5-Hydroxycyclopenicillone Inhibits β-Amyloid Oligomerization and Produces Anti-β-Amyloid Neuroprotective Effects In Vitro
Abstract
:1. Introduction
2. Results
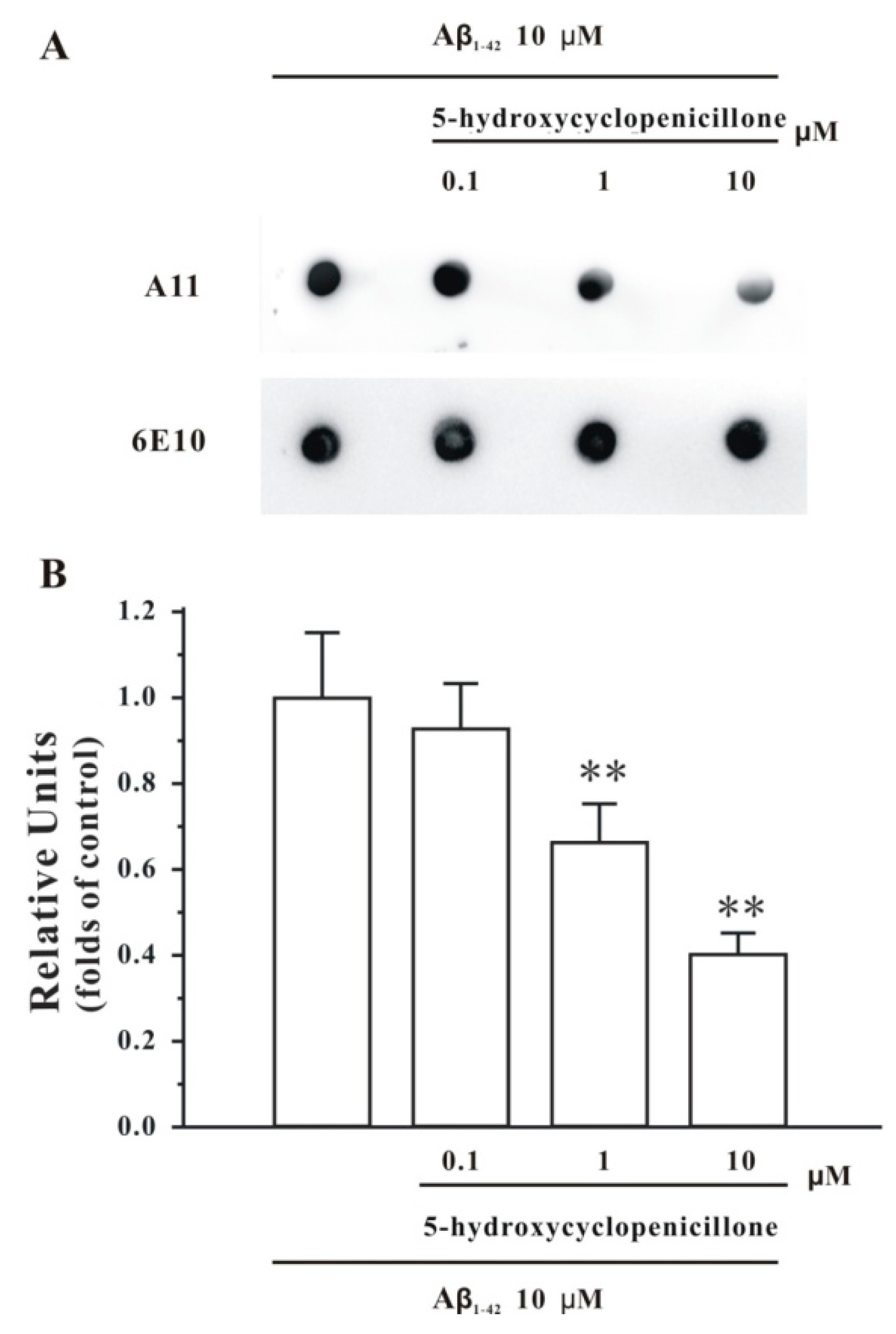
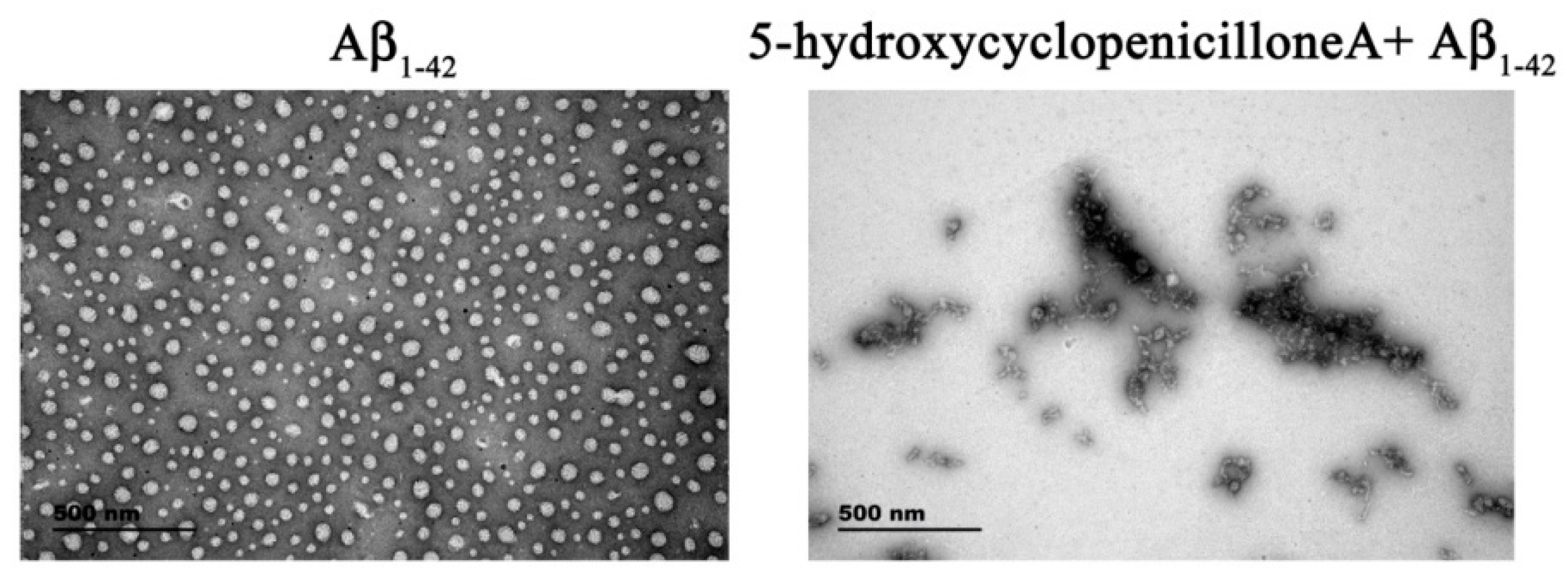
2.1. 5-Hydroxycyclopenicillone Inhibits Aβ1-42 Oligomer Formation
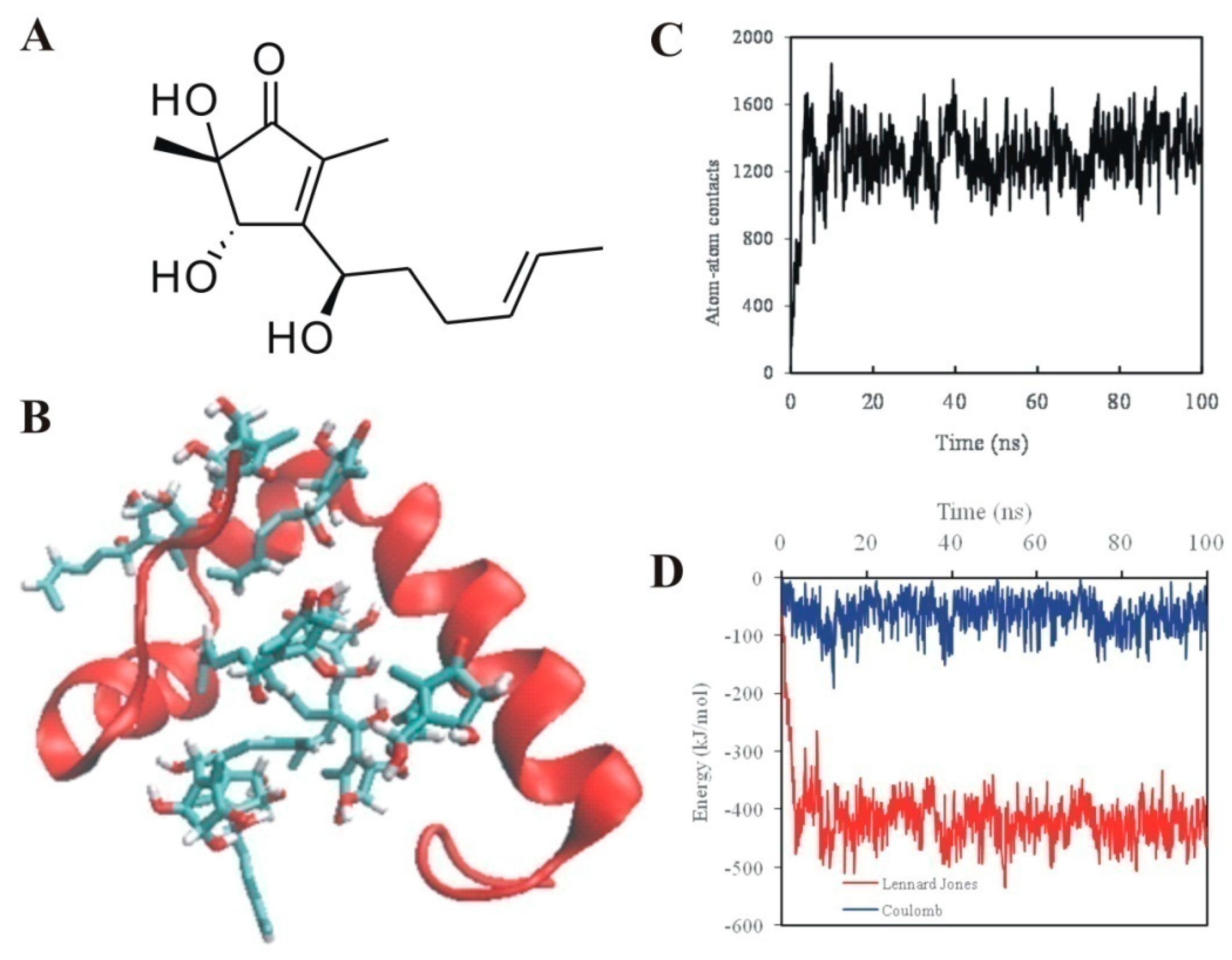
2.2. 5-Hydroxycyclopenicillone Likely Binds to Aβ1-42 Peptides via Hydrophobic Interactions
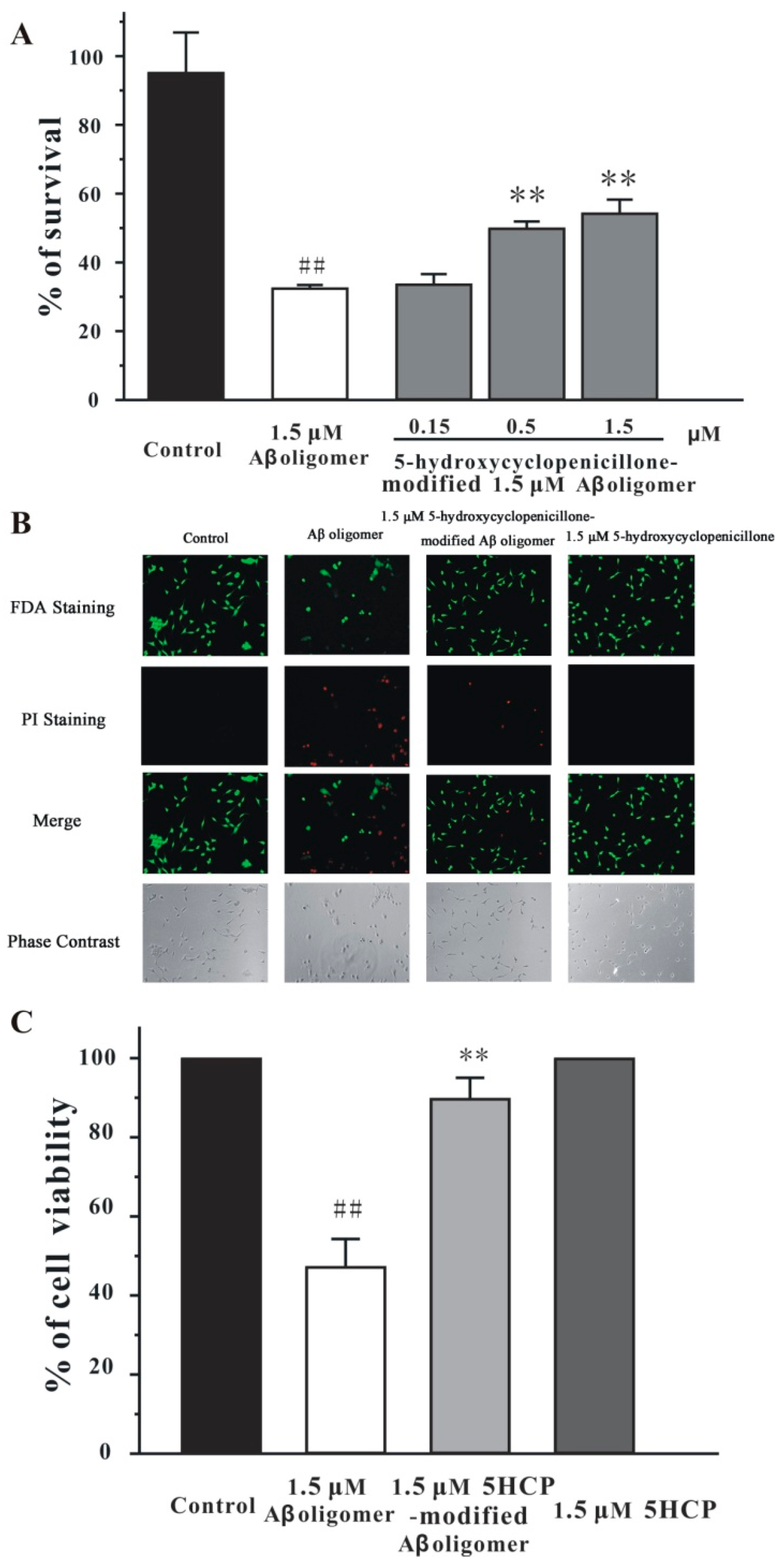
2.3. 5-Hydroxycyclopenicillone Decreased the Neurotoxicity of the Aβ1-42 Oligomer in SH-SY5Y Cells
2.4. 5-Hydroxycyclopenicillone Prevents Synaptic Toxicity of Aβ1-42 Oligomer in Primary Hippocampal Neurons
3. Discussion
4. Materials and Methods
4.1. Preparation of 5-Hydroxycyclopenicillone
4.2. Preparation of the Aβ1-42 Oligomer
4.3. Dot Blotting Analysis
4.4. TEM Analysis
4.5. Simulation System
4.6. Molecular Dynamics Simulation
4.7. Molecular Dynamics Simulation Analysis
4.8. Culture of SH-SY5Y Cells
4.9. Cell Viability Measurement
4.10. FDA/PI Double Staining
4.11. Primary Hippocampal Neuronal Cultures
4.12. Immunocyto Chemisty
4.13. Confocal Imaging and Analysis
4.14. Data Analysis and Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Viola, K.L.; Klein, W.L. Amyloid beta oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015, 129, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, P.; Fatima, M.T.; Abdelhameed, A.S.; Nusrat, S.; Khan, R.H. Structure of amyloid oligomers and their mechanisms of toxicities: Targeting amyloid oligomers using novel therapeutic approaches. Eur. J. Med. Chem. 2016, 114, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Jett, S.D.; Chi, E.Y. Curcumin Attenuates Arnyloid-beta Aggregate Toxicity and Modulates Amyloid-beta Aggregation Pathway. ACS Chem. Neurosci. 