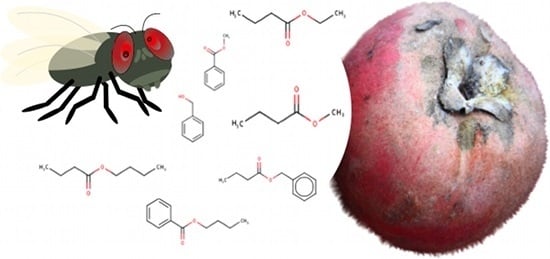
Molecular Affinity of Mabolo Extracts to an Octopamine Receptor of a Fruit Fly
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Extracts
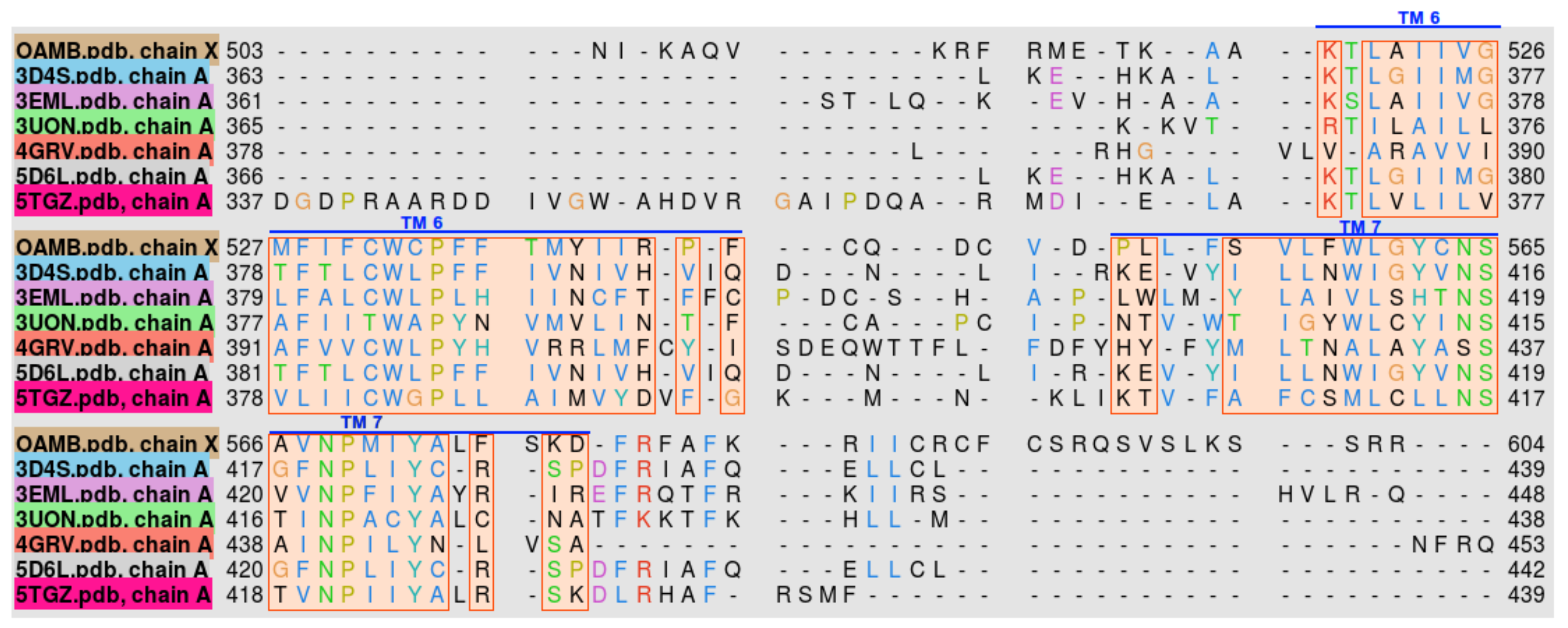
2.2. Protein Model Verification
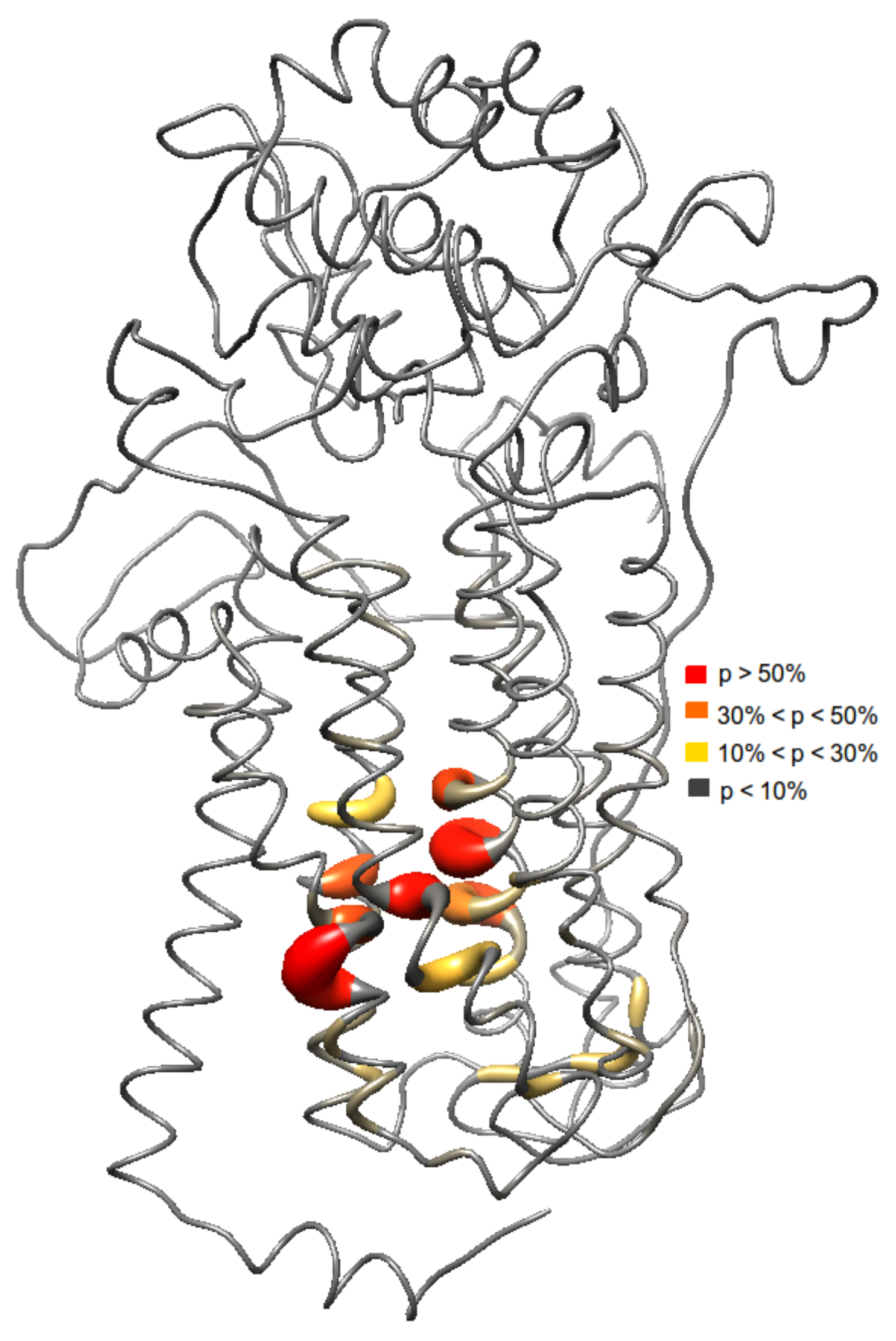
2.3. Ensemble Docking
3. Materials and Methods
3.1. Extraction of Essential Oil Components
3.2. Receptor Modeling and Ligand Preparation
3.3. Molecular Dynamics: Protein Conformational Ensemble
3.4. Ensemble Docking
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| cAMP | cyclic Adenosine monophosphate |
| GABA | -aminobutyric acid |
| GC-MS | Gas chromatography - Mass spectroscopy |
| GPCR | G-Protein Coupled Receptors |
| MD | Molecular dynamics |
| OA | Octopamine |
| OAMB | Octopamine Receptor in Mushroom Bodies |
| OAR | Octopamine Receptors |
| PDB | Protein Databank |
References
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.; Tripathi, A.; Aggarwal, K.; Khanuja, S. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Papachristos, D.; Stamopoulos, D. Repellent, toxic and reproduction inhibitory effects of essential oil vapours on Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2002, 38, 117–128. [Google Scholar] [CrossRef]
- Ngoh, S.P.; Choo, L.E.; Pang, F.Y.; Huang, Y.; Kini, M.R.; Ho, S.H. Insecticidal and repellent properties of nine volatile constituents of essential oils against the American cockroach, Periplaneta americana (L.). Pest. Manag. Sci. 1998, 54, 261–268. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E.E. Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera). J. Stored Prod. Res. 2009, 45, 212–214. [Google Scholar] [CrossRef]
- Collins, R.; Halim, A. Characterization of the Volatile Compounds of Diospyros blancoi. Econ. Bot. 1976, 30, 313–316. [Google Scholar] [CrossRef]
- The Plant List (2013) Version 1.1. Available online: http://www.theplantlist.org/ (accessed on 23 December 2016).
