Effect of High-Pressure Treatment on Catalytic and Physicochemical Properties of Pepsin
Abstract
:1. Introduction
2. Results and Discussion
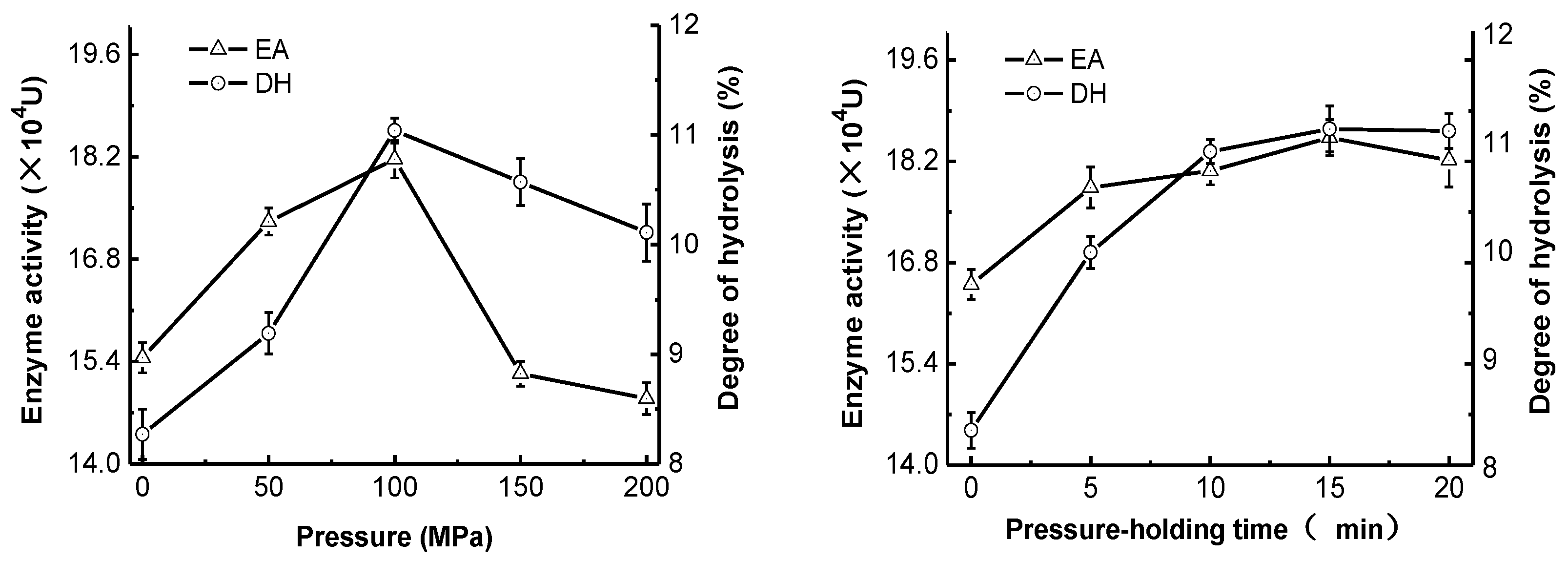
2.1. Effect of High-Pressure Treatment on Enzyme Activity and Degree of Hydrolysis
2.2. Effect of High-Pressure Treatment on Molecular Weight Distribution of Casein Hydrolysate
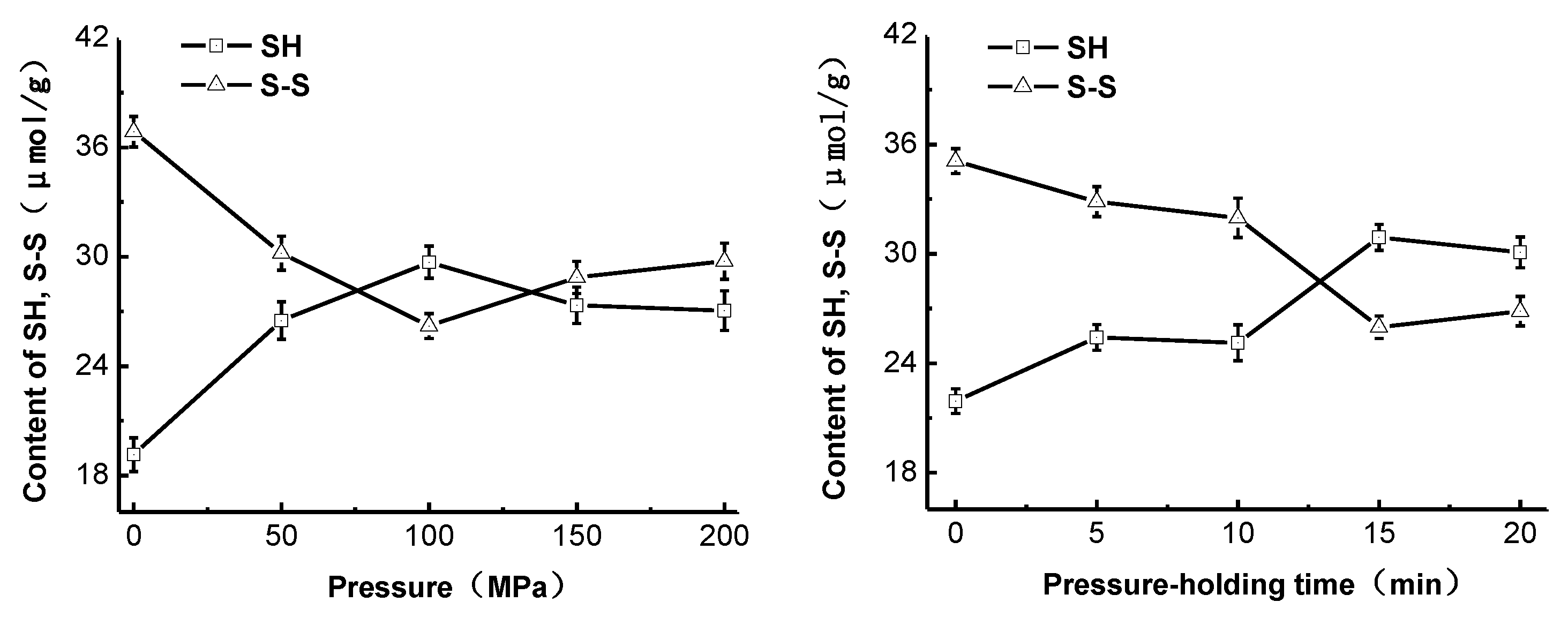
2.3. Effect of High-Pressure Treatment on the Sulfhydryl Group and Disulfide Bond Content of Pepsin
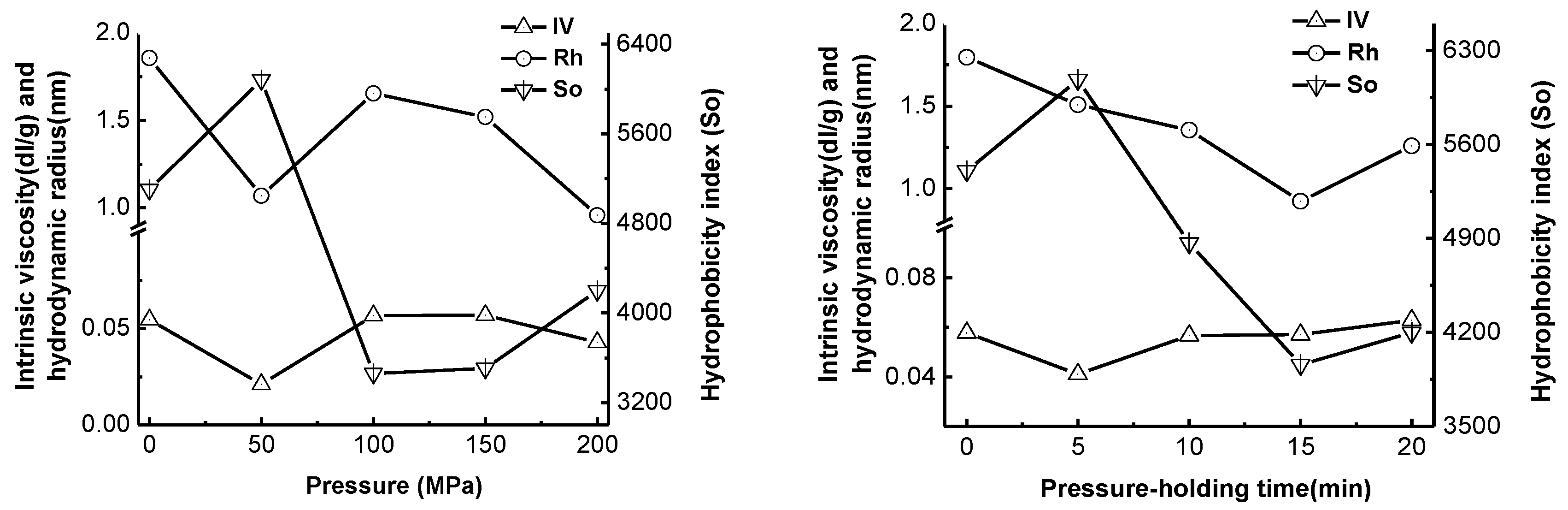
2.4. Effect of High-Pressure Treatment on Surface Hydrophobicity, Intrinsic Viscosity and Hydrodynamic Radius of Pepsin
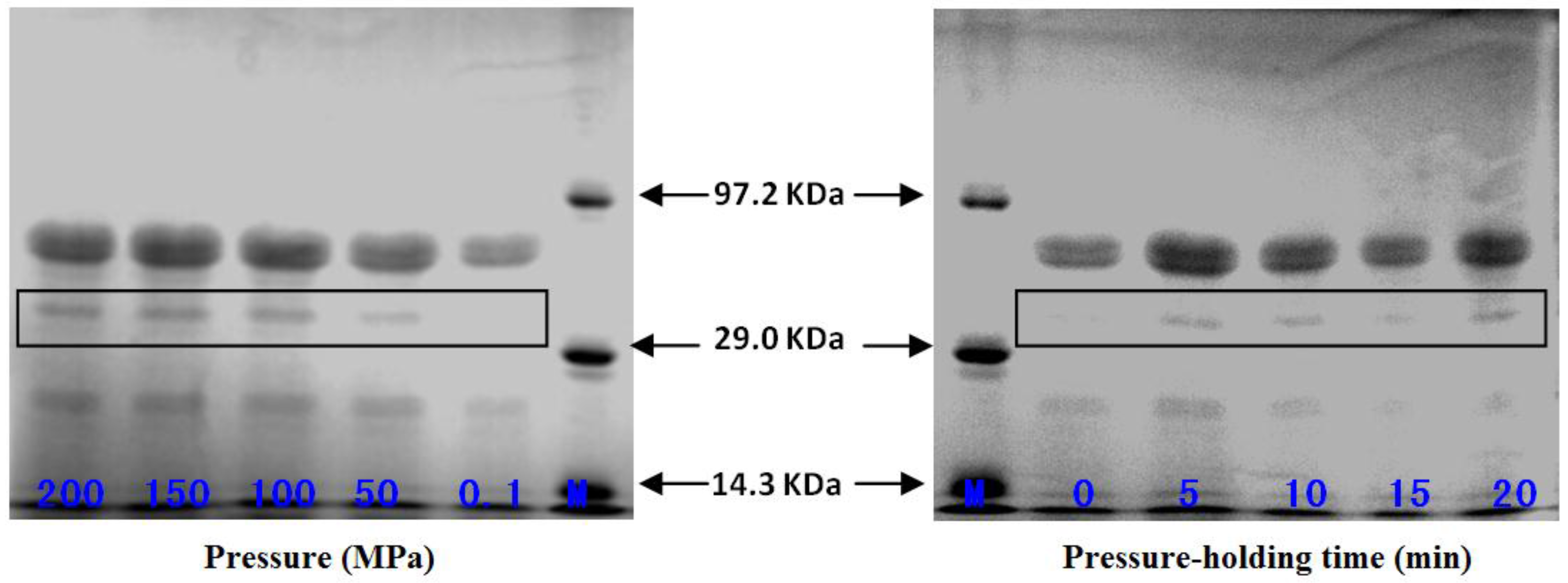
2.5. Effect of High-Pressure Treatment on Subunit Composition of Pepsin
3. Experimental Section
3.1. Chemicals
3.2. High-Pressure Treatment of Pepsin
3.3. Measurement of Enzymatic Activity of Pepsin
3.4. Determination of Degree of Hydrolysis
3.5. Determination of Molecular Weight Distribution of Casein Hydrolysate
3.6. Determination of the Sulfhydryl Group (SH) and Disulfide Bond (S-S) Contents of Pepsin
3.7. Determination of Surface Hydrophobicity Index of Pepsin
3.8. Determination of Intrinsic Viscosity and Hydrodynamic Radius of Pepsin
3.9. Determination of Subunit Composition of Pepsin
