Tetraphenylpyrimidine-Based AIEgens: Facile Preparation, Theoretical Investigation and Practical Application
Abstract
:1. Introduction
2. Results and Discussion
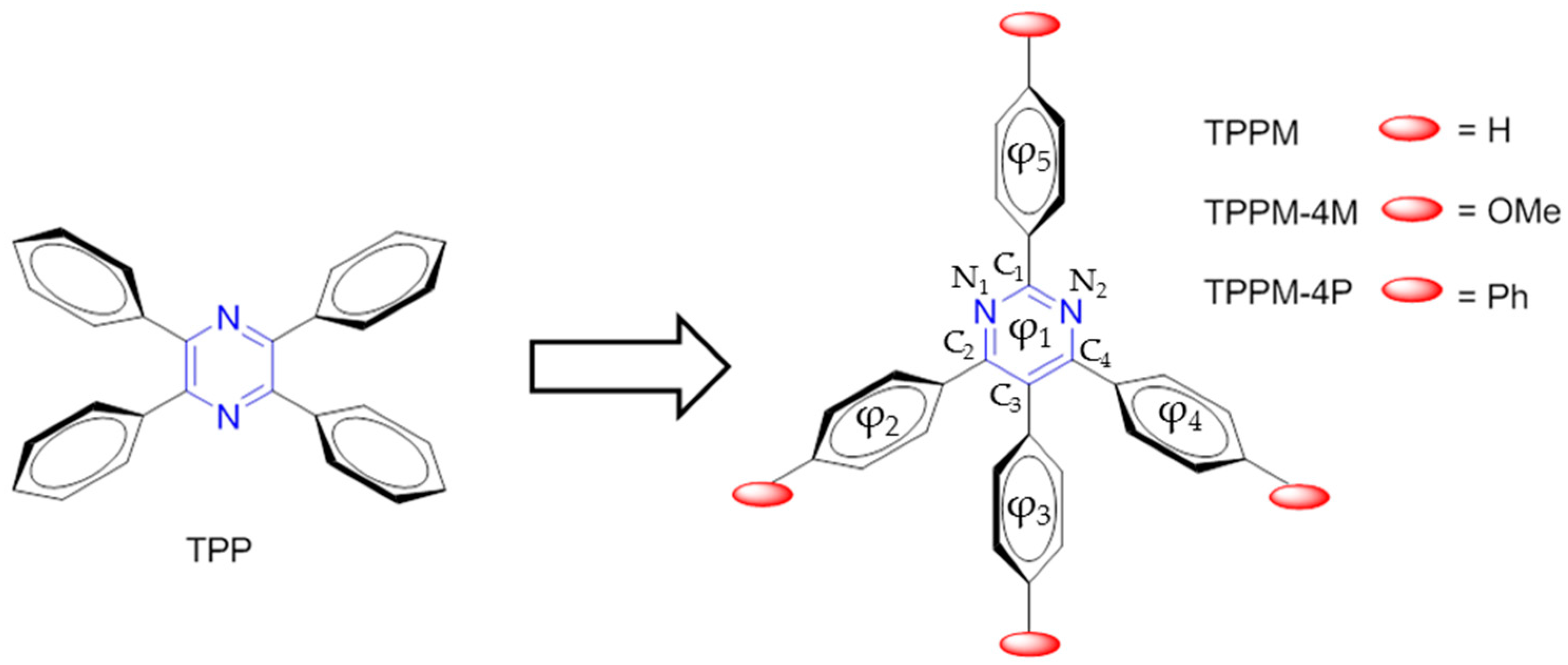
2.1. General Routes for Synthesis of TPPM Derivatives
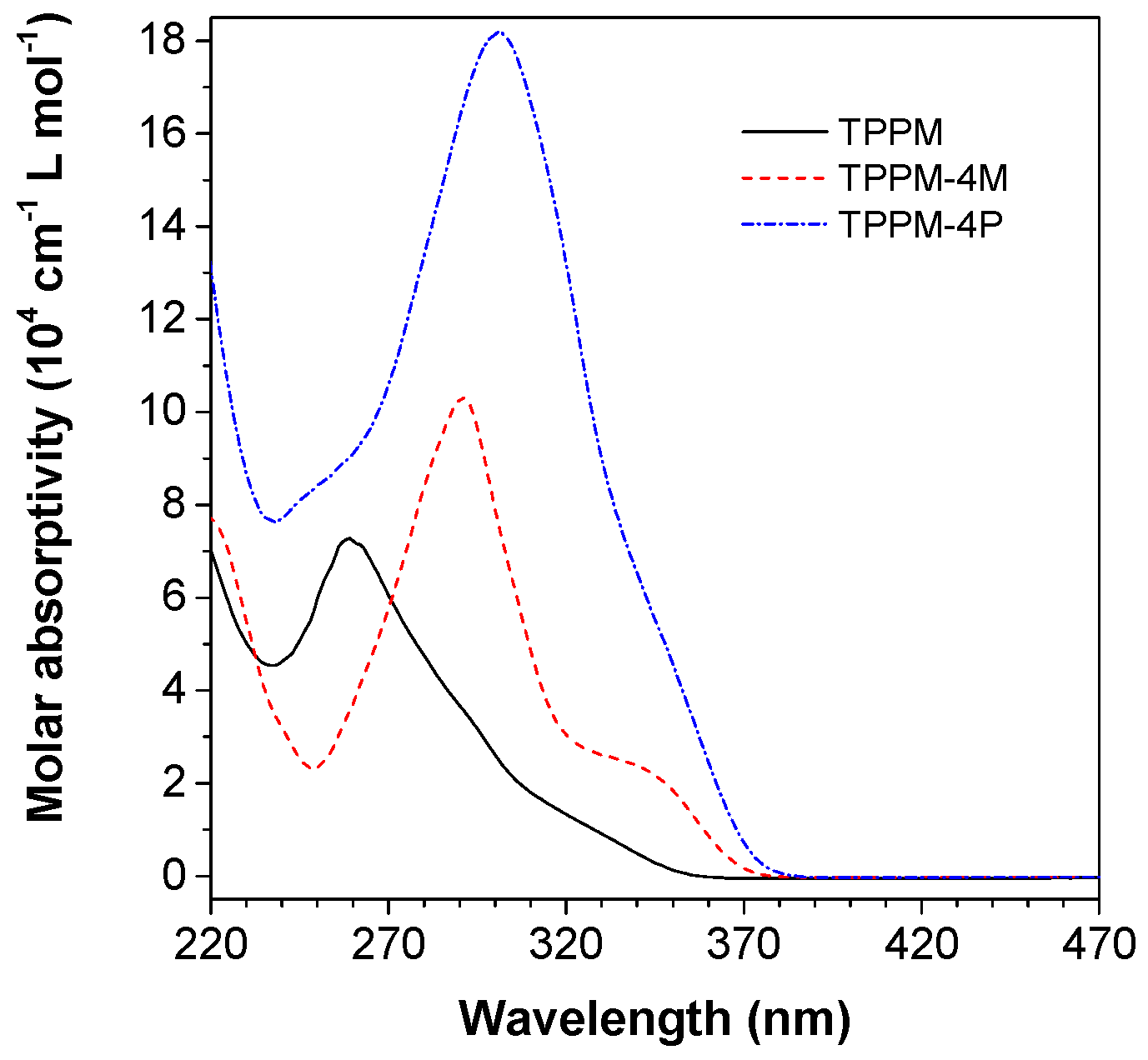
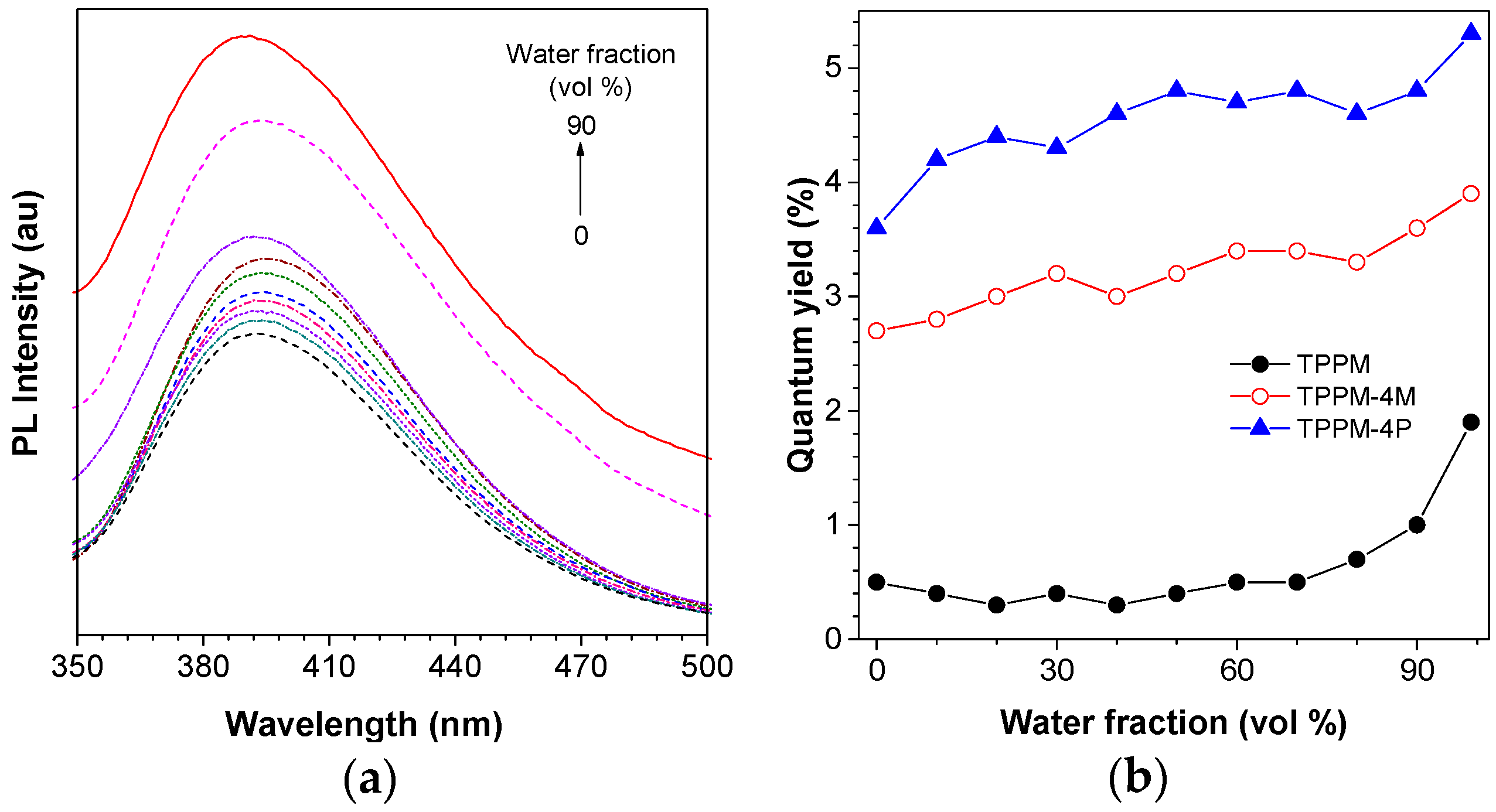
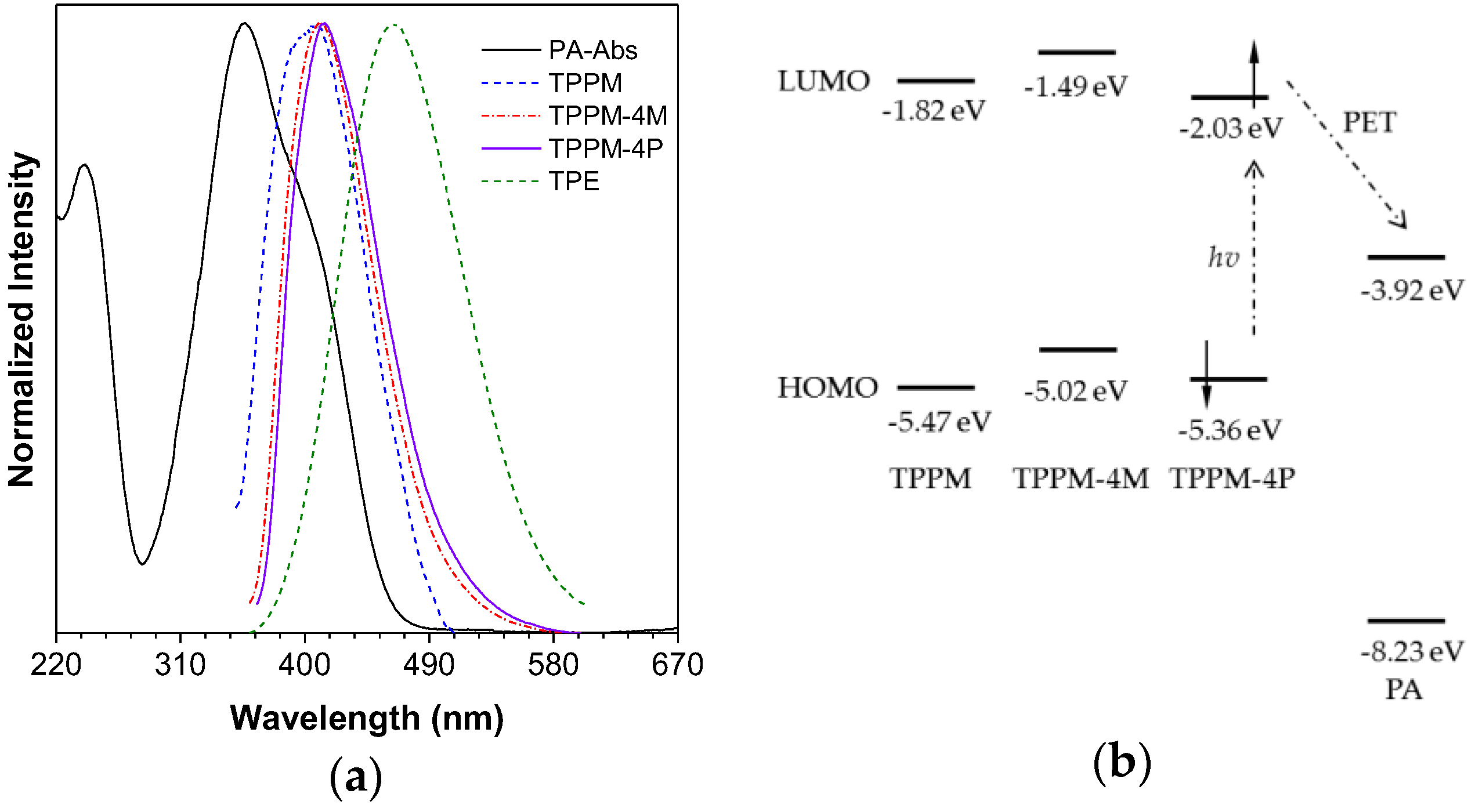
2.2. Photophysical Property of TPPM-Based AIEgens
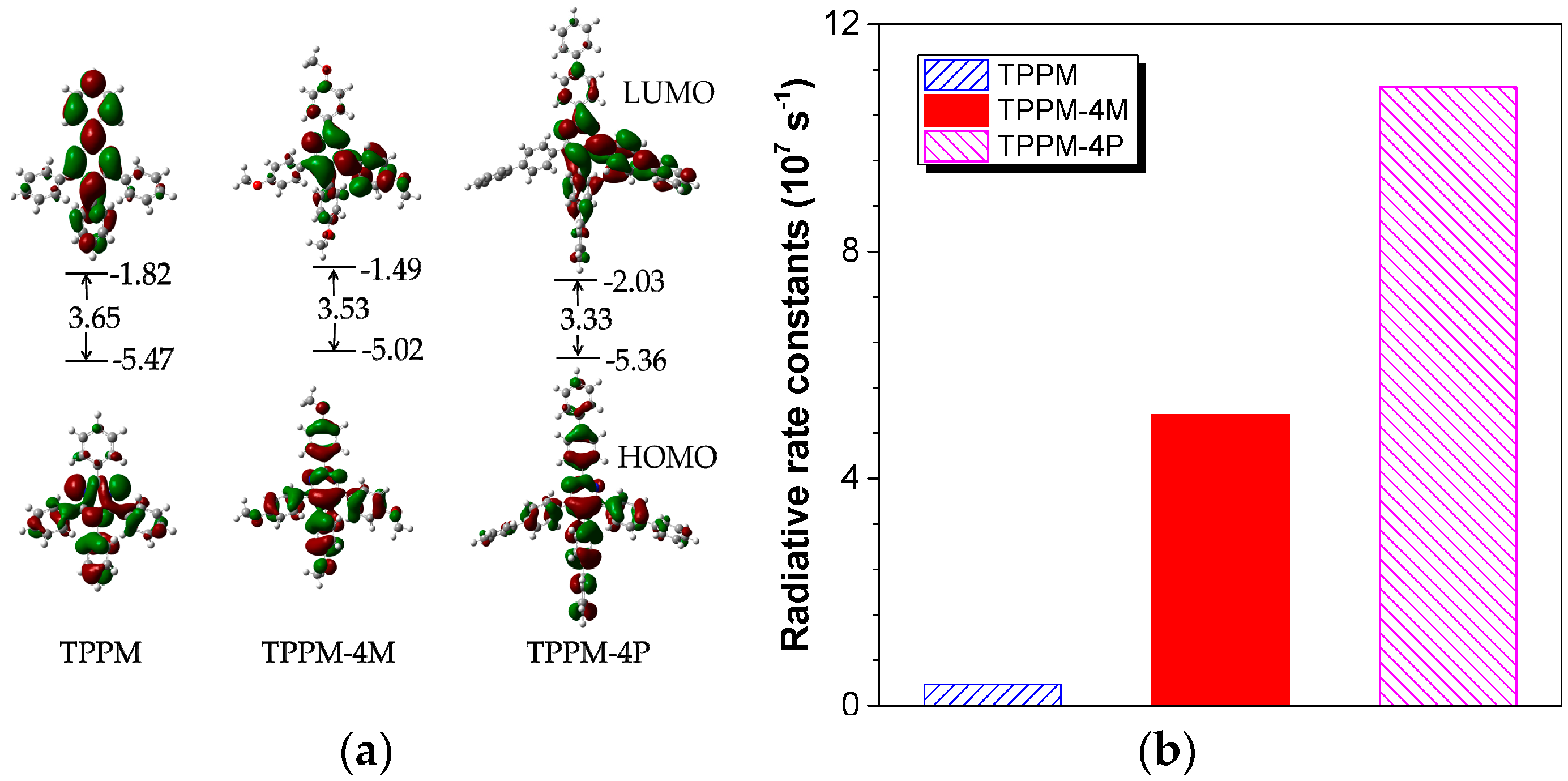
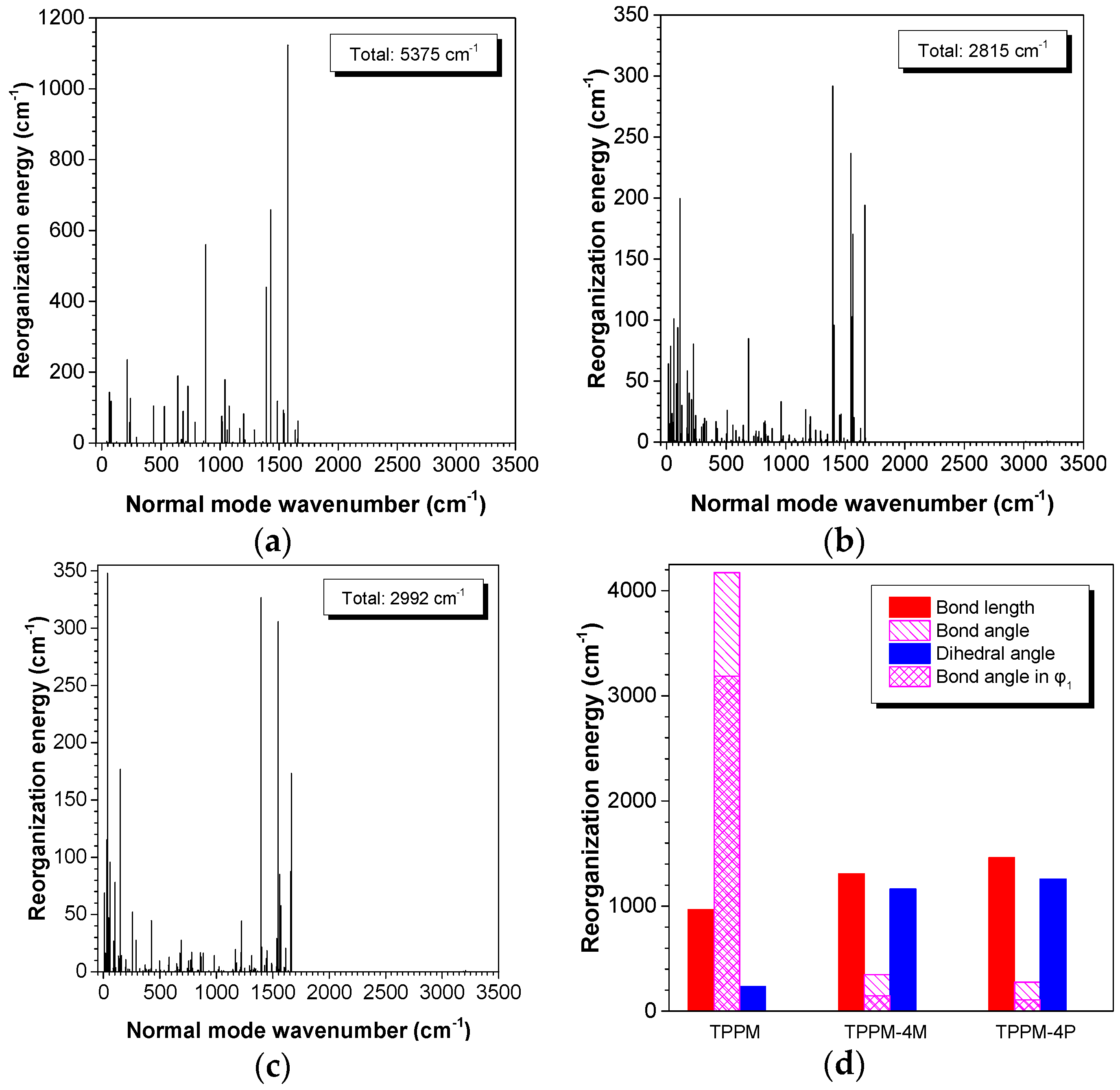
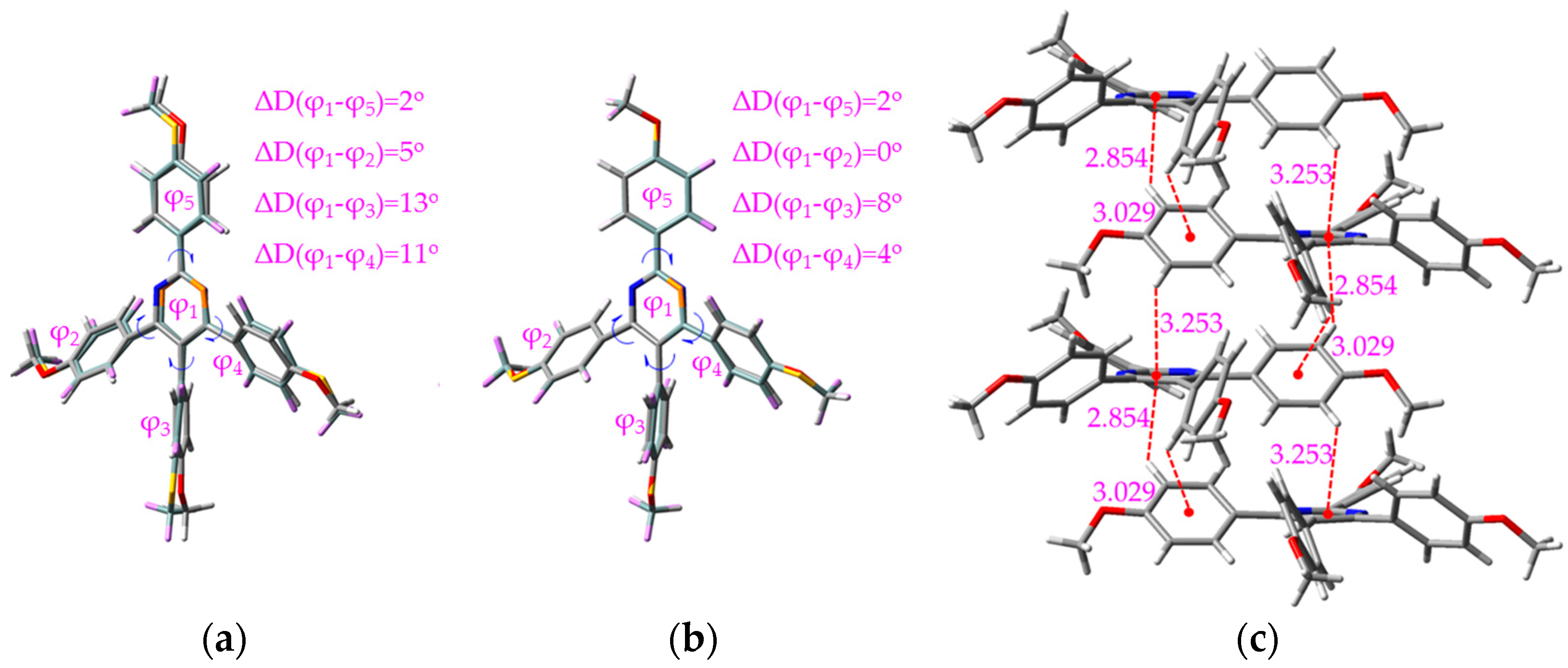
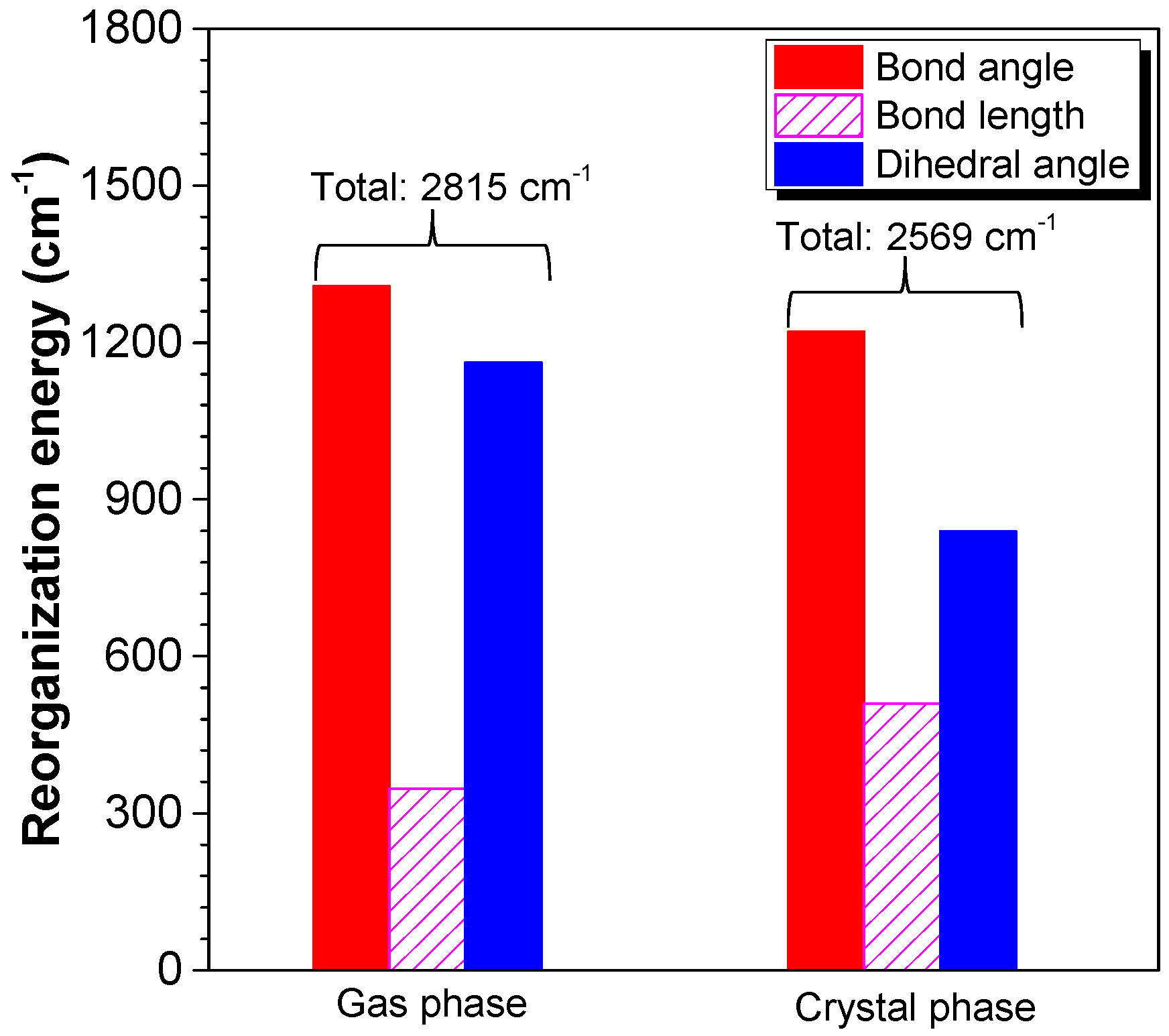
2.3. Working Mechanism
2.4. Application in Explosive Detection
3. Materials and Methods
3.1. Synthesis of TPPM
3.2. Synthesis of TPPM-4M
3.3. Synthesis of TPPM-4P
3.4. Methods for Theoretical Calculation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhang, G.X.; Zhan, C.; Zhang, W.; Yan, Y.L.; Zhao, Y.S.; Fu, H.B.; Zhang, D.Q. Highly