Characterization and Trypanocidal Activity of a Novel Pyranaphthoquinone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Analysis
2.2. X-ray Fluorescence
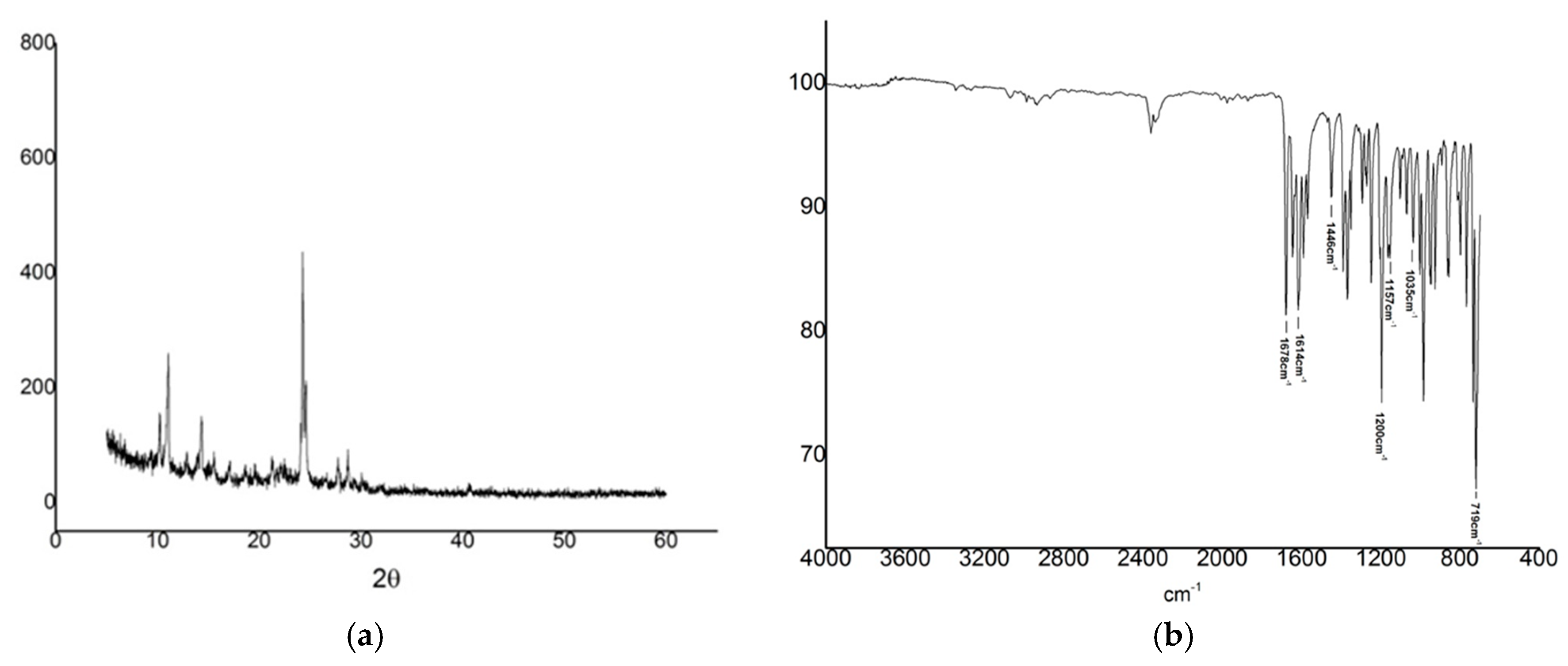
2.3. X-ray Diffraction
2.4. Fourier Transform Infrared (FT-IR)
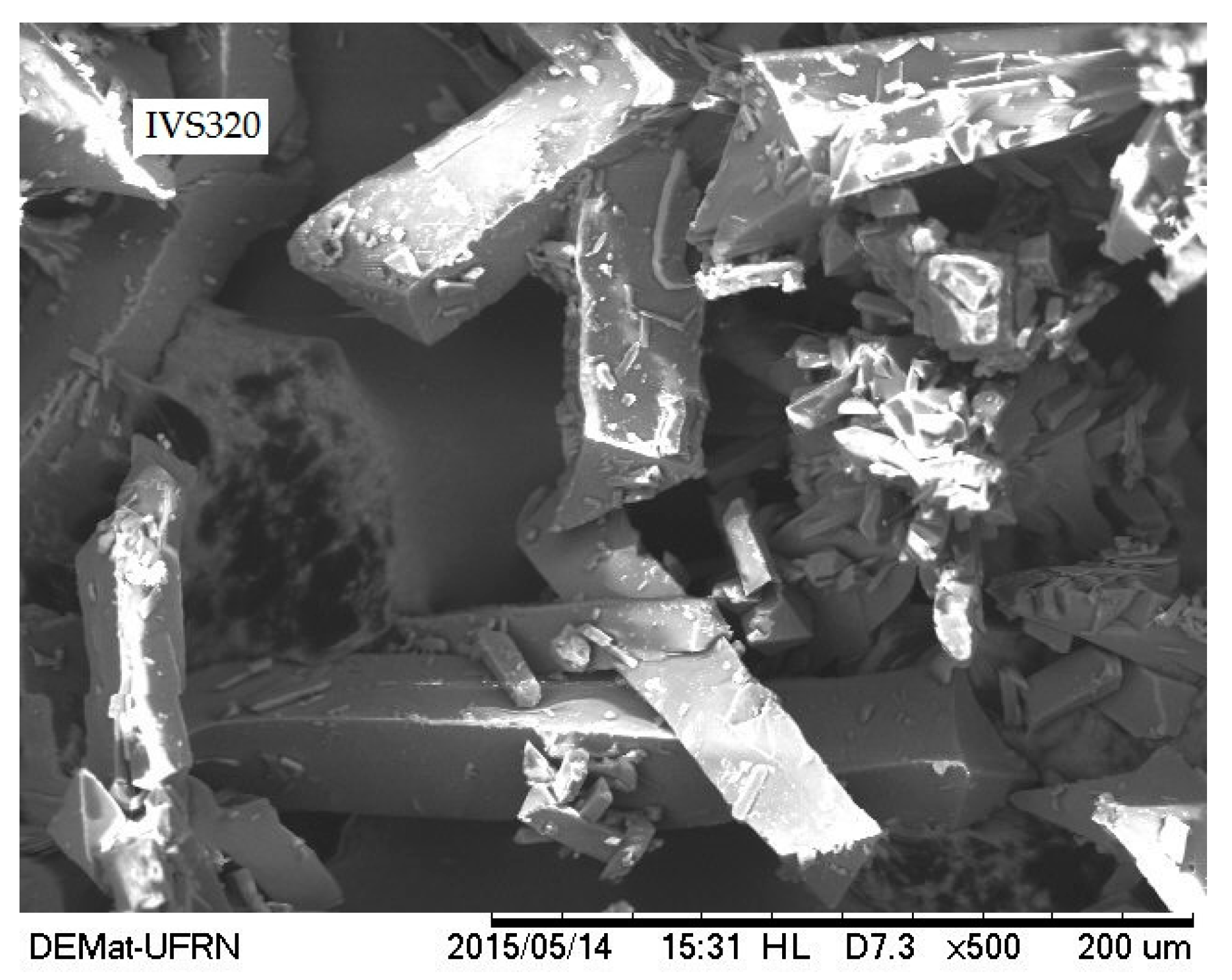
2.5. Scanning Electron Microscopy (SEM)
2.6. Hygroscopicity
2.7. Partition Coefficient/Lipophilicity
2.8. Solubility
3. Trypanocidal Activity
4. Materials and Methods
4.1. Material
