In Silico Prediction of the Anti-Depression Mechanism of a Herbal Formula (Tiansi Liquid) Containing Morinda officinalis and Cuscuta chinensis
Abstract
:1. Introduction
2. Methodology
2.1. Retrieval of Chemical Ingredients and Their Targets
2.2. Network Construction and Analysis
2.3. Gene Ontology and Pathway Enrichment Analysis
3. Results
3.1. Retrieval of Chemical Ingredients and Their Targets
3.2. Network Construction and Analysis
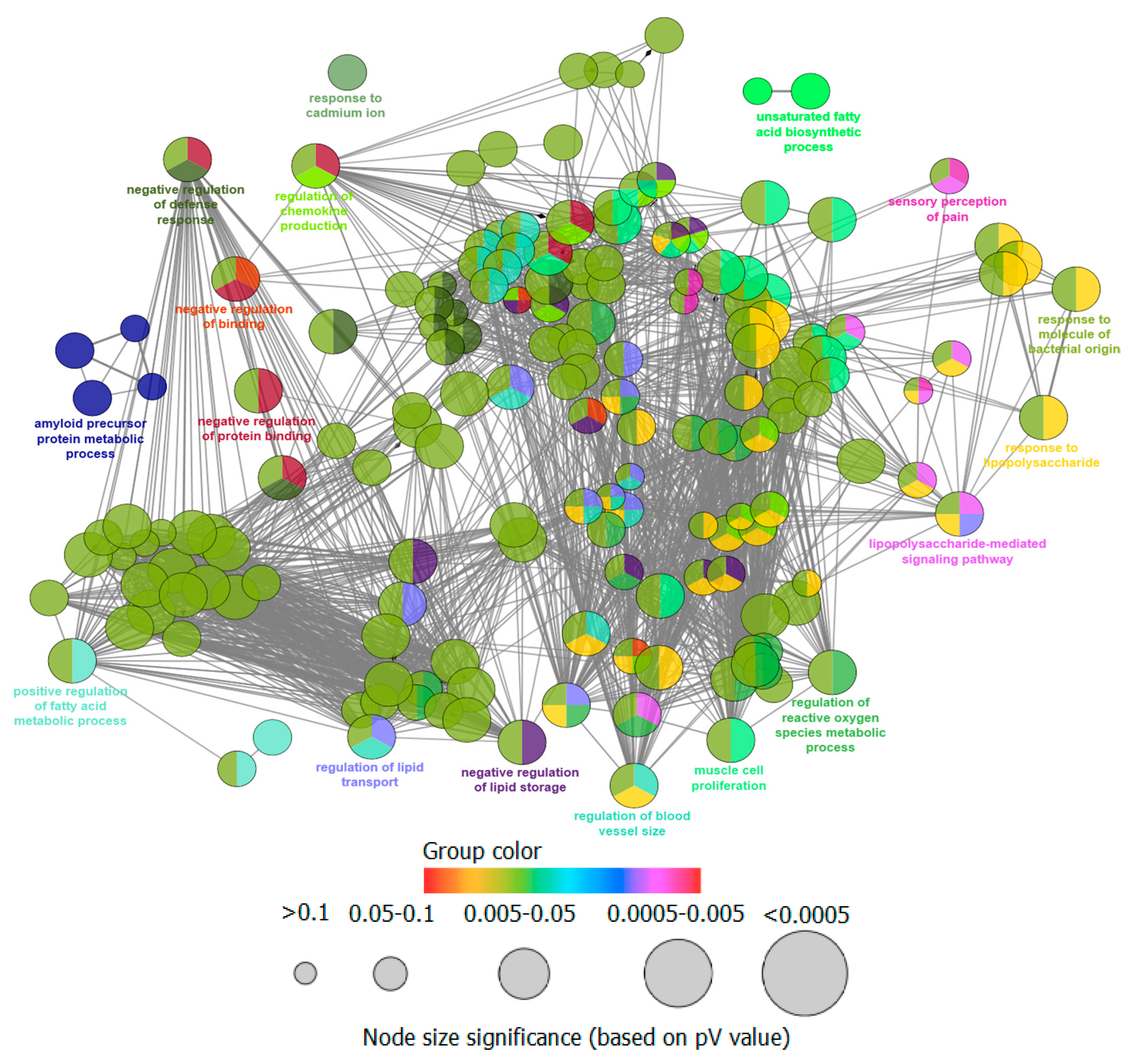
3.3. Gene Ontology and Pathway Enrichment Analysis
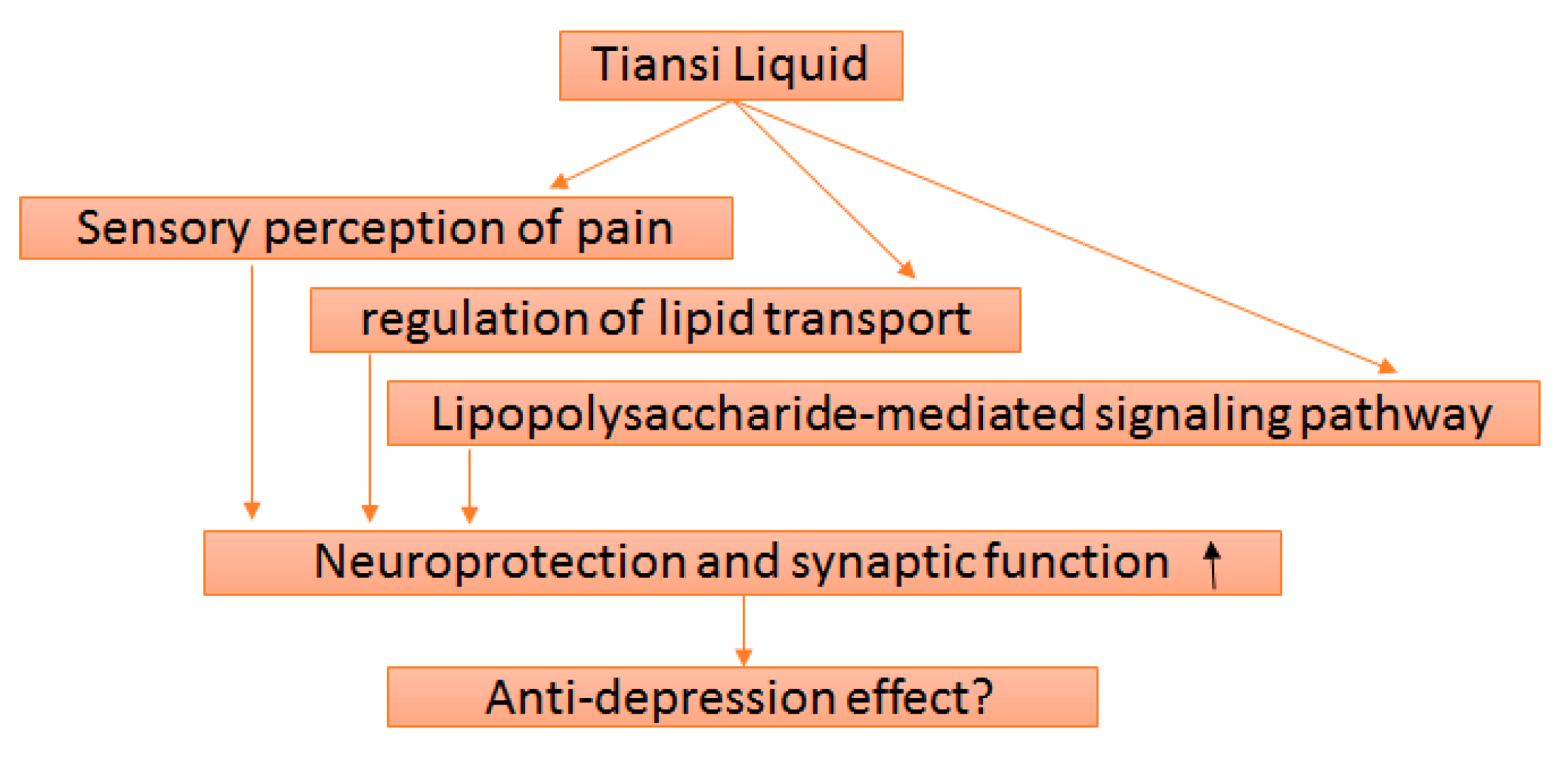
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liang, J.; Shu, L.; Luo, Z.; Liu, P.; Yang, M.; Tan, C. Preliminary clinical effectiveness of Morinda offcinalis water extract in the treatment of depression. China J. Chin. Mater. Med. 2002, 27, 75–78. [Google Scholar]
- Kong, Q.; Shu, L.; Zhang, H.; Jiao, F.; Han, Z.; Wang, J.; Du, B.; Shi, J.; Wang, X.; Ai, C.; et al. Efficacy and safety of Morinda officinalis oligose capsule in the treatment of depression. China J. Clin. Pharmacol. 2011, 27, 170–173. [Google Scholar]
- Sharma, B.; Gupta, V.K. Modulations of Mammalian Brain Functions by Antidepressant Drugs: Role of Some Phytochemicals as Prospective Antidepressants. Evid. Based Med. Pract. 2016, 2, 1–12. [Google Scholar]
- Mokhtarifar, N.; Sharif, B.; Naderi, N.; Mosaddegh, M.; Faizi, M. Evaluation of anti-depressant effects of Cuscuta chinensis in experimental models. Res. Pharm. Sci. 2012, 7, S826. [Google Scholar]
- Donnapee, S.; Li, J.; Jang, X.; Ge, A.; Donker, P.O.; Gao, X.; Chang, Y. Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J. Ethnopharmacol. 2014, 157, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-Y.; Lu, Y.; Xu, Y.-Q.; Zhang, J.; Li, H.-N.; Chang, H.-S. Effect of antidepression and mechanism of regulation on IDO of Tiansi Liquid. J. Beijing Univ. Tradit. Chin. Med. 2015, 38, 182–185. [Google Scholar]
- Xu, L.-Z.; Xu, D.-F.; Han, Y.; Liu, L.-J.; Sun, C.-Y.; Deng, J.-H.; Zhang, R.-X.; Yuan, M.; Zhang, S.-Z.; Li, Z.-M.; et al. BDNF-GSK-3β-β-Catenin Pathway in the mPFC Is Involved in Antidepressant-Like Effects of Morinda officinalis Oligosaccharides in Rats. Int. J. Neuropsychopharmacol. 2017, 20, 83–93. [Google Scholar] [PubMed]
- O’Keane, V.; Frodl, T.; Dinan, T.G. A review of atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinol. 2012, 37, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.; Berlund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R. The epidemiology of major depressive disorder: Results for the National Comorbidity Survey Replication (NCS-R). J. Am. Med. Assoc. 2003, 289, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Toda, H.; Palmar, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Iosif, R.E.; Ekdahl, C.T.; Ahlenius, H.; Pronk, C.J.; Bonde, S.; Kokaia, Z.; Jacobsen, S.E.; Lindvall, O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J. Neurosci. 2006, 26, 9703–9712. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.S.; Bahramsoltani, R.; Farzaei, M.H.; Mohammad, A.; Rahimi, R. Plant-derived natural medicines for the management of depression: An overview of mechanisms of action. Rev. Neurosci. 2015, 26, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Farzaei, M.H.; Farahani, M.S.; Rahimi, R. Phytochemical constituents as future antidepressants: A comprehensive review. Rev. Neurosci. 2015, 26, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Voleti, B. Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H.; Fava, M.; Wisniewski, S.R.; Thase, M.E.; Quitkin, F.; Warden, D.; Ritz, L.; Nierenberg, A.A.; Lebowitz, B.D.; Biggs, M.M. Medication augmentation after the failure of SSRIs for depression. N. Engl. J. Med. 2006, 354, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Potdar, V.H.; Kibile, S.J. Evaluation of antidepressant-like effect of Citrus maxima leaves in animal models of depression. Iran. J. Basic Med. Sci. 2011, 14, 478–483. [Google Scholar] [PubMed]