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bah, C.S.F.; Bekhit, A.E.D.A.; McConnell, M.A.; Carne, A. Generation of bioactive peptide hydrolysates from cattle plasma using plant and fungal proteases. Food Chem. 2016, 213, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Ripolles, D.; Parron, J.A.; Calvo, M.; Perez, M.D.; FitzGerald, R.J.; Sanchez, L. Antioxidant activity of co-products from milk fat processing and their enzymatic hydrolysates obtained with different proteolytic preparations. Int. Dairy J. 2016, 60, 70–77. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.; Xu, S.Y. Preparation of casein phosphorylated peptides and casein non-phosphorylated peptides using alcalase. Eur. Food Res. Technol. 2007, 225, 579–584. [Google Scholar] [CrossRef]
- Jakopovic, K.L.; Barukcic, I.; Bozanic, R. Physiological significance, structure and isolation of α-lactalbumin. Mljekarstvo 2016, 66, 3–11. [Google Scholar]
- Heinrich, M.; Kulozik, U. Study of chymosin hydrolysis of casein micelles under ultra high pressure: Effect on re-association upon pressure release. Int. Dairy J. 2011, 21, 664–669. [Google Scholar] [CrossRef]
- Hu, G.L.; Zheng, Y.R.; Liu, Z.M.; Deng, Y.; Zhao, Y.Y. Structure and IgE-binding properties of α-casein treated by high hydrostatic pressure, UV-C, and far-IR radiations. Food Chem. 2016, 204, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Bravo, F.I.; Felipe, X.; Lopez-Fandino, R.; Molina, E. Skim milk protein distribution as a result of very high hydrostatic pressure. Food Res. Int. 2015, 72, 74–79. [Google Scholar] [CrossRef]