2016, 7, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Du, W.J.; Guo, J.J.; Gao, M.T.; Hu, S.Q.; Dong, X.Y.; Han, Y.F.; Liu, F.F.; Jiang, S.Y.; Sun, Y. Brazilin inhibits amyloid beta-protein fibrillogenesis, remodels amyloid fibrils and reduces amyloid cytotoxicity functional. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Bieschke, J.; Herbst, M.; Wiglenda, T.; Friedrich, R.P.; Boeddrich, A.; Schiele, F.; Kleckers, D.; del Amo, J.M.L.; Gruning, B.A.; Wang, Q.W.; et al. Small-molecule conversion of toxic oligomers to nontoxic beta-sheet-rich amyloid fibrils. Nat. Chem. Biol. 2012, 8, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-Lopez, M.; Perez-Sanchez, A.; Galiano, V.; Barrajon-Catalan, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, B.; Malar, D.S.; Sathya, S.; Devi, K.P. Antiaggregation Potential of Padina gymnospora against the Toxic Alzheimer’s Beta-Amyloid Peptide(25–35) and Cholinesterase Inhibitory Property of Its Bioactive Compounds. PLoS ONE 2015, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.Y.; Liu, F.F.; Lin, J.J.; Chen, H.X.; Huang, C.H.; Chen, L.P.; Zhou, Y.Y.; Ye, L.Y.; Zhang, K.; Jin, J.K.; et al. Fucoxanthin Inhibits beta-Amyloid Assembly and Attenuates beta-Amyloid Oligomer-Induced Cognitive Impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Bryukhovetskiy, I.; Lyakhova, I.; Mischenko, P.; Milkina, E.; Zaitsev, S.; Khotimchenko, Y.; Bryukhovetskiy, A.; Polevshchikov, A.; Kudryavtsev, I.; Khotimchenko, M.; et al. Alkaloids of fascaplysin are effective conventional chemotherapeutic drugs, inhibiting the