- Farzaei, M.H.; Rahimi, R.; Attar, F.; Siavoshi, F.; Saniee, P.; Hajimahmoodi, M.; Mirnezami, T.; Khanavi, M. Chemical composition, antioxidant and antimicrobial activity of essential oil and extracts of Tragopogon graminifolius, a medicinal herb from Iran. Nat. Prod. Commun. 2014, 9, 121–124. [Google Scholar] [PubMed]
- Gan, Y.; Zheng, S.; Baak, J.P.A.; Zhao, S.; Zheng, Y.; Luo, N.; Liao, W.; Fu, C. Prediction of the anti-inflammatory mechanisms of curcumin by module-based protein interaction network analysis. Acta Pharm. Sin. B 2015, 5, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.C.; Xiao, P.G. Network pharmacology: A Rosetta Stone for traditional Chinese medicine. Drug Dev. Res. 2014, 75, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, X.; Wang, S.; Zhai, C.; He, Y.; Zhang, Y.; Qiao, Y. Mechanism of action of salvianolic acid B by module-based network analysis. Biomed. Mater. Eng. 2014, 24, 1333–1340. [Google Scholar] [PubMed]
- Janga, S.C.; Tzakos, A. Structure and organization of drug-target networks: Insights from genomic approaches for drug discovery. Mol. Biosyst. 2009, 5, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Szklarczyk, D.; Pletscher-Frankild, S.; Blicher, T.H.; von Mering, C.; Jensen, L.J. STITCH 4: Integration of protein-chemical interactions with user data. Nucleic Acids Res. 2014, 42, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. Cluego: A cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Hao, H.; Li, Y.; Li, S. Modularity-based credible prediction of disease genes and detection of disease subtypes on the phenotype-gene heterogeneous network. BMC Syst. Biol. 2011, 5, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, Z.; Fei, D.; Liu, Y.; Zhang, M.; Liu, S. Evaluation of the Sedative and Hypnotic Effects of Astragalin Isolated From Eucommia Ulmoides Leaves in Mice. Nat. Prod. Res. 2016, 31, 2072–2076. [Google Scholar] [CrossRef] [PubMed]
- Subarnas, A.; Tadano, T.; Nakahata, N.; Arai, Y.; Kinemuchi, H.; Oshima, Y.; Kisara, K.; Ohizumi, Y. A possible mechanism of antidepressant activity of beta-amyrin palmitate isolated from Lobelia inflata leaves in the forced swimming test. Life Sci. 1993, 52, 289–296. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Yamada, T.; Masuya, J.; Matsushita, K.; Tahara, M.; Iimori, M.; Matsumiya, T. Caffeic acid attenuates the decrease in cortical BDNF mRNA expression induced by exposure to forced swimming stress in mice. Eur. J. Pharmacol. 2006, 534, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Capra, J.C.; Cunha, M.P.; Machado, D.G.; Zomkowski, A.D.; Mendes, B.G.; Santos, A.R.; Pizzolatti, M.G.; Rodrigues, A.L. Antidepressant-like effect of scopoletin, a coumarin isolated from Polygala sabulosa (Polygalaceae) in mice: Evidence for the involvement of monoaminergic systems. Eur. J. Pharmacol. 2010, 643, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.S.; Stolz, E.D.; Betti, A.H.; Stein, A.C.; Schripsema, J.; Poser, G.L.; Rates, S.M. The anti-immobility effect of hyperoside on the forced swimming test in rats is mediated by the D2-like receptors activation. Planta Med. 2011, 77, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Choi, R.C.Y.; Zhu, K.Y.; Leung, K.W.; Guo, A.J.Y.; Bi, D.; Xu, H.; Lau, S.T.W.; Dong, T.T.X.; Tsim, K.W.K. Isorhamnetin, A Flavonol Aglycone from Ginkgo biloba L., Induces Neuronal Differentiation of Cultured PC12 Cells: Potentiating the Effect of Nerve Growth Factor. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.X.; Lang, J.L.; Song, Y.Y.; Wu, Y.Z.; Lv, M.H.; Zhao, X.; Liu, Y.H.; Xu, C.Y. Studies on Anti-Depressant Activity of Four Flavonoids Isolated from Apocynum venetum Linn (Apocynaceae) Leaf in Mice. Trop. J. Pharm. Res. 2015, 14, 2269–2277. [Google Scholar] [CrossRef]
