Trace Oxygen Sensitive Material Based on Two Porphyrin Derivatives in a Heterodimeric Complex
Abstract
:1. Introduction
2. Results and Discussions
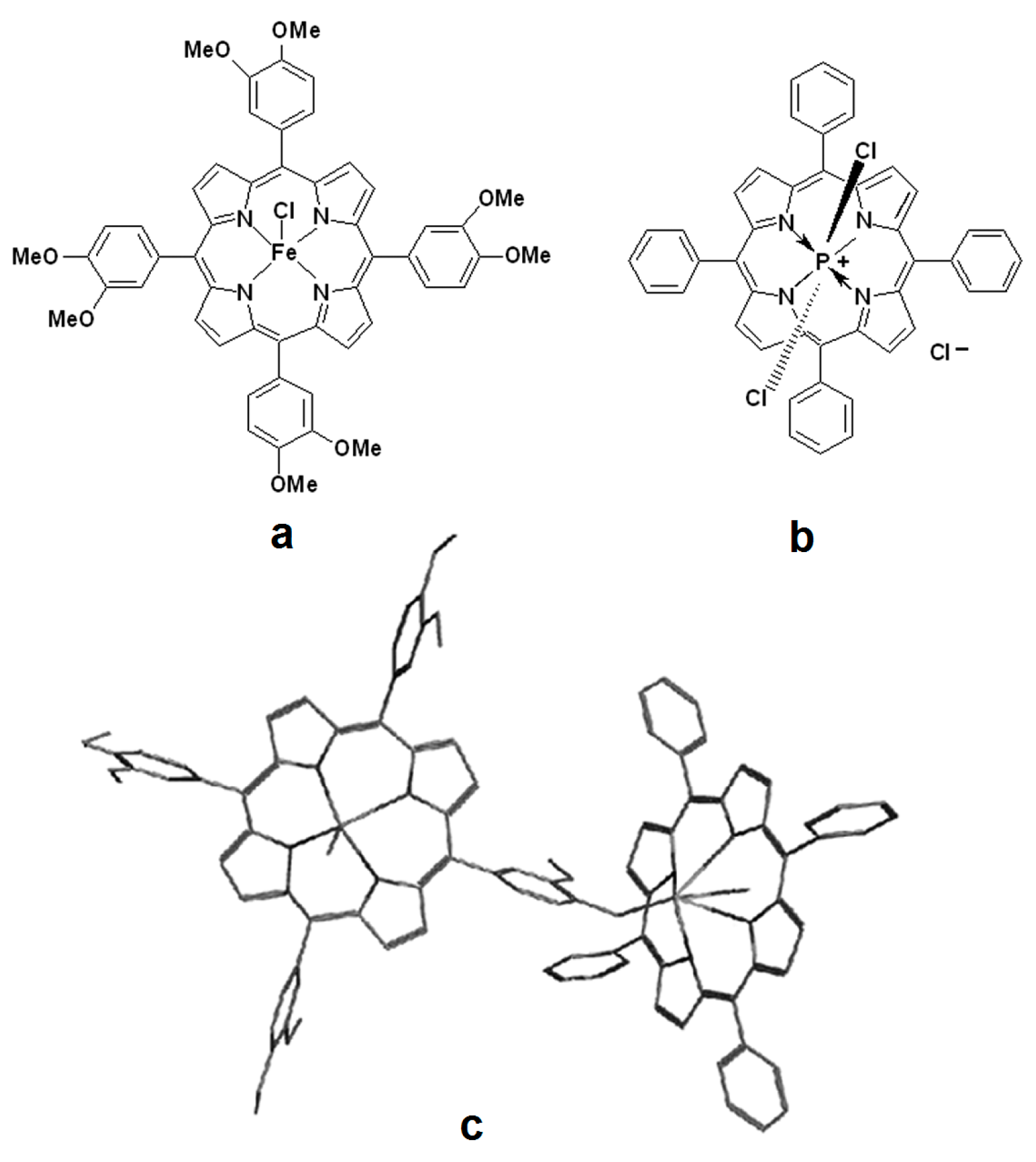
2.1. NMR Characterization of 5,10,15,20-tetrakis(3,4-dimethoxy-phenyl)-porphyrin