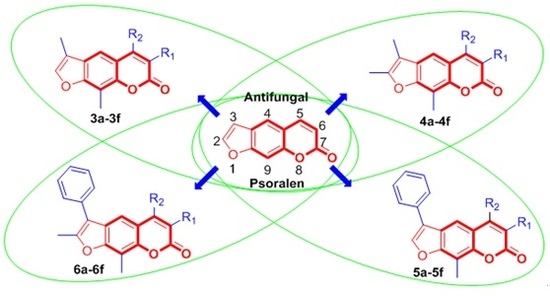
Design, Synthesis and Antifungal Activity of Psoralen Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Methods
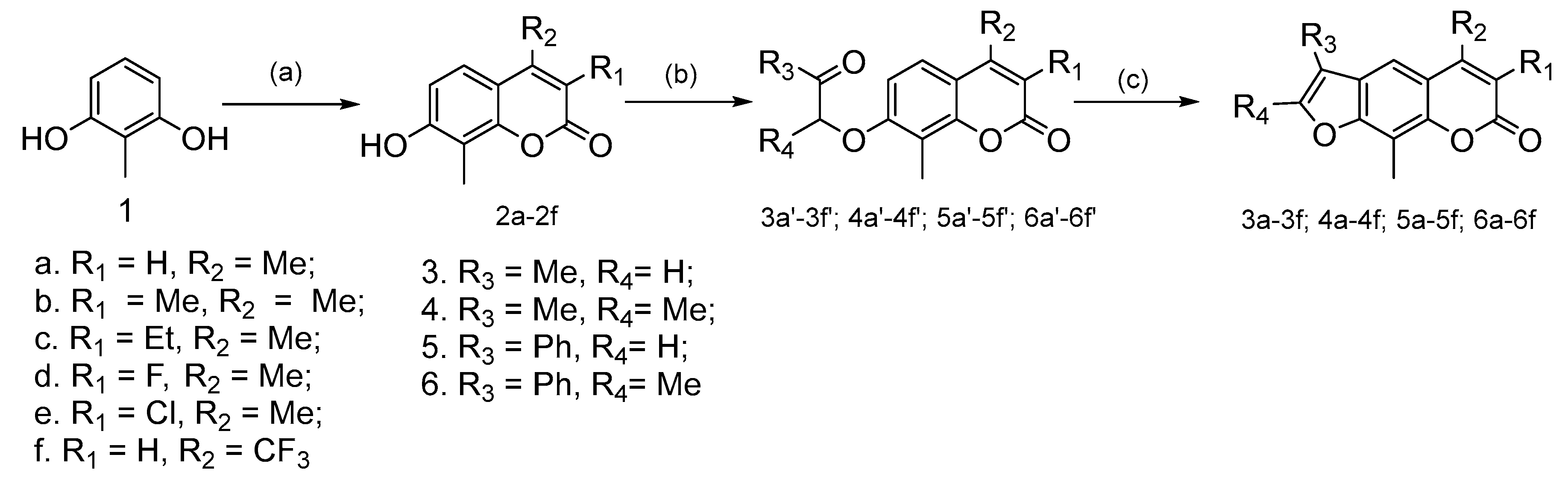
2.1.1. General Procedure for the Preparation of Compounds 2a–2f
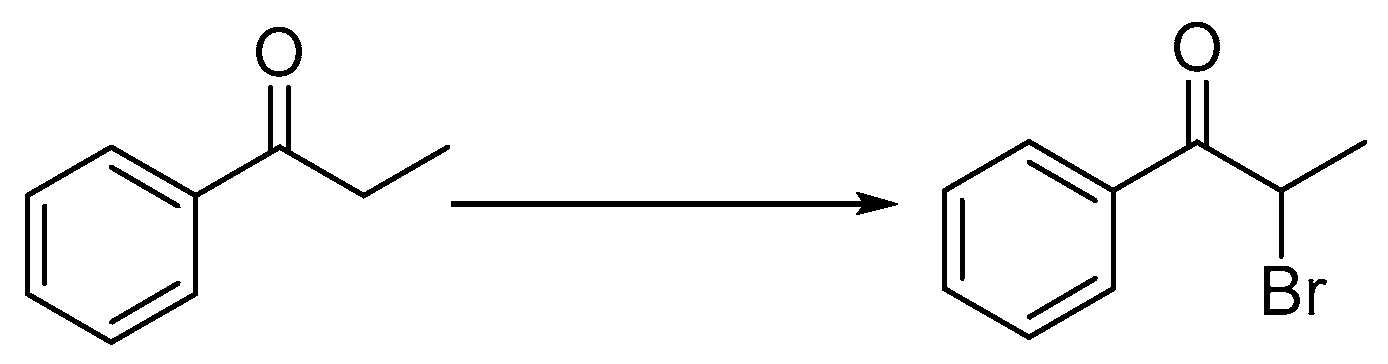
2.1.2. Preparation of 2-Bromo-1-phenylpropan-1-one
2.1.3. General Procedure for the Preparation of Compounds 3a′–3f′, 4a′–4f′, 5a′–5f′ and 6a′–6f′
2.1.4. General Procedure for the Preparation of Compounds 3a–3f, 4a–4f, 5a–5f, and 6a–6f
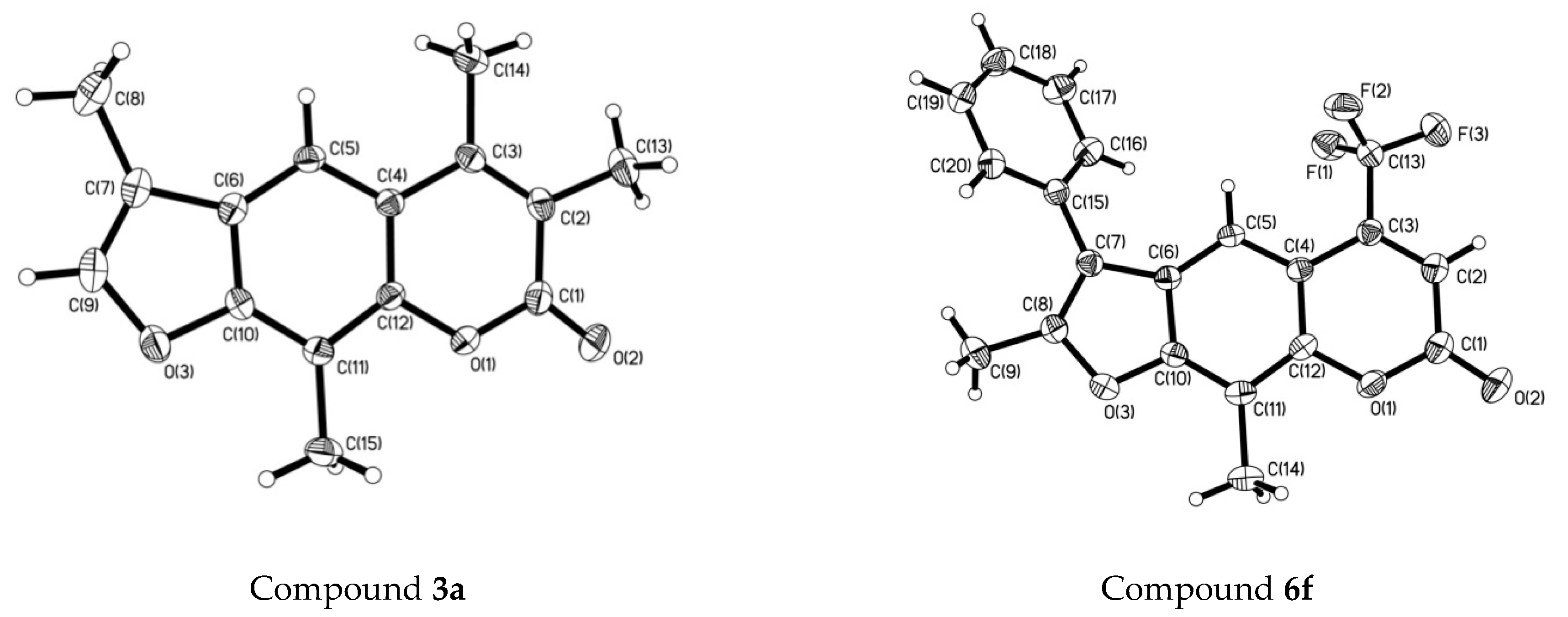
2.2. The Crystal Structure of Compounds 3a and 6f
2.3. Biological Assays
3. Results and Discussion
3.1. Synthetic Chemistry
3.2. Antifungal Activity and the Structure-Activity Relationships
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Z.; Chen, S.; Zhu, S.; Luo, J.; Zhang, Y.; Weng, Q. Synthesis and Fungicidal Activity of β-Carboline Alkaloids and Their Derivatives. Molecules 2015, 20, 13941–13957. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Talbot, N.J. Fungal physiology—A future perspective. Microbiology 2009, 155, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Vrchotová, N. Insecticidal effect of furanocoumarins from fruits of Angelica archangelica L. against larvae Spodoptera littoralis. Boisd. Ind. Crops Prod. 2013, 43, 33–39. [Google Scholar] [CrossRef]
- Marumoto, S.; Miyazawa, M. Structure-activity relationships for naturally occurring coumarins as β-secretase inhibitor. Bioorg. Med. Chem. 2012, 20, 784–788. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, H.D.; Pereira, A.C.; Brancini, G.T.P.; de Leão, H.C.; Júnior, N.S.M.; Bachmann, L. Furocoumarins and coumarins photoinactivate Colletotrichum acutatum and Aspergillus nidulans fungi under solar radiation. J. Photochem. Photobiol. B 2014, 131, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Chauthe, S.K.; Mahajan, S.; Rachamalla, M.; Tikoo, K.; Singh, I.P. Synthesis and evaluation of linear furanocoumarins as potential anti-breast and anti-prostate cancer agents. Med. Chem. Res. 2015, 24, 2476–2484. [Google Scholar] [CrossRef]