- Bandaruk, Y.; Mukai, R.; Terao, J. Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-A in human neuroblastoma SH-SY5Y cells. Toxicol. Rep. 2014, 1, 639–649. [Google Scholar] [CrossRef]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Supplementation with macular carotenoids reduces psychological stress, serum cortisol, and sub-optimal symptoms of physical and emotional health in young adults. Nutr. Neurosci. 2017, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Kanada, N.; Shimada, A.; Ogata, M.; Suzuki, I.; Hayashi, I.; Nakashima, K. Effect of sesamin in Acanthopanax senticosus HARMS on behavioral dysfunction in rotenone-induced parkinsonian rats. Biol. Pharm. Bull. 2005, 28, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Bettio, L.E.; Cunha, M.P.; Santos, A.R.; Pizzolatti, M.G.; Brighente, I.M.; Rodrigues, A.L. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008, 587, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhu, K.; Xu, Q.; Wang, G.; Zhang, J.; Cao, R.; Ye, J.; Yu, X. The antidepressant-like effect of trans-astaxanthin involves the serotonergic system. Oncotarget 2017, 8, 25552–25563. [Google Scholar] [CrossRef] [PubMed]
- Coffey, E.T.; Smiciene, G.; Hongisto, V.; Cao, J.; Brecht, S.; Herdegen, T.; Courtney, M.J. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Junactivation, in the presence of constitutive JNK1 activity in cerebellar neurons. J. Neurosci. 2002, 22, 4335–4345. [Google Scholar] [PubMed]
- Mohammad, H.; Marchisella, F.; Ortega-Martinez, S.; Hollos, P.; Eerola, K.; Komulainen, E.; Kulesskaya, N.; Freemantle, E.; Fagerholm, V.; Savontous, E.; et al. JNK1 controls adult hippocampal neurogenesis and imposes cell-autonomous control of anxiety behaviour from the neurogenic niche. Mol. Psychiatry 2016. [Google Scholar] [CrossRef] [PubMed]
- Gautron, L.; Lafon, P.; Chaigniau, M.; Tramu, G.; Layé, S. Spatiotemporal analysis of signal transducer and activator of transcription 3 activation in rat brain astrocytes and pituitary following peripheral immune challenge. Neuroscience 2002, 112, 717–729. [Google Scholar] [CrossRef]
- Baranowski, A.; Abrams, P.; Berger, R.; Buffington, T.; Collett, B.; Emmanuel, A.; Fall, M.; Hanno, P.; Howard, F.; Hughes, J.; et al. Classification of chronic pain: Descriptions of chronic pain syndromes and definition of pain terms. In Task Force on Taxonomy of the International Association for the Study of Pain; Merskey, H., Bogduk, N., Eds.; IASP: Seattle, WA, USA, 1994. [Google Scholar]
- Kara, H.; Abay, E. Kronik ag˘rıya psikiyatrik yaklas¸ım [Psychiatric Approachs to Chronic Pain Patients]. Anadolu Psikiyatr. Derg. 2000, 1, 89–99. [Google Scholar]
- Quadros, A.U.; Cunha, T.M. C5a and pain development: An old molecule, a new target. Pharmacol. Res. 2016, 112, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kobuch, S.; Fazalbhoy, A.; Brown, R.; Macefield, V.G. Inter-individual responses to experimental muscle pain: Baseline physiological parameters do not determine whether muscle sympathetic nerve activity increases or decreases during pain. Front Neurosci. 2015, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Domenici, E.; Willé, D.R.; Tozzi, F.; Prokopenko, I.; Miller, S.; McKeown, A.; Brittain, C.; Rujescu, D.; Giegling, I.; Turck, C.W.; et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS ONE 2010, 5, e9166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, R.W.; Jenkins, C.M.; Yang, J.; Mancuso, D.J.; Han, X. Functional lipidomics: The roles of specialized lipids and lipid-protein interactions in modulating neuronal function. Prostaglandins Other Lipid Mediat. 2005, 77, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Tsui-Pierchala, B.A.; Encinas, M.; Milbrandt, J.; Johnson, E.M. Lipid rafts in neuronal signaling and function. Trends Neurosci. 2002, 25, 412–417. [Google Scholar] [CrossRef]