4.2. Differential Scanning Calorimetry (DSC)
4.3. Termogravimetry & Differential Thermal Analysis (TG and DTA)
4.4. X-ray Fluorescence
4.5. X-ray Diffraction
4.6. Fourrier Transform Infrared (FT-IR)
4.7. Scanning Electron Microscopy (SEM)
4.8. Hygroscopicity
4.9. Partition Coefficient/Lipophilicity
4.10. Solubility Studies
4.11. Parasites
4.12. Anti-Parasitic Activity
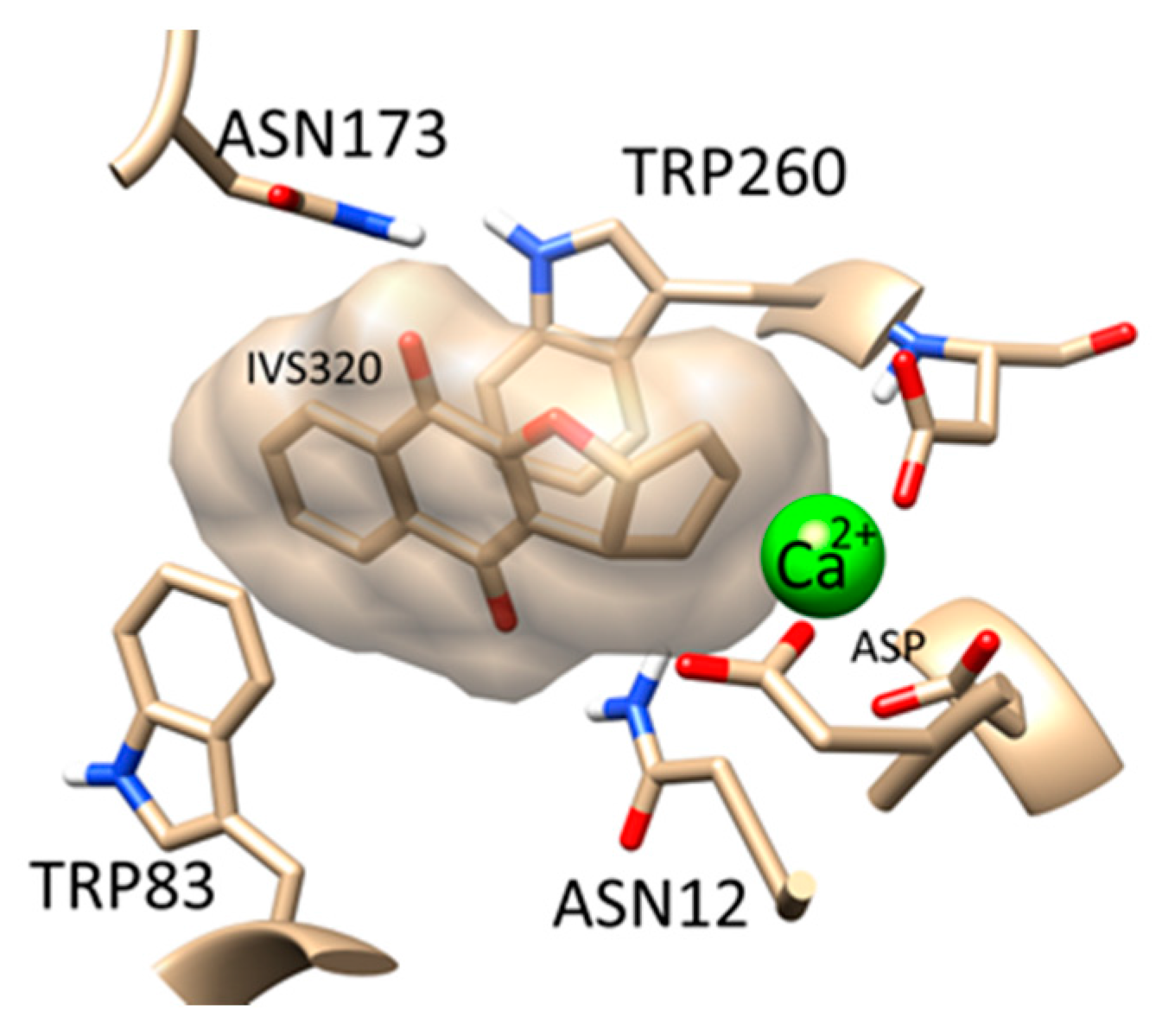
4.13. Inverse Virtual Screening
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McKerrow, J.; Doyle, P.; Engel, J.; Podust, L.; Robertson, S.; Ferreira, R.; Saxton, T.; Arkin, M.; Kerr, I.D.; Brinen, L.S.; et al. Two approaches to discovering and developing new drugs for Chagas disease. Mem. Inst. Oswaldo Cruz 2009, 104, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Chagas Disease (American Trypanosomiasis)—World Health Organization. Available online: http://www.who.int/chagas/home_more/en/ (accessed on 15 December 2016).