- Hafez, O.M.A.; Amin, K.M.; Abdel-Latif, N.A.; Mohamed, T.K.; Ahmed, E.Y.; Maher, T. Synthesis and antitumor activity of some new xanthotoxin derivatives. Eur. J. Med. Chem. 2009, 44, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, N.M.M.; Abd-Alla, H.I.; Aly, H.F.; Albalawy, M.A.; Shaker, K.H.; Bouajila, J. Preliminary In Vitro and In Vivo Evaluation of Antidiabetic Activity of Ducrosia anethifolia Boiss. and Its Linear Furanocoumarins. Biomed. Res. Int. 2014, 2014, 480545. [Google Scholar] [CrossRef] [PubMed]
- Karamat, F.I.; Olry, A.; Doerper, S.; Vialart, G.; Ullmann, P.; Werck-Reichhart, D. CYP98A22, a phenolic ester 30-hydroxylase specialized in the synthesis of chlorogenic acid, as a new tool for enhancing the furanocoumarin concentration in Rutagraveolens. BMC Plant Biol. 2012, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Sardari, S.; Mori, Y.; Horita, K.; Micetich, R.G.; Nishibe, S.; Daneshtalab, M. Synthesis and Antifungal Activity of Coumarins and Angular Furanocoumarins. Bioorg. Med. Chem. 1999, 7, 1933–1940. [Google Scholar] [CrossRef]
- Verotta, L.; Lovagli, E.; Vidari, G.; Finzi, P.V.; Neri, M.G.; Raimondi, A.; Parapini, S.; Taramelli, D.; Riva, A.; Bombardelli, E. Raimondi, 4-Alkyl- and 4-phenylcoumarins from Mesua ferrea as promising multidrug resistant antibacterials. Phytochemistry 2004, 65, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Zhang, R.R.; Wang, J.Q. Microwave-assisted synthesis and antifungal activity of novel fused Osthole derivatives. Eur. J. Med. Chem. 2016, 97, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Xu, Z.C. Microwave-Assisted Synthesis and Antifungal Activities of Polysubstituted Furo[3,2-c]chromen-4-ones and 7,8,9,10-Tetrahydro-6H-benzofuro[3,2-c]chromen-6-ones. Synth. Commun. 2015, 46, 3257–3263. [Google Scholar] [CrossRef]
- Shen, Q.; Peng, Q.; Shao, J.; Liu, X.; Huang, Z.; Pu, X. Synthesis and biological evaluation of functionalized coumarins as acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2005, 40, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Finn, G.J.; Creaven, B.; Egan, D.A. Study of the in vitro cytotoxic potential of natural and synthetic coumarin derivatives using human normal and neoplastic skin cell lines. Melanoma Res. 2001, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- El-Gogary, S.; Hashem, N.; Khodeir, M.N. Synthesis and photooxygenation of angular furocoumarins: Isopsedopsoralen and allopsoralen. Res. Chem. Intermed. 