- Parekh, A.; Smeeth, D.; Milner, Y.; Thuret, S. The Role of Lipid Biomarkers in Major Depression. Healthcare 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Mizunoya, W.; Ohnuki, K.; Baba, K.; Miyahara, H.; Shimizu, N.; Tabata, K.; Kino, T.; Sato, Y.; Tatsumi, R.; Ikeuchi, Y. Effect of dietary fat type on anxiety-like and depression-like behavior in mice. Springerplus 2013, 2, 165. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Luo, L.; Hou, F.; Fan, X.; Yu, J.; Ma, W.; Tang, W.; Yang, X.; Zhu, J.; Kang, W.; et al. Agmatine Reduces Lipopolysaccharide-Mediated Oxidant Response via Activating PI3K/Akt Pathway and up-Regulating Nrf2 and HO-1 Expression in Macrophages. PLoS ONE 2016, 11, e0163634. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.D.; Matthies, H.J.G.; Owens, A.W.; Sathananthan, V.; Christianson, N.S.B.; Kennedy, J.P.; Lindsley, C.W.; Daws, L.C.; Galli, A. Insulin reveals Akt signaling as a novel regulator of norepinephrine transporter trafficking and norepinephrine homeostasis. J. Neurosci. 2010, 30, 11305–11316. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Nicodemus, K.K.; Chen, Q.; Li, Z.; Brooke, J.K.; Honea, R.; Kolachana, B.S.; Straub, R.E.; Meyer-Lindenberg, A.; Sei, Y.; et al. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J. Clin. Investig. 2008, 118, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.H.; Lochan, A.C.; Cowen, D.S. Activation of Akt1 by human 5-hydroxytryptamine (serotonin)1B receptors is sensitive to inhibitors of MEK. J. Pharmacol. Exp. Ther. 2001, 298, 825–832. [Google Scholar] [PubMed]
- Wu, J.B.; Shih, J.C. Valproic Acid Induces Monoamine Oxidase A via Akt/Forkhead Box O1 Activation. Mol. Pharmacol. 2011, 80, 714–723. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

 ) inhibition (
) inhibition (  ), binding (
), binding (  ), catalysis (-
), catalysis (-  ), phenotype (
), phenotype (  ), posttranslational modification (
), posttranslational modification (  ), reaction (
), reaction (  ) and transcriptional regulation (
) and transcriptional regulation (  ). Action effects are shown by following signs: Positive (
). Action effects are shown by following signs: Positive (  ), negative (
), negative (  ) and unspecified (
) and unspecified (  ). Note: MIF—macrophage migration inhibitory factor; MAPK1—mitogen-activated protein kinase 1; HMOX1—heme oxygenase (decycling) 1; PON2—paraoxonase 2; CCND1—cyclin D1; CSN1S1—casein alpha s1; APOE—apolipoprotein E; NR1H2—nuclear receptor subfamily 1, group H, member 2; PPARA—peroxisome proliferator-activated receptor alpha; UCP1—uncoupling protein 1; STK17B—serine/threonine kinase 17b; AKT1—v-akt murine thymoma viral oncogene homolog 1; EGFR—epidermal growth factor receptor; NOS3—nitric oxide synthase 3; ACHE—Acetylcholinesterase; IL8—interleukin 8; CASP3—caspase 3; ADIPOQ—adiponectin, C1Q; NOS2—nitric oxide synthase 2; PRKCD—protein kinase C; NR1I2—nuclear receptor subfamily 1, group I, member 2; FDPS—farnesyl diphosphate synthase; MAPK8—mitogen-activated protein kinase 8; COMT—catechol-O-methyltransferase; NPY1R—neuropeptide Y receptor Y1; PTGS2—prostaglandin-endoperoxide synthase 2; MCL1—myeloid cell leukemia sequence 1 (BCL2 -related); DHCR24—24-dehydrocholesterol reductase; RUNX2—runt-related transcription factor 2; ALOX5—arachidonate 5-lipoxygenase; PRNP—prion protein; RPS6KA3—ribosomal protein S6 kinase, 90 kDa, polypeptide 3; CDK1—cyclin-dependent kinase 1; KDM1A—lysine (K)-specific demethylase 1A; NR1H3—nuclear receptor subfamily 1, group H, member 3; MAPK9—mitogen-activated protein kinase 9; IL10—interleukin 10; BDNF—brain-derived neurotrophic factor; RGS6—regulator of G-protein signaling 6; S1PR2—Sphingosine 1-phosphate receptor 2; CCNB1—cyclin B1; CDK4—cyclin-dependent kinase 4; JUN—jun proto-oncogene; MTOR—mechanistic target of rapamycin; CDK6—cyclin-dependent kinase 6; SHC1—SHC transforming protein 1; GRB2—growth factor receptor-bound protein 2; RCOR1—REST corepressor 1; CCNA2—cyclin A2; STAT3—signal transducer and activator of transcription 3.