- Ra, M.M.; Howl, M.S.I.; Khal, A.B.R.; Rahman, M.M.; Ahmed, F. Antioxidant and Antidiarrhoeal Potentiality of Diospyros blancoi. Int. J. Pharmacol. 2012, 8, 403–409. [Google Scholar]
- Amin, C.M.I.; Mitali, D.; Foyez, A.D.; Nazmul, A.M.; Mohammad, S.A.; Sudipta, C.; Rajesh, B.; Uddin, M.M.M.; Mustafa, K.A.H. Potential Phytochemical, Analgesic and Anticancerous Activities of Cymbopogon citratus Leaf. Am. J. Biomed. Res. 2015, 3, 66–70. [Google Scholar]
- DeFilipps, R.A.; Maina, S.L.; Crepin, J. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana); Department of Botany, National Museum of Natural History, Smithsonian Institution: Washington, DC, USA, 2004. [Google Scholar]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotechnol. 2008, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.L.; Kogan, M. Plant volatiles as insect attractants. Crit. Rev. Plant Sci. 1987, 5, 251–301. [Google Scholar] [CrossRef]
- Szendrei, Z.; Rodriguez-Saona, C. A meta-analysis of insect pest behavioral manipulation with plant volatiles. Entomol. Exp. Appl. 2010, 134, 201–210. [Google Scholar] [CrossRef]
- Evans, P.D.; Maqueira, B. Insect octopamine receptors: A new classification scheme based on studies of cloned Drosophila G-Protein Coupled Receptors. Invertebr. Neurosci. 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.M.; Rasmussen, S.G.F.; Kobilka, B.K. The structure and function of G-Protein-Coupled Receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.D. Biogenic Amines in the Insect Nervous System. In Advances in Insect Physiology Volume 15; Elsevier BV: Amsterdam, The Netherlands, 1980; pp. 317–473. [Google Scholar]
- Evans, P.D.; Robb, S. Octopamine receptor subtypes and their modes of action. Neurochem. Res. 1993, 18, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Seong, C.S.; Kim, Y.C.; Davis, R.L.; Han, K.A. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev. Biol. 2003, 264, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Han, K.A.; Millar, N.S.; Davis, R.L. A Novel Octopamine Receptor with Preferential Expression in Drosophila Mushroom Bodies. J. Neurosci. 1998, 18, 3650–3658. [Google Scholar] [PubMed]
- Balfanz, S.; Strünker, T.; Frings, S.; Baumann, A. A family of octapamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 2005, 93, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Buckingham, S.; Matsuda, K.; Sattelle, D. Modes of action, resistance and toxicity of insecticides targeting nicotinic acetylcholine receptors. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.; Ihara, M.; Sattelle, D.; Matsuda, K. Mechanisms of action, resistance and toxicity of insecticides targeting GABA receptors. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.F. Fruits of Warm Climates; Florida Flair Books: Boynton Beach, FL, USA, 1987. [Google Scholar]
- Smith, R.M.; Oliveros-Belardo, L. Volatile Compounds from the Fruit Peelings of Diospyros discolor Willd. J. Essent. Oil Res. 1992, 4, 287–289. [Google Scholar] [CrossRef]
- Pino, J.A.; Cuevas-Glory, L.; Fuentes, V. Volatile components of mabolo (Diospyros blancoi A. DC.) grown in Cuba. J. Essent. Oil Res. 2008, 20, 506–508. [Google Scholar] [CrossRef]
- Wong, K.; Zul Rusdi, N.; Chee, S. Volatile constituents of Diospyros blancoi DC. fruit. J. Essent. Oil Res. 1997, 9, 699–702. [Google Scholar] [CrossRef]
- Hung, S.F.; Roan, S.F.; Chang, T.L.; King, H.B.; Chen, I.Z. Analysis of aroma compounds and nutrient contents of Mabolo (Diospyros blancoi A. DC.), an ethnobotanical fruit of Austronesian Taiwan. J. Food Drug Anal. 2016, 24, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Poling, S.M.; Hsu, W.J.; Koehrn, F.J.; Yokoyama, H. Chemical induction of β-carotene biosynthesis. Phytochemistry 1977, 16, 551–555. [Google Scholar] [CrossRef]