- Belloque, J.; Chicon, R.; Lopez-Fandino, R. Unfolding and refolding of β-lactoglobulin subjected to high hydrostatic pressure at different pH values and temperatures and its influence on proteolysis. J. Agric. Food Chem. 2007, 55, 5282–5288. [Google Scholar] [CrossRef] [PubMed]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High pressure enhancement of enzymes: A review. Enzyme Microb. Technol. 2009, 45, 331–347. [Google Scholar] [CrossRef]
- Synowiecki, J.; Shahidi, F. Heat-induced changes in sulfhydryl groups of harp seal muscle proteins. J. Agric. Food Chem. 1991, 39, 2006–2009. [Google Scholar] [CrossRef]
- Lothrop, A.P.; Snider, G.W.; Ruggles, E.L.; Patel, A.S.; Lees, W.J.; Hondal, R.J. Selenium as an electron acceptor during the catalytic mechanism of thioredoxin reductase. Biochemistry 2014, 53, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Kok, C.M.; Rudin, A. Relationship between the hydrodynamic radius and the radius of gyration of a polymer in solution. Macromol. Rapid Commun. 1981, 2, 655–659. [Google Scholar] [CrossRef]
- Samloff, I.M. Pepsinogens I and II: Purification from gastric mucosa and radioimmunoassay in serum. Gastroenterology 1982, 82, 26–33. [Google Scholar] [PubMed]