). Note: MIF—macrophage migration inhibitory factor; MAPK1—mitogen-activated protein kinase 1; HMOX1—heme oxygenase (decycling) 1; PON2—paraoxonase 2; CCND1—cyclin D1; CSN1S1—casein alpha s1; APOE—apolipoprotein E; NR1H2—nuclear receptor subfamily 1, group H, member 2; PPARA—peroxisome proliferator-activated receptor alpha; UCP1—uncoupling protein 1; STK17B—serine/threonine kinase 17b; AKT1—v-akt murine thymoma viral oncogene homolog 1; EGFR—epidermal growth factor receptor; NOS3—nitric oxide synthase 3; ACHE—Acetylcholinesterase; IL8—interleukin 8; CASP3—caspase 3; ADIPOQ—adiponectin, C1Q; NOS2—nitric oxide synthase 2; PRKCD—protein kinase C; NR1I2—nuclear receptor subfamily 1, group I, member 2; FDPS—farnesyl diphosphate synthase; MAPK8—mitogen-activated protein kinase 8; COMT—catechol-O-methyltransferase; NPY1R—neuropeptide Y receptor Y1; PTGS2—prostaglandin-endoperoxide synthase 2; MCL1—myeloid cell leukemia sequence 1 (BCL2 -related); DHCR24—24-dehydrocholesterol reductase; RUNX2—runt-related transcription factor 2; ALOX5—arachidonate 5-lipoxygenase; PRNP—prion protein; RPS6KA3—ribosomal protein S6 kinase, 90 kDa, polypeptide 3; CDK1—cyclin-dependent kinase 1; KDM1A—lysine (K)-specific demethylase 1A; NR1H3—nuclear receptor subfamily 1, group H, member 3; MAPK9—mitogen-activated protein kinase 9; IL10—interleukin 10; BDNF—brain-derived neurotrophic factor; RGS6—regulator of G-protein signaling 6; S1PR2—Sphingosine 1-phosphate receptor 2; CCNB1—cyclin B1; CDK4—cyclin-dependent kinase 4; JUN—jun proto-oncogene; MTOR—mechanistic target of rapamycin; CDK6—cyclin-dependent kinase 6; SHC1—SHC transforming protein 1; GRB2—growth factor receptor-bound protein 2; RCOR1—REST corepressor 1; CCNA2—cyclin A2; STAT3—signal transducer and activator of transcription 3.
 ) inhibition (
) inhibition (  ), binding (
), binding (  ), catalysis (-
), catalysis (-  ), phenotype (
), phenotype (  ), posttranslational modification (
), posttranslational modification (  ), reaction (
), reaction (  ) and transcriptional regulation (
) and transcriptional regulation (  ). Action effects are shown by following signs: Positive (
). Action effects are shown by following signs: Positive (  ), negative (
), negative (  ) and unspecified (
) and unspecified (  ). Note: MIF—macrophage migration inhibitory factor; MAPK1—mitogen-activated protein kinase 1; HMOX1—heme oxygenase (decycling) 1; PON2—paraoxonase 2; CCND1—cyclin D1; CSN1S1—casein alpha s1; APOE—apolipoprotein E; NR1H2—nuclear receptor subfamily 1, group H, member 2; PPARA—peroxisome proliferator-activated receptor alpha; UCP1—uncoupling protein 1; STK17B—serine/threonine kinase 17b; AKT1—v-akt murine thymoma viral oncogene homolog 1; EGFR—epidermal growth factor receptor; NOS3—nitric oxide synthase 3; ACHE—Acetylcholinesterase; IL8—interleukin 8; CASP3—caspase 3; ADIPOQ—adiponectin, C1Q; NOS2—nitric oxide synthase 2; PRKCD—protein kinase C; NR1I2—nuclear receptor subfamily 1, group I, member 2; FDPS—farnesyl diphosphate synthase; MAPK8—mitogen-activated protein kinase 8; COMT—catechol-O-methyltransferase; NPY1R—neuropeptide Y receptor Y1; PTGS2—prostaglandin-endoperoxide synthase 2; MCL1—myeloid cell leukemia sequence 1 (BCL2 -related); DHCR24—24-dehydrocholesterol reductase; RUNX2—runt-related transcription factor 2; ALOX5—arachidonate 5-lipoxygenase; PRNP—prion protein; RPS6KA3—ribosomal protein S6 kinase, 90 kDa, polypeptide 3; CDK1—cyclin-dependent kinase 1; KDM1A—lysine (K)-specific demethylase 1A; NR1H3—nuclear receptor subfamily 1, group H, member 3; MAPK9—mitogen-activated protein kinase 9; IL10—interleukin 10; BDNF—brain-derived neurotrophic factor; RGS6—regulator of G-protein signaling 6; S1PR2—Sphingosine 1-phosphate receptor 2; CCNB1—cyclin B1; CDK4—cyclin-dependent kinase 4; JUN—jun proto-oncogene; MTOR—mechanistic target of rapamycin; CDK6—cyclin-dependent kinase 6; SHC1—SHC transforming protein 1; GRB2—growth factor receptor-bound protein 2; RCOR1—REST corepressor 1; CCNA2—cyclin A2; STAT3—signal transducer and activator of transcription 3.