2015, 41, 1591–1600. [Google Scholar] [CrossRef]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Zhang, R.R.; Yin, W.Z.; Yu, X.; Zhang, Y.L.; Liu, P.; Gu, Y.C.; Zhang, W.H. Microwave-assisted Synthesis and antifungal activity of coumarin[8,7-e][1,3]oxazine derivatives. Mol. Divers. 2016, 20, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Zhang, Y.; Wang, J.Q.; Zhang, W.H. Design, Synthesis and Antifungal Activity of Coumarin Ring-Opening Derivatives. Molecules 2016, 21, 1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Liu, J.; Zhang, Y.; Hou, M.Q.; Zhang, M.Z.; Zhou, F.; Zhang, W.H. Microwave-assisted synthesis and antifungal activity of novel coumarin derivatives: Pyrano[3,2-c]chromene-2,5-diones. Eur. J. Med. Chem. 2016, 116, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Podgoršek, A.; Stavber, S.; Zupan, M.; Iskra, J. Bromination of ketones with H2O2-HBr “on water”. Green Chem. 2007, 9, 1212–1218. [Google Scholar] [CrossRef]
- Jiang, Q.; Sheng, W.; Guo, C. Synthesis of phenacyl bromides via K2S2O8-mediated tandem hydroxybromination and oxidation of styrenes in water. Green Chem. 2013, 8, 2175–2179. [Google Scholar] [CrossRef]
- Zhang, W.H.; Jiang, M.G. Synthesis and antifungal activity of umbelliferone analogues. Chin. J. Org. Chem. 2010, 30, 254–259. [Google Scholar]
Sample Availability: Samples of the compounds 3a-3f are available from the authors. |


| Compound | R1, R2, R3, R4 | Inhibitory Rate (%) a | |||||
|---|---|---|---|---|---|---|---|
| Rhizoctonia solani | Botrytis cinerea | Alternaria solani | Gibberella zeae | Cucumber anthrax | Alternaria leaf spot | ||
| 3a | H, Me, Me, H | 42.4 | 67.9 | 10.0 b | 30.7 | 34.9 | 16.2 |
| 3b | Me, Me, Me, H | 26.3 | 52.4 | 10.0 | 10.0 | 10.0 | 18.2 |
| 3c | Et, Me, Me, H | 37.5 | 10.0 | 10.0 | 10.0 | 15.5 | 12.5 |