proliferation of C6 glioma cells and causing their death in vitro. Oncol. Lett. 2017, 13, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhao, J.Y.; Ding, L.J.; Huang, C.H.; Naman, C.B.; He, S.; Wu, B.; Zhu, P.; Luo, Q.J.; Gerwick, W.H.; et al. 5-Hydroxycyclopenicillone, a New beta-Amyloid Fibrillization Inhibitor from a Sponge-Derived Fungus Trichoderma sp HPQJ-34. Mar. Drugs 2017, 15, 260. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, C.Q.; Yang, X.J.; Ren, J.S.; Xu, C.; Qu, X.G. In Situ Monitoring Alzheimer’s Disease ss-Amyloid Aggregation and Screening of A ss Inhibitors Using a Perylene Probe. Small 2013, 9, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Culyba, E.K.; Powers, E.T.; Kelly, J.W. Amyloid-beta forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 2011, 7, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Laganowsky, A.; Liu, C.; Sawaya, M.R.; Whitelegge, J.P.; Park, J.; Zhao, M.L.; Pensalfini, A.; Soriaga, A.B.; Landau, M.; Teng, P.K.; et al. Atomic View of a Toxic Amyloid Small Oligomer. Science 2012, 335, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Morgado, I.; Wieligmann, K.; Bereza, M.; Ronicke, R.; Meinhardt, K.; Annamalai, K.; Baumann, M.; Wacker, J.; Hortschansky, P.; Malesevic, M.; et al. Molecular basis of beta-amyloid oligomer recognition with a conformational antibody fragment. Proc. Natl. Acad. Sci. USA 2012, 109, 12503–12508. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Q.; Wang, R.; Cui, W.; Mak, S.H.; Li, G.; Hu, Y.J.; Lee, M.Y.; Pang, Y.P.; Han, Y.F. Dimeric bis (heptyl)-Cognitin Blocks Alzheimer’s beta-Amyloid Neurotoxicity Via the Inhibition of A beta Fibrils Formation and Disaggregation of