). Note: MIF—macrophage migration inhibitory factor; MAPK1—mitogen-activated protein kinase 1; HMOX1—heme oxygenase (decycling) 1; PON2—paraoxonase 2; CCND1—cyclin D1; CSN1S1—casein alpha s1; APOE—apolipoprotein E; NR1H2—nuclear receptor subfamily 1, group H, member 2; PPARA—peroxisome proliferator-activated receptor alpha; UCP1—uncoupling protein 1; STK17B—serine/threonine kinase 17b; AKT1—v-akt murine thymoma viral oncogene homolog 1; EGFR—epidermal growth factor receptor; NOS3—nitric oxide synthase 3; ACHE—Acetylcholinesterase; IL8—interleukin 8; CASP3—caspase 3; ADIPOQ—adiponectin, C1Q; NOS2—nitric oxide synthase 2; PRKCD—protein kinase C; NR1I2—nuclear receptor subfamily 1, group I, member 2; FDPS—farnesyl diphosphate synthase; MAPK8—mitogen-activated protein kinase 8; COMT—catechol-O-methyltransferase; NPY1R—neuropeptide Y receptor Y1; PTGS2—prostaglandin-endoperoxide synthase 2; MCL1—myeloid cell leukemia sequence 1 (BCL2 -related); DHCR24—24-dehydrocholesterol reductase; RUNX2—runt-related transcription factor 2; ALOX5—arachidonate 5-lipoxygenase; PRNP—prion protein; RPS6KA3—ribosomal protein S6 kinase, 90 kDa, polypeptide 3; CDK1—cyclin-dependent kinase 1; KDM1A—lysine (K)-specific demethylase 1A; NR1H3—nuclear receptor subfamily 1, group H, member 3; MAPK9—mitogen-activated protein kinase 9; IL10—interleukin 10; BDNF—brain-derived neurotrophic factor; RGS6—regulator of G-protein signaling 6; S1PR2—Sphingosine 1-phosphate receptor 2; CCNB1—cyclin B1; CDK4—cyclin-dependent kinase 4; JUN—jun proto-oncogene; MTOR—mechanistic target of rapamycin; CDK6—cyclin-dependent kinase 6; SHC1—SHC transforming protein 1; GRB2—growth factor receptor-bound protein 2; RCOR1—REST corepressor 1; CCNA2—cyclin A2; STAT3—signal transducer and activator of transcription 3.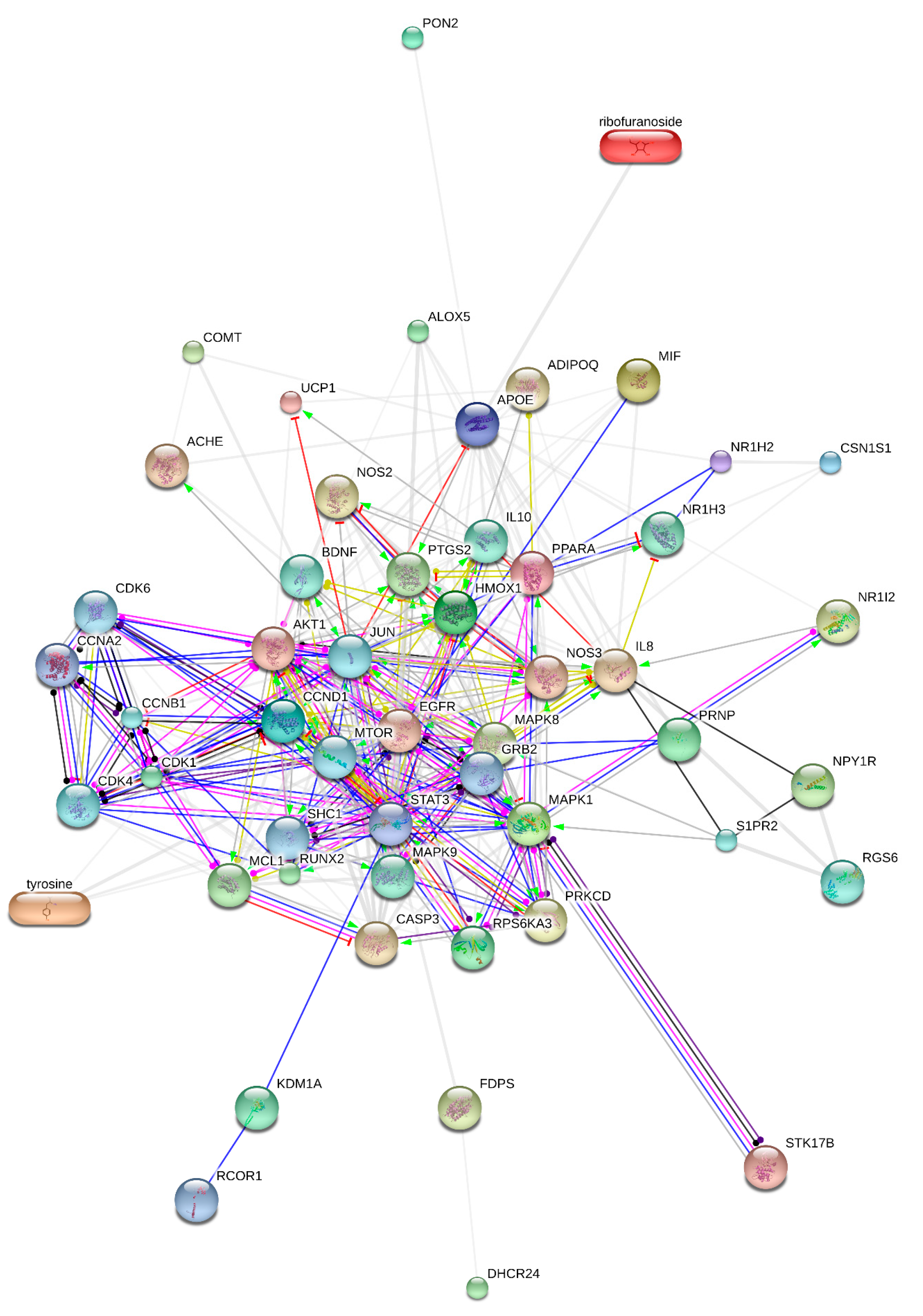
| Targets | Degree | Targets | Degree | Targets | Degree | Targets | Degree |
|---|---|---|---|---|---|---|---|
| Ribofuranoside | 1 | AKT1 | 33 | STAT3 | 29 | MAPK9 | 14 |
| Tyrosine | 5 | EGFR | 25 | COMT | 3 | IL10 | 15 |
| MIF | 6 | NOS3 | 23 | NPY1R | 3 | BDNF | 16 |
| MAPK1 | 23 | ACHE | 4 | PTGS2 | 16 | RGS6 | 3 |
| HMOX1 | 17 | IL8 | 21 | MCL1 | 15 | S1PR2 | 6 |
| PON2 | 1 | CASP3 | 17 | DHCR24 | 1 | CCNB1 | 16 |
| CCND1 | 22 | ADIPOQ | 8 | RUNX2 | 11 | CDK4 | 10 |
| CSN1S1 | 3 | NOS2 | 11 | ALOX5 | 6 | JUN | 33 |
| APOE | 22 | PRKCD | 11 | PRNP | 3 | MTOR | 24 |
| NR1H2 | 4 | NR1I2 | 3 | RPS6KA3 | 13 | CDK6 | 11 |
| PPARA | 22 | FDPS | 2 | CDK1 | 14 | SHC1 | 15 |
| UCP1 | 4 | MAPK8 | 26 | KDM1A | 2 | GRB2 | 16 |
| STK17B | 1 | CCNA2 | 6 | NR1H3 | 6 | RCOR1 | 1 |
| Activation | Inhibition | Binding | Phenotype | Catalysis | Post-Trans. Mod. | Reaction | Expression | Score | |
|---|---|---|---|---|---|---|---|---|---|
| CCNB1 | • | • | • | • | • | 0.999 | |||
| CDK4 | • | • | • | • | • | • | 0.999 | ||
| JUN | • | • | • | • | • | • | 0.999 | ||
| MTOR | • | • | • | • | • | 0.999 | |||