- Castillo-Yanez, F.J.; Pacheco-Aguilar, R.; Garcia-Carreno, F.L.; Navarrete-Del Toro, M.D.L.A. Characterization of acidic proteolytic enzymes from Monterey sardine (Sardinops sagax caerulea) viscera. Food Chem. 2004, 85, 343–350. [Google Scholar] [CrossRef]
- Manassero, C.A.; Vaudagna, S.R.; Sancho, A.M.; Anon, M.C.; Speroni, F. Combined high hydrostatic pressure and thermal treatments fully inactivate trypsin inhibitors and lipoxygenase and improve protein solubility and physical stability of calcium-added soymilk. Innov. Food. Sci. Emerg. 2016, 35, 86–95. [Google Scholar] [CrossRef]
- Thaiphanit, S.; Schleining, G.; Anprung, P. Effects of coconut (Cocos nucifera L.) protein hydrolysates obtained from enzymatic hydrolysis on the stability and rheological properties of oil-in-water emulsions. Food Hydrocoll. 2016, 60, 252–264. [Google Scholar] [CrossRef]
- Beveridge, T.; Toma, S.J.; Nakai, S. Determination of SH- and SS-group in some food proteins using Ellman’s reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Li, B.S.; Yang, X.Q.; Li, L.; Ma, C.Y. Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Singh, N.K.; Shepherd, K.W.; Cornish, G.B. A simplified SDS-PAGE procedure for separating LMW subunits of glutenin. J. Cereal Sci. 1991, 14, 203–208. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds pepsin and bovine serum albumin are available from the authors. |