- Coura, J.R.; Viñas, P.A.; Junqueira, A.C.V. Ecoepidemiology, short history and control of Chagas disease in the endemic countries and the new challenge for non-endemic countries. Mem. Inst. Oswaldo Cruz 2014, 109, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Vermelho, A.B.; Capaci, G.R.; Rodrigues, I.A.; Cardoso, V.S.; Mazotto, A.M.; Supuran, C.T. Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorg. Med. Chem. 2017, 25, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Lima, Á.A.; Soares-Sobrinho, J.L.; Silva, J.L.; Corrêa-Júnior, R.A.; Lyra, M.A.; Santos, F.L.; Oliveiran, B.G.; Hernandes, M.Z.; Rolim, L.A.; Rolim-Neto, P.J. The use of solid dispersion systems in hydrophilic carriers to increase benznidazole solubility. J. Pharm. Sci. 2011, 100, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.C.M.; Navarro, E.C. Challenges and perspectives of Chagas disease: A review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 34. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.P.V.; Ferreira, S.B.; Oliveira, N.S.M.; Matsuura, A.B.J.; Gama, I.L.; Silva, F.C.; Souza, M.C.B.V.; Lima, E.S.; Ferreira, V.F. Synthesis and biological evaluation of substituted α-and β-2, 3-dihydrofuran naphthoquinones as potent anticandidal agents. MedChemComm 2010, 1, 229–232. [Google Scholar] [CrossRef]
- Silva, F.C.; Ferreira, V.F. Natural naphthoquinones with great importance in medicinal chemistry. Curr. Org. Synth. 2016, 13, 334–371. [Google Scholar] [CrossRef]
- Bhasin, D.; Chettiar, S.N.; Etter, J.P.; Mok, M.; Li, P.K. Anticancer activity and SAR studies of substituted 1, 4-naphthoquinones. Bioorg. Med. Chem. 2013, 21, 4662–4669. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Bębenek, E.; Chrobak, E.; Latocha, M.; Boryczka, S. Alkoxy and Enediyne Derivatives Containing 1,4-Benzoquinone Subunits—Synthesis and Antitumor Activity. Molecules 2017, 22, 447. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, T.; Kandhasamy, S.; Dinesh, R.; Shruthy, S.; Shweta, S.; Mukesh, D.; Karunagaran, D.; Balaji, R.; Mathivanan, N.; Perumal, P.T. Synthesis and SAR study of novel anticancer and antimicrobial naphthoquinone amide derivatives. Bioorg. Med. Chem. 2014, 24, 3647–3651. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, M.; Demchuk, O.M.; Strzelecka, D.; Kubiński, K.; Masłyk, M. New family of antimicrobial agents derived from 1,4-naphthoquinone. Eur. J. Med. Chem. 2016, 124, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Efficacy of naphthoquinones as insecticides against the house fly, Musca domestica L. Ind. Crop. Prod. 2013, 43, 745–750. [Google Scholar] [CrossRef]