Preformed Fibrils. CNS Neurosci. Ther. 2015, 21, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.T.; Huang, M.; Xu, S.J.; Wang, Y.; An, P.Y.; Feng, C.X.; Chen, X.W.; Wei, X.F.; Han, Y.F.; Wang, Q.W. Bis(propyl)-cognitin Prevents beta-amyloid-induced Memory Deficits as Well as Synaptic Formation and Plasticity Impairments via the Activation of PI3-K Pathway. Mol. Neurobiol. 2016, 53, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Cui, W.; Yang, Y.; Xu, S.J.; Zhou, W.H.; Fu, H.J.; Hu, S.Q.; Mak, S.H.; Hu, J.W.; Wang, Q.; et al. Protection against beta-amyloid-induced synaptic and memory impairments via altering beta-amyloid assembly by bis(heptyl)-cognitin. Sci. Rep-Uk 2015, 5, 10256. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Liu, G.L.; Bao, X.M.; Wu, J.; Li, S.M.; Zheng, B.X.; Anwyl, R.; Wang, Q.W. Rosiglitazone Prevents Amyloid-beta Oligomer-Induced Impairment of Synapse Formation and Plasticity via Increasing Dendrite and Spine Mitochondrial Number. J. Alzheimers Dis. 2014, 39, 239–251. [Google Scholar] [PubMed]
- Laport, M.S.; Santos, O.C.S.; Muricy, G. Marine Sponges: Potential Sources of New Antimicrobial Drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.C.S.; Soares, A.R.; Machado, F.L.S.; Romanos, M.T.V.; Muricy, G.; Giambiagi-deMarval, M.; Laport, M.S. Investigation of biotechnological potential of sponge-associated bacteria collected in Brazilian coast. Lett. Appl. Microbiol. 2015, 60, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Indraningrat, A.A.G.; Smidt, H.; Sipkema, D. Bioprospecting Sponge-Associated Microbes for Antimicrobial Compounds. Marinedrugs 2016, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, J.A. Diversity and biotechnological potential of microorganisms associated with marine sponges. Appl. Microbiol. Biotechnol. 