| CDK6 | • | • | • | 0.999 | |||||
| SHC1 | • | • | • | • | • | 0.999 | |||
| GRB2 | • | • | • | • | 0.999 | ||||
| RCOR1 | 0.999 | ||||||||
| CCNA2 | • | • | • | • | • | 0.999 | |||
| STAT3 | • | • | • | • | 0.999 |
| GO ID | GO Term | Term p Value (¤) | Group p Value (¤) | Associated Genes Found | Related Diseases |
|---|---|---|---|---|---|
| 46686 | Response to cadmium ion | 13.0 × 10−6 (770.0 × 10−6) | 13.0 × 10−6 (39.0 × 10−6) | [CDK1, HMOX1, MAPK9, PRNP] | Fibrosis, Hypertensive Disease, Inflammation, Diarrhea, Nervousness, Neoplasms, Lung Diseases, Hypoxia, Anoxia, Acidosis |
| 06636 | Unsaturated fatty acid biosynthetic process | 870.0 × 10−6 (4.3 × 10−3) | 900.0 × 10−6 (900.0 × 10−6) | [ALOX5, MIF, PTGS2] | Neoplasms, Hyperpigmentation, Nervousness, Agitation, Pituitary Diseases, Infective Disorder, Albinism, Tumor Angiogenesis, Hemorrhage, Hypertrophy, Diarrhea |
| 42982 | Amyloid precursor protein metabolic process | 100.0 × 10−6 (3.2 × 10−3) | 130.0 × 10−6 (260.0 × 10−6) | [ACHE, APOE, DHCR24] | Alzheimer’s disease |
| 19233 | Sensory perception of pain | 10.0 × 10−6 (660.0 × 10−6) | 1.1 × 10−6 (4.5 × 10−6) | [COMT, IL10, MAPK1, NPY1R, PTGS2] | Pain, Nervousness, Ulcer, Depressive disorder, Ulcer, Diabetes Mellitus, Neuralgia, Sensation Disorders, Anxiety, Disorders, Inflammation, Perceptual Disorders |
| 31348 | Negative regulation of defense response | 310.0 × 10−9 (27.0 × 10−6) | 25.0 × 10−12 (300.0 × 10−12) | [ADIPOQ, APOE, IL10, NR1H2, NR1H3, PPARA, PRKCD] | Dehydration, Dysequilibrium syndrome (balance disorder), Physiological stress |
| 32368 | Regulation of lipid transport | 460.0 × 10−12 (53.0 × 10−9) | 410.0 × 10−15 (6.2 × 10−12) | [ADIPOQ, AKT1, APOE, MIF, NR1H2, NR1H3, PPARA, PRKCD] | Alzheimer’s disease, Motor neuron disease, Sphingolipidoses, Nervousness, Nervous system disorder, Hereditary Motor, Neurone Disease, Depression |
| 10888 | Negative regulation of lipid storage | 200.0 × 10−9 (19.0 × 10−6) | 20.0 × 10−12 (260.0 × 10−12) | [NR1H2, NR1H3, PPARA, TNF] | Obesity, Diabetes Mellitus, Pituitary Diseases |
| 32722 | Positive regulation of chemokine production | 17.0 × 10−6 (960.0 × 10−6) | 20.0 × 10−12 (260.0 × 10−12) | [ADIPOQ, HMOX1, MIF, TNF] | Cholangiocarcinoma, Inflammatory Response, Arthritis, Rheumatism, Bacterial Infections |
| 50880 | Regulation of blood vessel size | 210.0 × 10−9 (19.0 × 10−6) | 9.2 × 10−12 (120.0 × 10−12) | [AKT1, APOE, EGFR, HMOX1, NOS2, NOS3, PTGS2] | Retinal Degeneration, Albinism |
| 33002 | Muscle cell proliferation | 1.0 × 10−9 (110.0 × 10−9) | 35.0 × 10−12 (380.0 × 10−12) | [ADIPOQ, AKT1, CDK1, COMT, EGFR, HMOX1, IL10, PTGS2, TNF] | Atherosclerosis, Hyperplasia, Vascular Diseases, Cardiovascular Diseases, Inflammation, Stenosis, Dental Plaque |
