. 2005, 35, 154–160. [Google Scholar] [CrossRef]
- Li, Y.; Gao, F.; Wei, W.; Qu, J.B.; Ma, G.H.; Zhou, W.Q. Pore size of macroporous polystyrene microspheres affects lipase immobilization. J. Mol. Catal. B-Enzym. 2010, 66, 182–189. [Google Scholar] [CrossRef]
- Sarı, M.; Akgöl, S.; Karatas, M.; Denizli, A. Reversible immobilization of catalase by metal chelate affinity interaction on magnetic beads. Ind. Eng. Chem. Res. 2006, 45, 3036–3043. [Google Scholar] [CrossRef]
- Alfani, F.; Cantarella, M.; Spera, A.; Viparelli, P. Operational stability of Brevibacterium imperialis CBS 489-74 nitrile hydratase. J. Mol. Catal. B-Enzym. 2001, 11, 687–697. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Samples | NHase Loading Capacity (mg/g) | Specific Activity (U/mg) | Activity Recovery (%) |
|---|---|---|---|
| NHase@Dop-TAMS | 63.1 | 2.14 | 40.9 |
| NHase@TAMS | 47.7 | 1.85 | 35.2 |
| Samples | Free NHase | NHase@TAMS | NHase@Dop-TAMS |
|---|---|---|---|
| Km (mM) | 1.71 | 2.48 | 2.35 |
| Vmax. (mM/min) | 4.46 | 3.59 | 3.96 |
| Vmax./Km (s−1) | 2.61 | 1.45 | 1.69 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.-k.; Zhang, Z.-j.; Jiang, Y.-j.; Chen, Y.; Gao, S.-f. Biomimetic-Functionalized, Tannic Acid-Templated Mesoporous Silica as a New Support for Immobilization of NHase. Molecules 2017, 22, 1597. https://doi.org/10.3390/molecules22101597
Gao J-k, Zhang Z-j, Jiang Y-j, Chen Y, Gao S-f. Biomimetic-Functionalized, Tannic Acid-Templated Mesoporous Silica as a New Support for Immobilization of NHase. Molecules. 2017; 22(10):1597. https://doi.org/10.3390/molecules22101597
Chicago/Turabian StyleGao, Jun-kai, Zi-jun Zhang, Yan-jun Jiang, Yan Chen, and Shu-feng Gao. 2017. "Biomimetic-Functionalized, Tannic Acid-Templated Mesoporous Silica as a New Support for Immobilization of NHase" Molecules 22, no. 10: 1597. https://doi.org/10.3390/molecules22101597