2014, 98, 7331–7347. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Griffiths, H.H.; Noble, E.; Rushworth, J.V.; Hooper, N.M. Amyloid- Receptors: The Good, the Bad, and the Prion Protein. J. Biol. Chem. 2016, 291, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Hielscher-Michael, S.; Griehl, C.; Buchholz, M.; Demuth, H.U.; Arnold, N.; Wessjohann, L.A. Natural Products from Microalgae with Potential against Alzheimer’s Disease: Sulfolipids Are Potent Glutaminyl Cyclase Inhibitors. Marinedrugs 2016, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, L.; Jope, R.S. Cholinergic stimulation of AP-1 and NF kappa B transcription factors is differentially sensitive to oxidative stress in SH-SY5Y neuroblastoma: relationship to phosphoinositide hydrolysis. J. Neurosci. 1996, 16, 5914–5922. [Google Scholar] [PubMed]
- Nirmaladevi, D.; Venkataramana, M.; Chandranayaka, S.; Ramesha, A.; Jameel, N.M.; Srinivas, C. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell. Mol. Neurobiol. 2014, 34, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Guo, L.P.; Hu, X.L.; Huang, J.; Fan, Y.H.; Ren, T.S.; Zhao, Q.C. Protective effects of Arctium lappa L. roots against hydrogen peroxide-induced cell injury and potential mechanisms in SH-SY5Y cells. Cell. Mol. Neurobiol. 2015, 35, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, S.; Esposito, V.; Vangone, P.; van Nuland, N.A.; Bonvin, A.M.; Guerrini, R.; Tancredi, T.; Temussi, P.A.; Picone, D. The alpha-to-beta conformational transition of Alzheimer’s Abeta-(1–42) peptide in aqueous media is reversible: A step by