- Sodero, A.C.R.; Abrahim-Vieira, B.; Torres, P.H.M.; Pascutti, P.G.; Garcia, C.R.; Ferreira, V.F.; Rocha, D.R.; Ferreira, S.B.; Silva, F.P., Jr. Insights into cytochrome bc1 complex binding mode of antimalarial 2-hydroxy-1, 4-naphthoquinones through molecular modelling. Mem. Inst. Oswaldo Cruz 2017, 112, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Jun, D.Y.; Lee, J.Y.; Park, H.S.; Woo, M.H.; Park, S.J.; Kim, S.C.; Yang, C.H.; Kim, Y.H. Anti-inflammatory action of 2-carbomethoxy-2,3-epoxy-3-prenyl-1, 4-naphthoquinone (CMEP-NQ) suppresses both the MyD88-dependent and TRIF-dependent pathways of TLR4 signaling in LPS-stimulated RAW264. 7 cells. J. Ethnopharmacol. 2017, 205, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.V.; David, C.C.; Neto, J.C.; Oliveira, L.A.; Silva, K.C.; Santos, J.M.; Silva, J.K.S.; Brandão, V.B.C.A.; Silva, T.M.S.; Camara, C.A.; Alexandre-Moreira, M.S. Evaluation on the leishmanicidal activity of 2-N,N′-dialkylamino-1,4-naphthoquinone derivatives. Exp. Parasitol. 2017, 176, 46–51. [Google Scholar] [CrossRef]
- Naujorks, A.A.S.; Silva, A.O.; Silva Lopes, R.; Albuquerque, S.; Beatriz, A.; Marques, M.R.; Lima, D.P. Novel naphthoquinone derivatives and evaluation of their trypanocidal and leishmanicidal activities. Org. Biomol. Chem. 2015, 13, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Calvo, J.M.; Barbero, G.R.; Guerrero-Vásquez, G.; Durán, A.G.; Macías, M.; Rodríguez-Iglesias, M.A.; Molinillo, J.M.G.; Macías, F.A. Synthesis, antibacterial and antifungal activities of naphthoquinone derivatives: A structure–activity relationship study. Med. Chem. Res. 2016, 6, 1274–1285. [Google Scholar] [CrossRef]
- Nair, V.; Treesa, P.M.; Maliakal, D.; Rath, N.P. CAN Mediated oxidative addition of 2-hydroxynaphthoquinone to dienes: A facile synthesis of naphthofurandiones. Tetrahedron 2001, 57, 7705–7710. [Google Scholar] [CrossRef]
- Ferreira, M.P.S.B.C.; Cardoso, M.F.C.C.; Silva, F.C.; Ferreira, V.F.; Lima, E.S.; Souza, J.V.B. Antifungal activity of synthetic naphthoquinones against dermatophytes and opportunistic fungi: Preliminary mechanism-of-action tests. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Láng, P.; Kiss, V.; Ambrus, R.; Farkas, G.; Szabó-Révész, P.; Aigner, Z.; Várkonyi, E. Polymorph screening of an active material. J. Pharm. Biomed. Anal. 2013, 84, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Neto, J.L.; Presmich, G.M.A.; Rolim, L.A.; Alves, L.D.S.; Albuqueque, M.M.; Rolim-Neto, P.J. Caracterização físico-química do potencial agente antineoplásico β-lapachona. Rev. Ciênc. Farm. Básica Apl. 2013, 33, 545–553. [Google Scholar]
- Dinunzio, J.C.; Brough, C.; Miller, D.A.; Williams, R.O.; McGinitya, J.W. Applications of KinetiSol® Dispersing for the production of plasticizer free amorphous solid dispersions. Eur. J. Pharm. Sci. 2010, 40, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.S.; Vishwakarma, R.A. Impact of preformulation on drug development. Expert Opin. Drugs Deliv. 2013, 10, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.W.; Reutzel-Edens, S.M.; Zografi, G. Characterization of the “hygroscopic” properties of active pharmaceutical ingredients. J. Pharm. Sci. 2008, 97, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Murikipudi, V.; Gupta, P.; Sihorkar, V. Efficient throughput method for hygroscopicity classification of active and inactive pharmaceutical ingredients by water vapor sorption analysis. Pharm. Dev. Technol. 2013, 18, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Fassihi, A.R.; Persicaner, P.H.R. Solid state interaction of bromazepam with polyvinylpyrrolidone in the presence of moisture. Int. J. Pharm. 1987, 37, 167–170. [Google Scholar] [CrossRef]