| 32496 | Response to lipopolysaccharide | 200.0 × 10−15 (25.0 × 10−15) | 5.5 × 10−15 (88.0 × 10−15) | [AKT1, CASP3, COMT, CXCL8, IL10, MAPK1, MAPK8, MIF, NOS2, NOS3, NR1H3, PTGS2, RPS6KA3, TNF] | Inflammation, Systemic Infection, Innate Immune Response, Neoplasms, Decreased Immunologic Activity, Septic Shock, Atherosclerosis, Lung Injury, Autoimmune Reaction, Nervousness |
| 71219 | Cellular response to molecule of bacterial origin | 37.0 × 10−12 (4.4 × 10−9) | 5.5 × 10−15 (88.0 × 10−15) | [AKT1, CXCL8, IL10, MAPK1, MAPK8, MIF, NOS2, NOS3, NR1H3, TNF] | Septic Shock, Innate Immune Response, Communicable , Diseases |
| 51100 | Negative regulation of binding | 120.0 × 10−9 (11.0 × 10−6) | 9.7 × 10−18 (160.0 × 10−18) | [ADIPOQ, HMOX1, IL10, KDM1A, MAPK8, PPARA, PRKCD] | Tissue Adhesions, Inflammation, Escherichia Coli Infections, Edema, Neoplasms, Hemorrhage, Basophilic Leukemia, Dwarfism, Pertussis, Nervousness, Headache, Hyperglycemia, Pituitary Diseases, Mammary Neoplasms, Diabetes Mellitus, Kidney Diseases, Obesity, Diabetes Mellitus, Hiv Infections, Neoplasms, Leukemia |
| 2000377 | Regulation of reactive oxygen species metabolic process | 380.0 × 10−9 (33.0 × 10−6) | 9.7 × 10−18 (160.0 × 10−18) | [AKT1, EGFR, IL10, PRKCD, PTGS2, TNF, UCP1] | Neoplasm metastasis, Neoplasms, Cancer of nasopharynx, Carcinoma |
| 32091 | Negative regulation of protein binding | 120.0 × 10−9 (12.0 × 10−6) | 9.7 × 10−18 (160.0 × 10−18) | [ADIPOQ, IL10, KDM1A, MAPK8, PPARA, PRKCD] | Flushing, Tuberculosis |
| 31663 | Lipopolysaccharide-mediated signaling pathway | 620.0 × 10−9 (52.0 × 10−6) | 9.7 × 10−18 (160.0 × 10−18) | [AKT1, MAPK1, MIF, NOS3, TNF] | Ischemia, Neoplasms, Hepatic ischaemia, Fibrosis, Brain Injuries, Depressive Disorder, Cystic Fibrosis, Neurodegenerative Disorders, Anoxia, Neuroblastoma, Alzheimer’s Disease |
| 45923 | Positive regulation of fatty acid metabolic process | 31.0 × 10−9 (3.2 × 10−6) | 9.7 × 10−18 (160.0 × 10−18) | [ADIPOQ, NR1H2, NR1H3, PPARA, PTGS2] | Obesity, Diabetes Mellitus, Ischemia, Neoplasms, Inflammation |
| Tumor Angiogenesis, Fatty Liver, Hyperthyroidism |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, D.; Murtaza, G.; Ma, S.; Li, L.; Li, X.; Tian, F.; Zheng, J.; Lu, Y. In Silico Prediction of the Anti-Depression Mechanism of a Herbal Formula (Tiansi Liquid) Containing Morinda officinalis and Cuscuta chinensis. Molecules 2017, 22, 1614. https://doi.org/10.3390/molecules22101614
Cheng D, Murtaza G, Ma S, Li L, Li X, Tian F, Zheng J, Lu Y. In Silico Prediction of the Anti-Depression Mechanism of a Herbal Formula (Tiansi Liquid) Containing Morinda officinalis and Cuscuta chinensis. Molecules. 2017; 22(10):1614. https://doi.org/10.3390/molecules22101614
Chicago/Turabian StyleCheng, Dan, Ghualm Murtaza, Suya Ma, Lingling Li, Xinjie Li, Fangze Tian, Junchao Zheng, and Yi Lu. 2017. "In Silico Prediction of the Anti-Depression Mechanism of a Herbal Formula (Tiansi Liquid) Containing Morinda officinalis and Cuscuta chinensis" Molecules 22, no. 10: 1614. https://doi.org/10.3390/molecules22101614