step conformational analysis suggests the location of beta conformation seeding. ChemBioChem 2006, 7, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhang, Z.; Li, W.; Hu, S.; Mak, S.; Zhang, H.; Han, R.; Yuan, S.; Li, S.; Sa, F.; et al. The anti-cancer agent SU4312 unexpectedly protects against MPP(+) -induced neurotoxicity via selective and direct inhibition of neuronal NOS. Br. J. Pharmacol. 2013, 168, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhang, Z.J.; Hu, S.Q.; Mak, S.H.; Xu, D.P.; Choi, C.L.; Wang, Y.Q.; Tsim, W.K.; Lee, M.Y.; Rong, J.H.; et al. Sunitinib produces neuroprotective effect via inhibiting nitric oxide overproduction. CNS Neurosci. Ther. 2014, 20, 244–252. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Liu, F.; Huang, C.; Shentu, J.; Wang, M.; Sun, C.; Chen, L.; Yan, S.; Fang, F.; Wang, Y.; et al. 5-Hydroxycyclopenicillone Inhibits β-Amyloid Oligomerization and Produces Anti-β-Amyloid Neuroprotective Effects In Vitro. Molecules 2017, 22, 1651. https://doi.org/10.3390/molecules22101651
Zhao J, Liu F, Huang C, Shentu J, Wang M, Sun C, Chen L, Yan S, Fang F, Wang Y, et al. 5-Hydroxycyclopenicillone Inhibits β-Amyloid Oligomerization and Produces Anti-β-Amyloid Neuroprotective Effects In Vitro. Molecules. 2017; 22(10):1651. https://doi.org/10.3390/molecules22101651
Chicago/Turabian StyleZhao, Jiaying, Fufeng Liu, Chunhui Huang, Jieyi Shentu, Minjun Wang, Chenkai Sun, Liping Chen, Sicheng Yan, Fang Fang, Yuanyuan Wang, and et al. 2017. "5-Hydroxycyclopenicillone Inhibits β-Amyloid Oligomerization and Produces Anti-β-Amyloid Neuroprotective Effects In Vitro" Molecules 22, no. 10: 1651. https://doi.org/10.3390/molecules22101651