Solid-State Emissive Pyridinium-Substituted Tetraphenylethylene Salts: Emission Color-Tuning with Counter Anions and Application for Optical Waveguides. Small 2015, 11, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.L.; Li, K.; Chen, S.J.; Qin, A.J.; Ding, D.; Zhang, S.; Liu, Y.; Liu, B.; Sun, J.Z.; Tang, B. Aggregation-induced red-NIR emission organic nanoparticles as effective and photostable fluorescent probes for bioimaging. J. Mater. Chem. 2012, 22, 15128–15135. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Lam, J.W.Y.; Qin, A.J.; Liu, J.Z.; Li, Z.; Tang, B.Z. Aggregation-induced emissions of tetraphenylethene derivatives and their utilities as chemical vapor sensors and in organic light-emitting diodes. Appl. Phys. Lett. 2007, 91, 3. [Google Scholar] [CrossRef]
- Li, Z. Fabrication of high-performance non-doped OLEDs by combining aggregation-induced emission and thermally activated delayed fluorescence. Sci. China Chem. 2017, 60, 1107–1108. [Google Scholar] [CrossRef]
- Gao, Y.T.; Feng, G.X.; Jiang, T.; Goh, C.C.; Ng, L.G.; Liu, B.; Li, B.; Yang, L.; Hua, J.L.; Tian, H. Biocompatible Nanoparticles Based on Diketo-Pyrrolo-Pyrrole (DPP) with Aggregation-Induced Red/NIR Emission for In Vivo Two-Photon Fluorescence Imaging. Adv. Funct. Mater. 2015, 25, 2857–2866. [Google Scholar] [CrossRef]
- Shi, X.Y.; Wang, H.; Han, T.Y.; Feng, X.; Tong, B.; Shi, J.B.; Zhi, J.G.; Dong, Y.P. A highly sensitive, single selective, real-time and “turn-on” fluorescent sensor for Al3+ detection in aqueous media. J. Mater. Chem. 2012, 22, 19296–19302. [Google Scholar] [CrossRef]
- Liu, Z.T.; Xue, W.X.; Cai, Z.X.; Zhang, G.X.; Zhang, D.Q. A facile and convenient fluorescence detection of gamma-ray radiation based on the aggregation-induced emission. J. Mater. Chem. 2011, 21, 14487–14491. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, L.Z.; Nie, H.; Tong, J.Q.; Yan, L.L.; Xu, B.; Sun, J.Z.; Tian, W.J.; Zhao, Z.J.; Qin, A.J.; et al. Tetraphenylpyrazine-based AIEgens: Facile preparation and tunable light emission. Chem. Sci. 2015, 6, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.X.; Luo, W.W.; Chen, M.; Liu, J.K.; Xu, L.; Hu, R.R.; Zhao, Z.J.; Qin, A.J.; Tang, B.Z. Tetraphenylpyrazine-Based Luminogens with Aggregation-Enhanced Emission Characteristics: Preparation and Property. Chin. J. Org. Chem. 2016, 36, 1316–1324. [Google Scholar] [CrossRef]
- Achelle, S.; Plé, N. Pyrimidine Ring as Building Block for the Synthesis of Functionalized π-Conjugated Materials. Curr. Org. Synth. 2012, 9, 163–187. [Google Scholar] [CrossRef]
- Li, D.D.; Yu, X. Mesoporous aluminium organophosphonates: A reusable chemsensor for the detection of explosives. J. Solid State Chem. 2016, 239, 17–22. [Google Scholar] [CrossRef]
- Hussain, M.; Hung, N.T.; Khera, R.A.; Malik, I.; Zinad, D.S.; Langer, P. Synthesis of Aryl-Substituted Pyrimidines by Site-Selective Suzuki-Miyura Cross-Coupling Reactions of 2,4,5,6-Tetrachloropyrimidine. Adv. Synth. Catal. 2010, 352, 1429–1433. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z. Molecular conformation and packing: Their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 2017. [Google Scholar] [CrossRef]
- Wang, C.; Xu, B.J.; Li, M.S.; Chi, Z.G.; Xie, Y.J.; Li, Q.Q.; Li, Z. A stable tetraphenylethene derivative: Aggregation-induced emission, different crystalline polymorphs, and totally different mechanoluminescence properties. Mater. Horizons 2016, 3, 220–225. [Google Scholar] [CrossRef]