- Callahan, J.C.; Cleary, G.W.; Elefant, M.; Kaplan, G.; Kensler, T.; Nash, R.A. Equilibrium moisture content of pharmaceutical excipients. Drug Dev. Ind. Pharm. 1982, 8, 355–369. [Google Scholar] [CrossRef]
- Yang, Y.; Engkvist, O.; Llinàs, A.; Chen, H. Beyond size, ionization state, and lipophilicity: Influence of molecular topology on absorption, distribution, metabolism, excretion, and toxicity for druglike compounds. J. Med. Chem. 2012, 55, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.F.; Jorqueira, A.; Souza, A.M.; Silva, M.N.; Souza, M.C.; Gouvêa, R.M.; Rodrigues, C.R.; Pinto, A.V.; Castro, H.C.; Santos, D.O.; et al. Trypanocidal agents with low cytotoxicity to mammalian cell line: A comparison of the theoretical and biological features of lapachone derivatives. Bioorg. Med. Chem. 2006, 14, 5459–5466. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Miller, J.M.; Hoffman, A.; Amidon, G.E.; Amidon, G.L. The solubility–permeability interplay in using cyclodextrins as pharmaceutical solubilizers: Mechanistic modeling and application to progesterone. J. Pharm. Sci. 2010, 99, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Silva Júnior, E.N.; Melo, I.M.M.; Diogo, E.B.T.; Costa, V.A.; Souza Filho, J.D.; Valença, W.O.; Camara, C.A.; Oliveira, R.N.; Araujo, A.S.; Emery, F.S.; et al. On the search for potential anti-Trypanosoma cruzi drugs: Synthesis and biological evaluation of 2-hydroxy-3-methylamino and 1,2,3-triazolic naphthoquinoidal compounds obtained by click chemistry reactions. Eur. J. Med. Chem. 2012, 52, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Sieveking, I.; Thomas, P.; Estévez, J.C.; Quiñones, N.; Cuéllar, M.A.; Villena, J.; Espinosa-Bustos, C.; Fierro, A.; Tapia, R.A.; Maya, J.D.; et al. 2-Phenylaminonaphthoquinones and related compounds: Synthesis, trypanocidal and cytotoxic activities. Bioorg. Med. Chem. 2014, 22, 4609–4620. [Google Scholar] [CrossRef] [PubMed]
- Versées, W.; Decanniere, K.; Van-Holsbeke, E.; Devroede, N.; Steyaert, J. Enzyme-substrate interactions in the purine-specific nucleoside hydrolase from Trypanosoma vivax. J. Biol. Chem. 2002, 277, 15938–15946. [Google Scholar] [CrossRef] [PubMed]