- Jordán, M.J.; Goodner, K.L.; Shaw, P.E. Characterization of the aromatic profile in aqueous essence and fruit juice of yellow passion fruit (Passiflora edulis Sims F. Flavicarpa degner) by GC-MS and GC/O. J. Agric. Food Chem. 2002, 50, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Tapia, T.; Perich, F.; Pardo, F.; Palma, G.; Quiroz, A. Identification of volatiles from differently aged red clover (Trifolium pratense) root extracts and behavioural responses of clover root borer (Hylastinus obscurus) (Marsham) (Coleoptera: Scolytidae) to them. Biochem. Syst. Ecol. 2007, 35, 61–67. [Google Scholar] [CrossRef]
- Kolosova, N.; Gorenstein, N.; Kish, C.M.; Dudareva, N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 2001, 13, 2333–2347. [Google Scholar] [CrossRef] [PubMed]
- Rohrbeck, D.; Buss, D.; Effmert, U.; Piechulla, B. Localization of methyl benzoate synthesis and emission in Stephanotis floribunda and Nicotiana suaveolens flowers. Plant Biol. 2006, 8, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Atkins, E.; Macdonald, R.; Greywood-Hale, E. Repellent additives to reduce pesticide hazards to honey bees: Field tests. Environ. Entomol. 1975, 4, 207–210. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Schneidmiller, R.G.; Hoover, D.R. Essential oils and their compositions as spatial repellents for pestiferous social wasps. Pest Manag. Sci. 2013, 69, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.E. A receptor sensitive to oviposition site attractants on the antennae of the mosquito, Aedes aegypti. J. Insect Physiol. 1976, 22, 1371–1376. [Google Scholar] [CrossRef]
- Landolt, P.J. Chemical attractants for trapping yellowjackets Vespula germanica and Vespula pensylvanica (Hymenoptera: Vespidae). Environ. Entomol. 1998, 27, 1229–1234. [Google Scholar] [CrossRef]
- Haga, K.; Kruse, A.C.; Asada, H.; Yurugi-Kobayashi, T.; Shiroishi, M.; Zhang, C.; Weis, W.I.; Okada, T.; Kobilka, B.K.; Haga, T.; Kobayashi, T. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 2012, 482, 547–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, M.A.; Cherezov, V.; Griffith, M.T.; Roth, C.B.; Jaakola, V.P.; Chien, E.Y.; Velasquez, J.; Kuhn, P.; Stevens, R.C. A Specific Cholesterol Binding Site Is Established by the 2.8 Å Structure of the Human β2-Adrenergic Receptor. Structure 2008, 16, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762.e14. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Noinaj, N.; Shibata, Y.; Love, J.; Kloss, B.; Xu, F.; Gvozdenovic-Jeremic, J.; Shah, P.; Shiloach, J.; Tate, C.G.; Grisshammer, R. Structure of the agonist-bound neurotensin receptor. Nature 2012, 490, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, V.P.; Griffith, M.T.; Hanson, M.A.; Cherezov, V.; Chien, E.Y.T.; Lane, J.R.; IJzerman, A.P.; Stevens, R.C. The 2.6 Angstrom Crystal Structure of a Human A2A Adenosine Receptor Bound to an Antagonist. Science 2008, 322, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Pikyee, M.; Dietmar, W.; Luba, AA.; Timothy, J.J.; John, R.R.; Liu, X.; Brian, K.K.; Martin, C. The cubicon method for concentrating membrane proteins in the cubic mesophase. Nat. Protoc. 2017, 9, 1745. [Google Scholar]
- Kim, Y.C.; Lee, H.G.; Lim, J.; Han, K.A. Appetitive learning requires the alpha1-like octopamine receptor OAMB in the Drosophila mushroom body neurons. J. Neurosci. 2013, 33, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- UniProt: A hub for protein information. Nucleic Acids Res. 2014, 43, D204–D212.