| 3d | F, Me, Me, H | 23.9 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| 3e | Cl, Me, Me, H | 30.2 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| 3f | H, CF3, Me, H | 56.1 | 61.5 | 10.0 | 10.0 | 10.0 | 10.0 |
| 4a | H, Me, Me, Me | 37.9 | 40.0 | 10.0 | 10.0 | 11.2 | 13.0 |
| 4b | Me, Me, Me, Me | 62.4 | 10.0 | 20.8 | 11.5 | 16.8 | 10.0 |
| 4c | Et, Me, Me, Me | 30.2 | 10.0 | 10.0 | 11.2 | 10.0 | 10.0 |
| 4d | F, Me, Me, Me | 14.5 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| 4e | Cl, Me, Me, Me | 41.5 | 58.2 | 10.0 | 17.7 | 12.7 | 16.2 |
| 4f | H, CF3, Me, Me | 40.8 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| 5a | H, Me, Ph, H | 47.8 | 10.0 | 10.0 | 10.0 | 10.0 | 15.3 |
| 5b | Me, Me, Ph, H | 37.6 | 38.5 | 10.0 | 10.0 | 15.0 | 20.8 |
| 5c | Et, Me, Ph, H | 50.3 | 58.2 | 10.0 | 10.0 | 10.0 | 10.0 |
| 5d | F, Me, Ph, H | 23.9 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| 5e | Cl, Me, Ph, H | 19.4 | 50.0 | 10.0 | 10.0 | 10.0 | 13.8 |
| 5f | H, CF3, Ph, H | 24.3 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| 6a | H, Me, Ph, Me | 45.9 | 10.0 | 10.0 | 11.9 | 27.4 | 23.1 |
| 6b | Me, Me, Ph, Me | 10.0 | 10.0 | 10.0 | 10.0 | 22.2 | 10.0 |
| 6c | Et, Me, Ph, Me | 40.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| 6d | F, Me, Ph, Me | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| 6e | Cl, Me, Ph, Me | 49.1 | 44.5 | 26.0 | 22.5 | 10.0 | 36.9 |
| 6f | H, CF3, Ph, Me | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Osthole | - | 69.5 | 66.1 | 29.8 | 66.7 | 92.4 | 50.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Wen, Y.; Liang, C.-G.; Liu, J.; Ding, Y.-B.; Zhang, W.-H. Design, Synthesis and Antifungal Activity of Psoralen Derivatives. Molecules 2017, 22, 1672. https://doi.org/10.3390/molecules22101672
Yu X, Wen Y, Liang C-G, Liu J, Ding Y-B, Zhang W-H. Design, Synthesis and Antifungal Activity of Psoralen Derivatives. Molecules. 2017; 22(10):1672. https://doi.org/10.3390/molecules22101672
Chicago/Turabian StyleYu, Xiang, Ya Wen, Chao-Gen Liang, Jia Liu, Yu-Bin Ding, and Wei-Hua Zhang. 2017. "Design, Synthesis and Antifungal Activity of Psoralen Derivatives" Molecules 22, no. 10: 1672. https://doi.org/10.3390/molecules22101672