- Li, H.Y.; Chi, Z.G.; Xu, B.J.; Zhang, X.Q.; Li, X.F.; Liu, S.W.; Zhang, Y.; Xu, J.R. Aggregation-induced emission enhancement compounds containing triphenylamine-anthrylenevinylene and tetraphenylethene moieties. J. Mater. Chem. 2011, 21, 3760–3767. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, A.; Tang, B.Z. AIE-active polymers for explosive detection. Chin. J. Polym. Sci. 2016, 35, 141–154. [Google Scholar] [CrossRef]
- Meher, N.; Iyer, P.K. Pendant chain engineering to fine-tune the nanomorphologies and solid state luminescence of naphthalimide AIEEgens: Application to phenolic nitro-explosive detection in water. Nanoscale 2017, 9, 7674–7685. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Shyamal, M.; Das, D.; Mazumdar, P.; Sahoo, G.P.; Misra, A. Aggregation induced emission enhancement from antipyrine-based schiff base and its selective sensing towards picric acid. Sens. Actuator B Chem. 2017, 248, 223–233. [Google Scholar] [CrossRef]
- Dong, W.Y.; Pan, Y.Y.; Fritsch, M.; Scherf, U. High sensitivity sensing of nitroaromatic explosive vapors based on polytriphenylamines with AIE-active tetraphenylethylene side groups. J. Polym. Sci. Pol. Chem. 2015, 53, 1753–1761. [Google Scholar] [CrossRef]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Peng, H.N.; Liu, T.H.; Yang, M.N.; Zhang, Y.; Fang, Y. A novel picric acid film sensor via combination of the surface enrichment effect of chitosan films and the aggregation-induced emission effect of siloles. J. Mater. Chem. 2009, 19, 7347–7353. [Google Scholar] [CrossRef]
- Feng, H.T.; Zheng, Y.S. Highly Sensitive and Selective Detection of Nitrophenolic Explosives by Using Nanospheres of a Tetraphenylethylene Macrocycle Displaying Aggregation-Induced Emission. Chem. Eur. J. 2014, 20, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2010.