- Zhang, J.; Yang, J.; Jang, R.; Zhang, Y. GPCR-I-TASSER: A Hybrid Approach to G Protein-Coupled Receptor Structure Modeling and the Application to the Human Genome. Structure 2015, 23, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- MarvinSketch (Version 16.8.15, Calculation Module Developed by ChemAxon). Available online: http://www.chemaxon.com/products/marvin/marvinsketch/ (accessed on 3 October 2017).
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Dickson, C.J.; Madej, B.D.; Skjevik, Å.A.; Betz, R.M.; Teigen, K.; Gould, I.R.; Walker, R.C. Lipid14: The amber lipid force field. J. Chem. Theory Comput. 2014, 10, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Joung, I.S.; Cheatham III, T.E. Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J. Phys. Chem. B 2008, 112, 9020–9041. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Humphrey, W.; Gursoy, A.; Dalke, A.; Kalé, L.V.; Skeel, R.D.; Schulten, K. NAMD: A parallel, object-oriented molecular dynamics program. Int. J. High Perform. Comput. Appl. 1996, 10, 251–268. [Google Scholar] [CrossRef]
- Phillips, J.C.; Zheng, G.; Kumar, S.; Kalé, L.V. NAMD: Biomolecular simulation on thousands of processors. In Proceedings of the 2002 ACM/IEEE Conference on Supercomputing, Baltimore, MD, USA, 16–22 November 2002; p. 36. [Google Scholar]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Adelman, S.; Doll, J. Generalized Langevin equation approach for atom/solid-surface scattering: General formulation for classical scattering off harmonic solids. J. Chem. Phys. 1976, 64, 2375–2388. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N? log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham III, T.E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Greene, D.; Homeyer, N.; et al. AMBER 2017; University of California: San Francisco, CA, USA, 2017. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Michino, M.; Abola, E.; Brooks, C.L.; Dixon, J.S.; Moult, J.; Stevens, R.C. Community-wide assessment of GPCR structure modelling and ligand docking: GPCR Dock 2008. Nat. Rev. Drug Discov. 2009, 8, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kufareva, I.; Rueda, M.; Katritch, V.; Stevens, R.C.; Abagyan, R. Status of GPCR Modeling and Docking as Reflected by Community-wide GPCR Dock 2010 Assessment. Structure 2011, 19, 1108–1126. [Google Scholar] [CrossRef] [PubMed]