| Pressure (MPa) | Molecular Weight Distribution (%) | ||||
|---|---|---|---|---|---|
| >3000 | 3000–2000 | 2000–1000 | 1000–500 | <500 | |
| 0 | 7.82 | 9.5 | 20.61 | 17.65 | 44.42 |
| 50 | 6.25 | 8.79 | 21.35 | 17.55 | 46.06 |
| 100 | 6.22 | 8.66 | 21.48 | 17.52 | 46.39 |
| 150 | 6.24 | 8.77 | 21.11 | 17.58 | 46.31 |
| 200 | 6.43 | 8.85 | 20.94 | 17.38 | 46.11 |
| Pressure Holding Time (min) | Molecular Weight Distribution (%) | ||||
|---|---|---|---|---|---|
| >3000 | 3000–2000 | 2000–1000 | 1000–500 | <500 | |
| 0 | 8.3 | 9.39 | 20.8 | 17.59 | 43.91 |
| 5 | 6.98 | 8.75 | 21 | 17.57 | 45.64 |
| 10 | 6.96 | 8.71 | 21.26 | 17.41 | 45.73 |
| 15 | 6.48 | 8.7 | 21.41 | 17.4 | 45.93 |
| 20 | 6.91 | 8.79 | 21.18 | 17.4 | 45.85 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Bai, T.; Ma, Y.; Ma, H. Effect of High-Pressure Treatment on Catalytic and Physicochemical Properties of Pepsin. Molecules 2017, 22, 1659. https://doi.org/10.3390/molecules22101659
Wang J, Bai T, Ma Y, Ma H. Effect of High-Pressure Treatment on Catalytic and Physicochemical Properties of Pepsin. Molecules. 2017; 22(10):1659. https://doi.org/10.3390/molecules22101659
Chicago/Turabian StyleWang, Jianan, Tenghui Bai, Yaping Ma, and Hanjun Ma. 2017. "Effect of High-Pressure Treatment on Catalytic and Physicochemical Properties of Pepsin" Molecules 22, no. 10: 1659. https://doi.org/10.3390/molecules22101659