- Sherwood, P.; de Vries, A.H.; Guest, M.F.; Schreckenbach, G.; Catlow, R.C.A.; French, S.A.; Sokol, A.A.; Bromley, S.T.; Thiel, W.; Turner, A.J.; et al. QUASI: A General Purpose Implementation of the QM/MM Approach and Its Application to Problems in Catalysis. J. Mol. Struct. THEOCHEM 2003, 632, 1–28. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic Structure Calculations on Workstation Computers: The Program System Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Smith, W.; Forester, T.R. DL_POLY_2.0: A General-Purpose Parallel Molecular Dynamics Simulation Package. J. Mol. Graph. 1996, 14, 136–141. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Z.G.; Peng, Q.; Niu, Y.L.; Geng, H. MOMAP (Molecular Materials Property Prediction Package) is A Suite of Codes to Study the Spectroscopy; Revision 0.2.004; Shuai Group: Beijing, China, 2017; Available online: http://www.momap.net.cn/ (accessed on 9 October 2017).
- Shuai, Z.G.; Peng, Q. Excited states structure and processes: Understanding organic light-emitting diodes at the molecular level. Phys. Rep. Rev. Sec. Phys. Lett. 2014, 537, 123–156. [Google Scholar] [CrossRef]
- Peng, Q.; Yi, Y.P.; Shuai, Z.G.; Shao, J.S. Toward quantitative prediction of molecular fluorescence quantum efficiency: Role of Duschinsky rotation. J. Am. Chem. Soc. 2007, 129, 9333–9339. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Yi, Y.P.; Shuai, Z.G.; Shao, J.S. Excited state radiationless decay process with Duschinsky rotation effect: Formalism and implementation. J. Chem. Phys. 2007, 126, 114302. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.L.; Peng, Q.; Deng, C.M.; Gao, X.; Shuai, Z.G. Theory of Excited State Decays and Optical Spectra: Application to Polyatomic Molecules. J. Phys. Chem. A 2010, 114, 7817–7831. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Peng, Q.; Shuai, Z. Promoting-mode free formalism for excited state radiationless decay process with Duschinsky rotation effect. Sci. China Ser. B Chem. 2008, 51, 1153–1158. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| TPPM | TPPM-4M | TPPM-4P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | Δ(S1 − S0) | S0 | S1 | Δ(S1 − S0) | S0 | S1 | Δ(S1 − S0) | |
| D(ϕ1–ϕ2) | 40 | 27 | −13 | 37 | 42 | 5 | 40 | 47 | 7 |
| D(ϕ1–ϕ3) | 63 | 65 | 2 | 62 | 49 | −13 | 62 | 42 | −20 |
| D(ϕ1–ϕ4) | 40 | 27 | −13 | 37 | 26 | −11 | 40 | 25 | −15 |
| D(ϕ1–ϕ5) | 4 | 7 | 3 | 5 | 7 | 2 | 4 | 7 | 3 |
| A(C1-N1-C2) | 118 | 128 | 10 | 118 | 117 | −1 | 118 | 117 | −1 |
| A(C1-N2-C4) | 118 | 128 | 10 | 118 | 121 | 3 | 118 | 121 | 3 |
| A(N1-C2-C3) | 122 | 121 | −1 | 122 | 123 | 1 | 122 | 123 | 1 |
| A(N2-C4-C3) | 122 | 121 | −1 | 121 | 118 | −3 | 122 | 118 | −4 |
| A(N1-C1-N2) | 125 | 111 | −14 | 125 | 125 | 0 | 125 | 125 | 0 |
| A(C2-C3-C4) | 116 | 112 | −4 | 116 | 116 | 0 | 116 | 116 | 0 |
| 300 K | µ (Debye) | kr (s−1) | kic (s−1) |
|---|---|---|---|
| Gas | 3.63 | 5.12 × 107 | 3.30 × 1011 |
| Crystal | 3.21 | 3.54 × 107 | 2.34 × 106 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Pan, L.; Peng, Q.; Qin, A. Tetraphenylpyrimidine-Based AIEgens: Facile Preparation, Theoretical Investigation and Practical Application. Molecules 2017, 22, 1679. https://doi.org/10.3390/molecules22101679
Liu J, Pan L, Peng Q, Qin A. Tetraphenylpyrimidine-Based AIEgens: Facile Preparation, Theoretical Investigation and Practical Application. Molecules. 2017; 22(10):1679. https://doi.org/10.3390/molecules22101679
Chicago/Turabian StyleLiu, Junkai, Lingxiang Pan, Qian Peng, and Anjun Qin. 2017. "Tetraphenylpyrimidine-Based AIEgens: Facile Preparation, Theoretical Investigation and Practical Application" Molecules 22, no. 10: 1679. https://doi.org/10.3390/molecules22101679