- Versées, W.; Decanniere, K.; Pellé, R.; Depoorter, J.; Brosens, E.; Parkin, D.W.; Steyaert, J. Structure and function of a novel purine specific nucleoside hydrolase from Trypanosoma vivax. J. Mol. Biol. 2001, 307, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- Schormann, N.; Pal, B.; Senkovich, O.; Carson, M.; Howard, A.; Smith, C.; Delucas, L.; Chattopadhyay, D. Crystal structure of Trypanosoma cruzi pteridine reductase 2 in complex with a substrate and an inhibitor. J. Struct. Biol. 2005, 152, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). Test No. 123: Partition Coefficient (1-Octanol/Water): Slow-Stirring Method, OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2006; Section 1; Available online: http://www.oecd-ilibrary.org/environment/test-no-123-partition-coefficient-1-octanol-water-slow-stirring-method_9789264015845-en (accessed on 5 November 2015). [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E.J. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound IVS320 are available from the authors. |


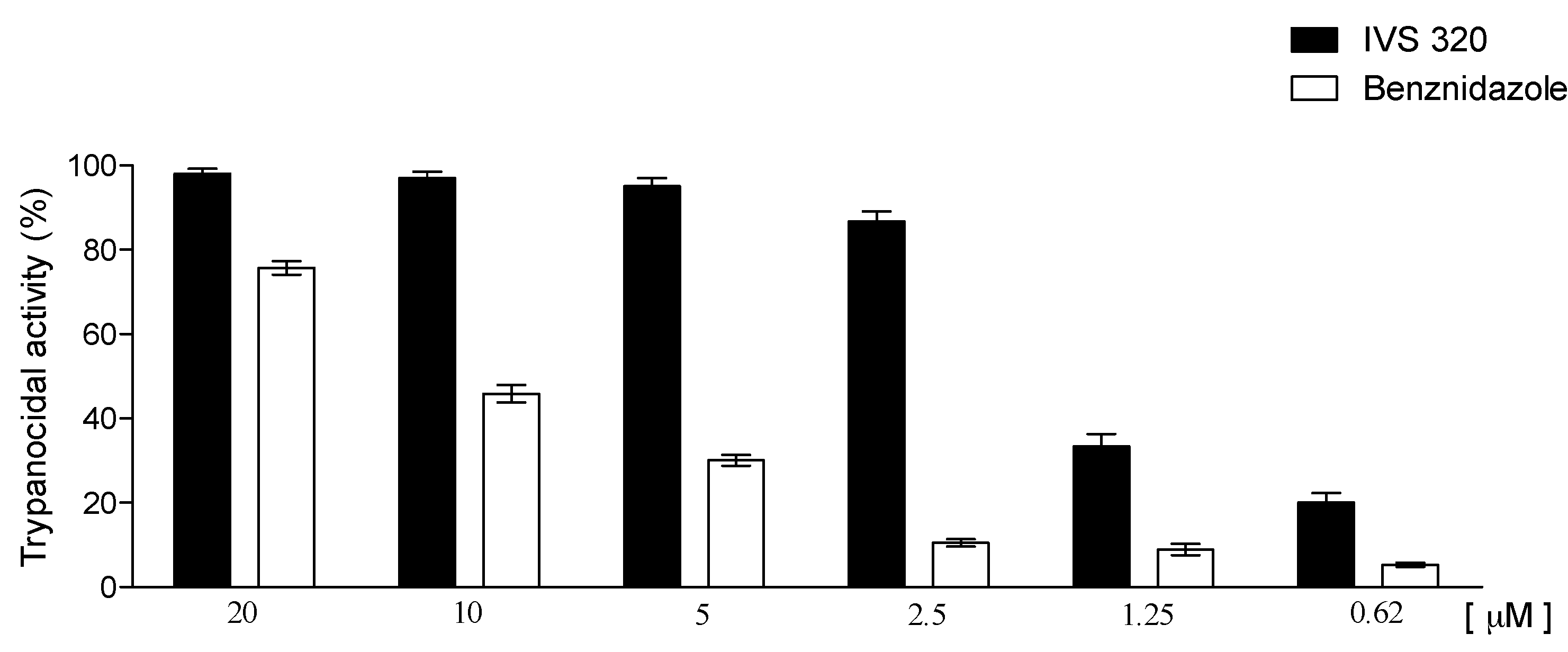
| Chemical Element | Concentration ‰ (w/w) |
|---|---|
| Na | 22.13 |
| Mg | 0.579 |
| Al | 0.615 |
| S | 0.03 |
| Cu | 0.01 |
| Classification | Criteria |
|---|---|
| Non-hygroscopic | No moisture increase at humidity levels below 90%. Less than 20% (w/w) increase in moisture content at humidity levels above 90% after 1 week of storage. |
| Slightly hygroscopic | No moisture increase at humidity levels below 80%. Less than 40% (w/w) increase in moisture content at humidity levels above 80% after 1 week of storage. |
| Moderately hygroscopic | Moisture content does not increase >5% (w/w) at humidity levels below 60%. Less than 50% (w/w) increase in moisture content at humidity levels above 80% after 1 week of storage. |
| Very hygroscopic | Moisture content increases at humidity levels as low as 40–50%. Greater than 20% (w/w) increase in moisture content at humidity levels above 90% after week of storage. |
| Data | LogP * |
|---|---|
| 1 | 2.04 |
| 2 | 2.13 |
| 3 | 2.07 |
| Data | Solubility (μg/mL) * |
|---|---|
| 1 | 0.0108 |
| 2 | 0.0134 |
| 3 | 0.0122 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantas, E.D.; De Souza, F.J.J.; Nogueira, W.N.L.; Silva, C.C.; De Azevedo, P.H.A.; Soares Aragão, C.F.; Almeida, P.D.O.d.; Cardoso, M.F.d.C.; Da Silva, F.D.C.; De Azevedo, E.P.; et al. Characterization and Trypanocidal Activity of a Novel Pyranaphthoquinone. Molecules 2017, 22, 1631. https://doi.org/10.3390/molecules22101631
Dantas ED, De Souza FJJ, Nogueira WNL, Silva CC, De Azevedo PHA, Soares Aragão CF, Almeida PDOd, Cardoso MFdC, Da Silva FDC, De Azevedo EP, et al. Characterization and Trypanocidal Activity of a Novel Pyranaphthoquinone. Molecules. 2017; 22(10):1631. https://doi.org/10.3390/molecules22101631
Chicago/Turabian StyleDantas, Elen Diana, Fabia Julliana Jorge De Souza, William Nascimento Litaiff Nogueira, Cláudia Cândida Silva, Pedro Henrique Antunes De Azevedo, Cícero Flávio Soares Aragão, Patricia Danielle Oliveira de Almeida, Mariana Filomena do Carmo Cardoso, Fernando De Carvalho Da Silva, Eduardo Pereira De Azevedo, and et al. 2017. "Characterization and Trypanocidal Activity of a Novel Pyranaphthoquinone" Molecules 22, no. 10: 1631. https://doi.org/10.3390/molecules22101631