- Kufareva, I.; Katritch, V.; Stevens, R.C.; Abagyan, R. Advances in GPCR Modeling Evaluated by the GPCR Dock 2013 Assessment: Meeting New Challenges. Structure 2014, 22, 1120–1139. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds along with the 3D structure files of the receptors and ligands are available from the authors. |
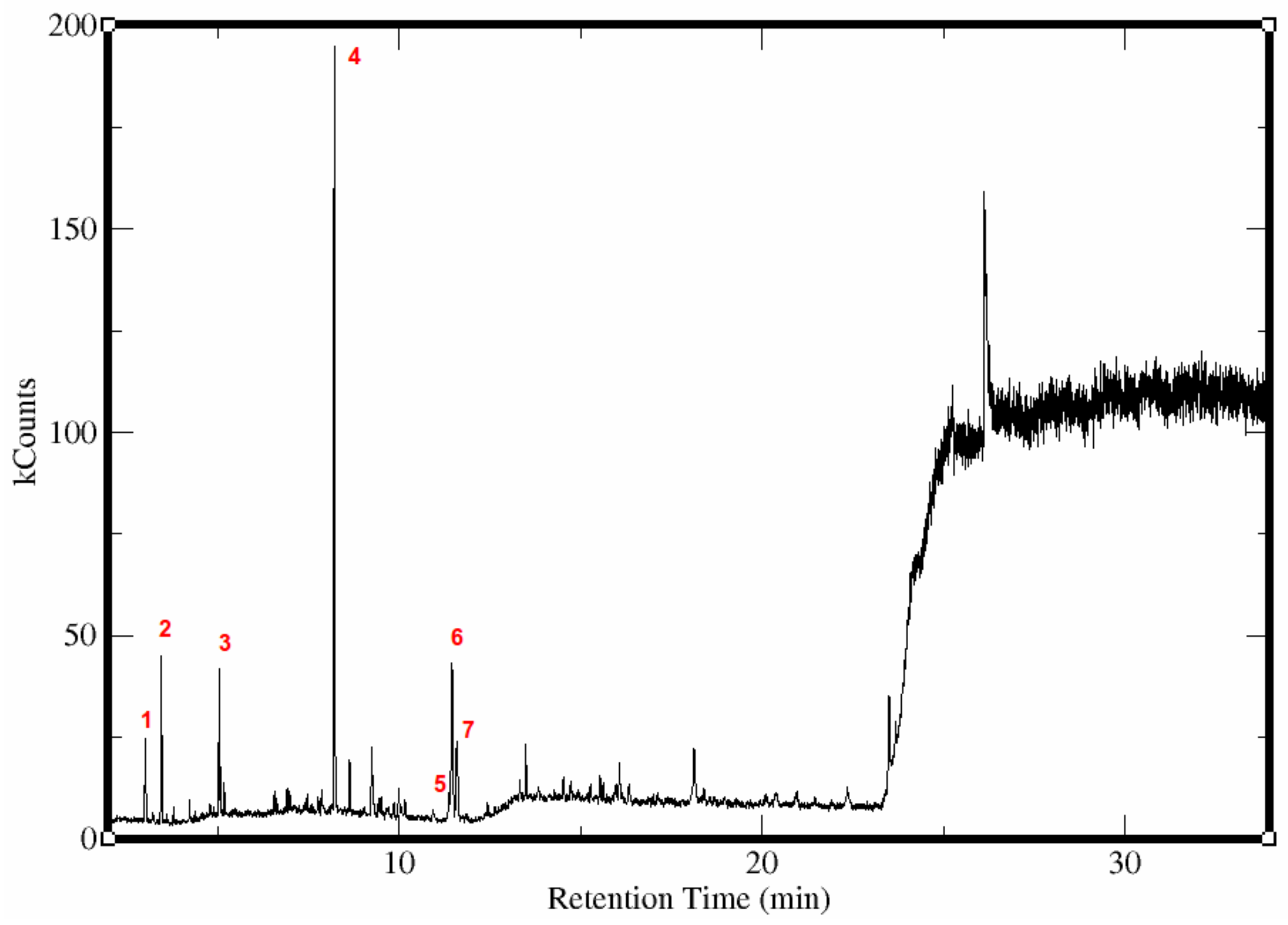

| Compounds Identified | Retention Time (min) | Relative Abundance |
|---|---|---|
| Methyl benzoate | 8.2 | 53.4 |
| Benzyl butanoate | 11.5 | 15.2 |
| Benzyl alcohol | 11.6 | 9.8 |
| Ethyl butanoate | 3.4 | 8.5 |
| Butyl butanoate | 5.1 | 6.0 |
| Methyl butanoate | 3.0 | 5.6 |
| Butyl benzoate | 11.4 | 1.4 |
| Ligand | Average Binding Affinity (kcal·mol) | Average (molar) |
|---|---|---|
| Octopamine | −5.18 ± 0.07 | 191.9 ± 27.4 |
| Benzyl butanoate | −6.03 ± 0.09 | 54.0 ± 12.3 |
| Butyl benzoate | −6.02 ± 0.01 | 47.6 ± 7.2 |
| Methyl benzoate | −5.61 ± 0.07 | 94.2 ± 15.0 |
| Benzyl alcohol | −4.93 ± 0.06 | 280.3 ± 37.9 |
| Butyl butanoate | −4.88 ± 0.06 | 304.1 ± 32.4 |
| Ethyl butanoate | −4.41 ± 0.06 | 657.8 ± 65.2 |
| Methyl butanoate | −4.06 ± 0.05 | 1140.7 ± 93.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dacanay, F.N.D.; Ladra, M.C.J.A.; Junio, H.A.; Nellas, R.B. Molecular Affinity of Mabolo Extracts to an Octopamine Receptor of a Fruit Fly. Molecules 2017, 22, 1677. https://doi.org/10.3390/molecules22101677
Dacanay FND, Ladra MCJA, Junio HA, Nellas RB. Molecular Affinity of Mabolo Extracts to an Octopamine Receptor of a Fruit Fly. Molecules. 2017; 22(10):1677. https://doi.org/10.3390/molecules22101677
Chicago/Turabian StyleDacanay, Francoise Neil D., Ma. Carmina Joyce A. Ladra, Hiyas A. Junio, and Ricky B. Nellas. 2017. "Molecular Affinity of Mabolo Extracts to an Octopamine Receptor of a Fruit Fly" Molecules 22, no. 10: 1677. https://doi.org/10.3390/molecules22101677