Fe(III) Chloride, Compound 1
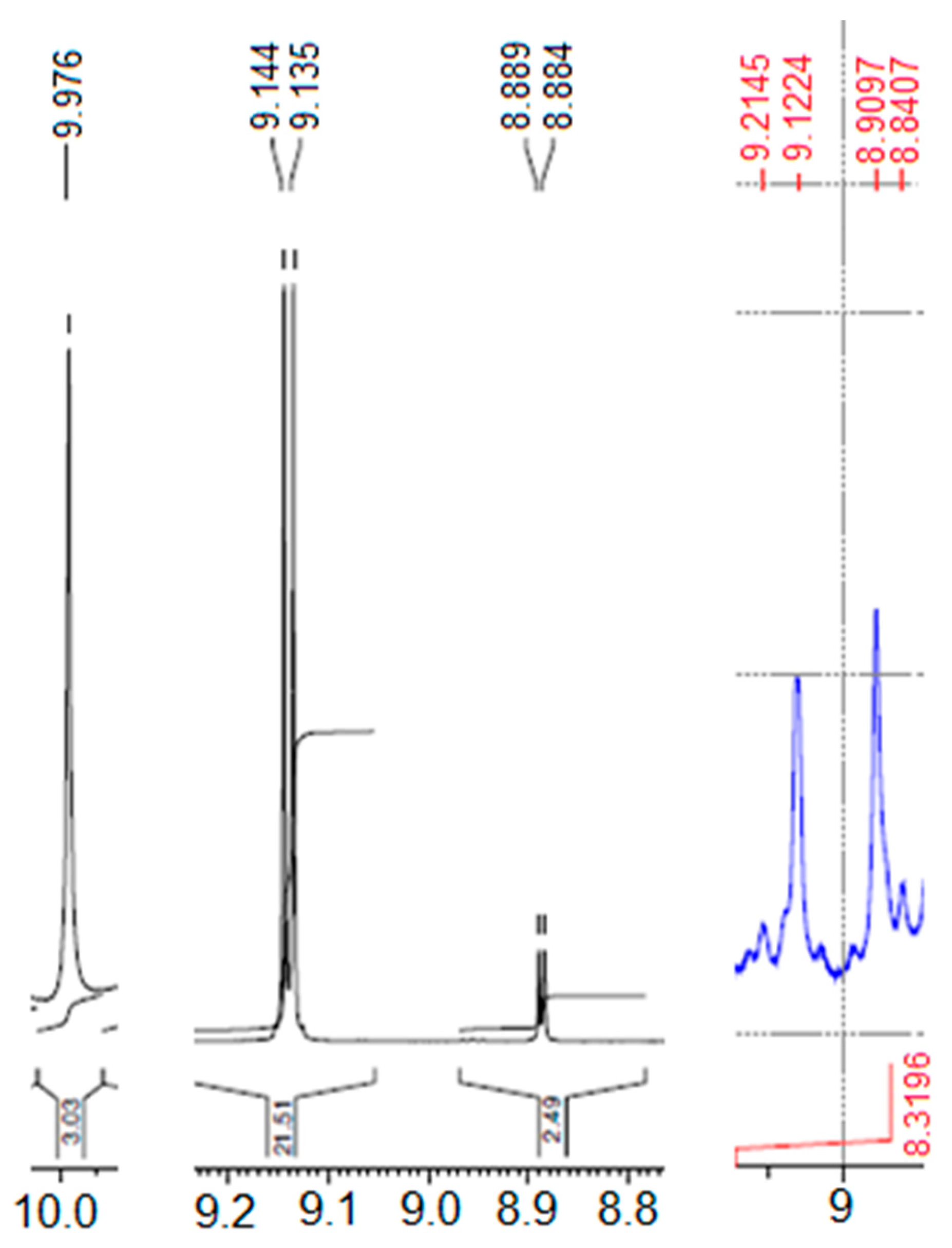
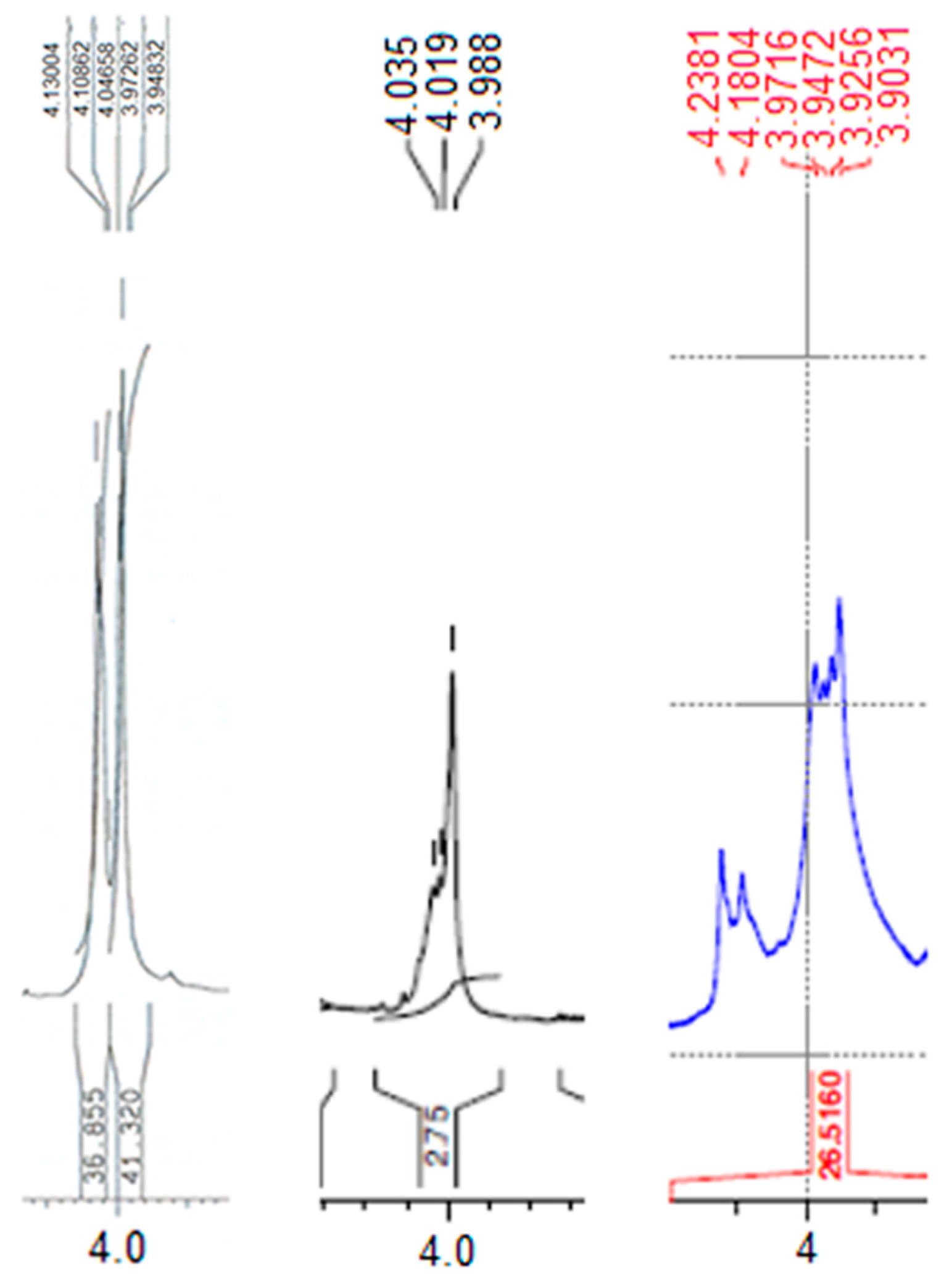
2.1.1. The 1H-NMR Spectrum of Compound 1
2.1.2. The 13C-NMR Spectrum of Compound 1
2.2. NMR Characterization of (5,10,15,20-tetraphenylporphinato)dichlorophosphorus (V) Chloride, Compound 2
2.2.1. The 1H-NMR Spectrum of Compound 2 (Figure S3 from Supplementary Material)
2.2.2. The 13C NMR Spectrum of Compound 2
2.2.3. The 31P-NMR Spectrum of Compound 2
2.2.4. 2D Spectra 1H-13C, HSQC, and HMBC Experiments for Compound 2
2.3. Physical Characterization of the Dimer Complex, Compound 3
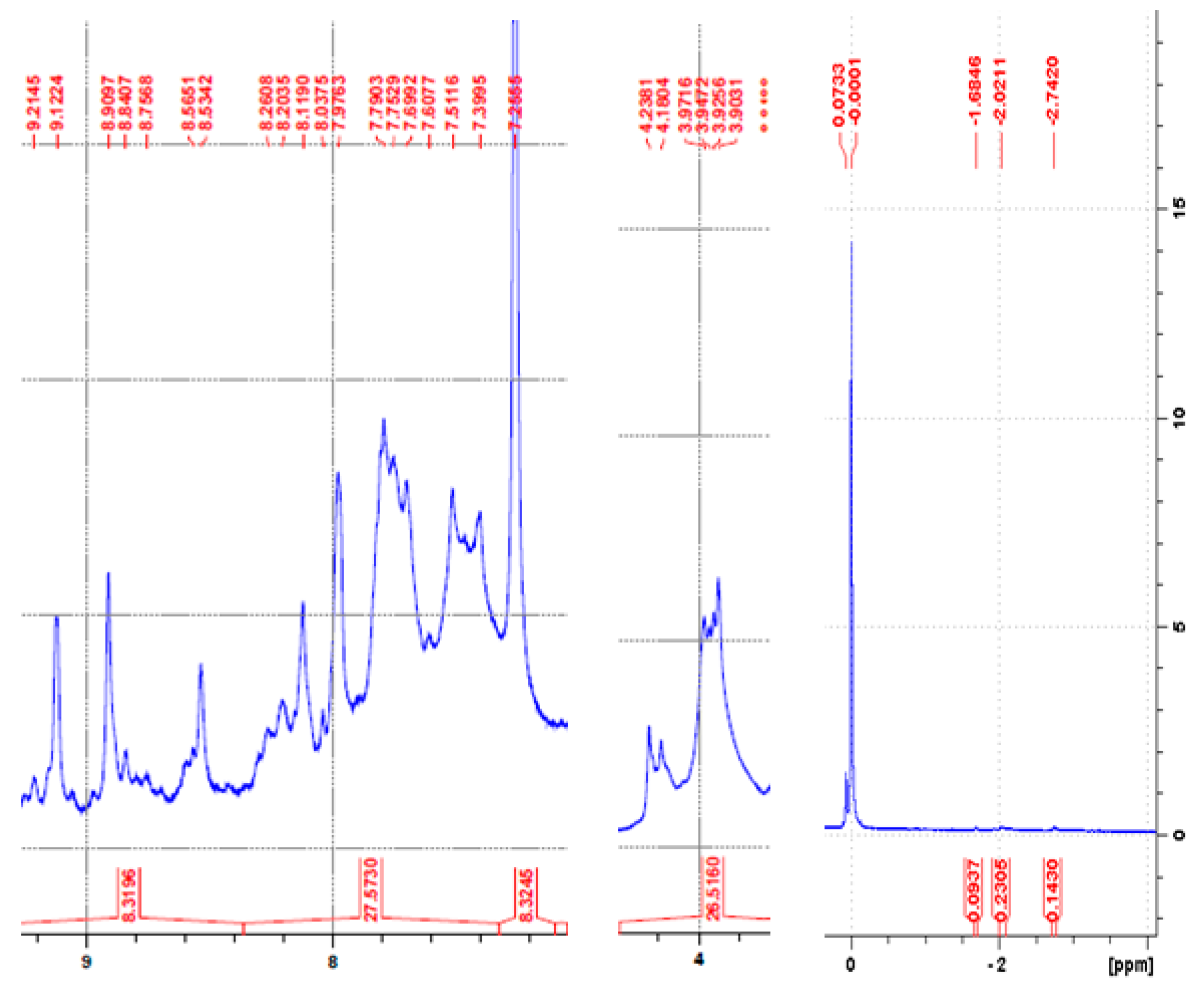
2.3.1. The 1H-NMR Spectrum of the Dimer Complex Compound 3
2.3.2. 31P-NMR Spectrum of the Heterodimer Compound 3
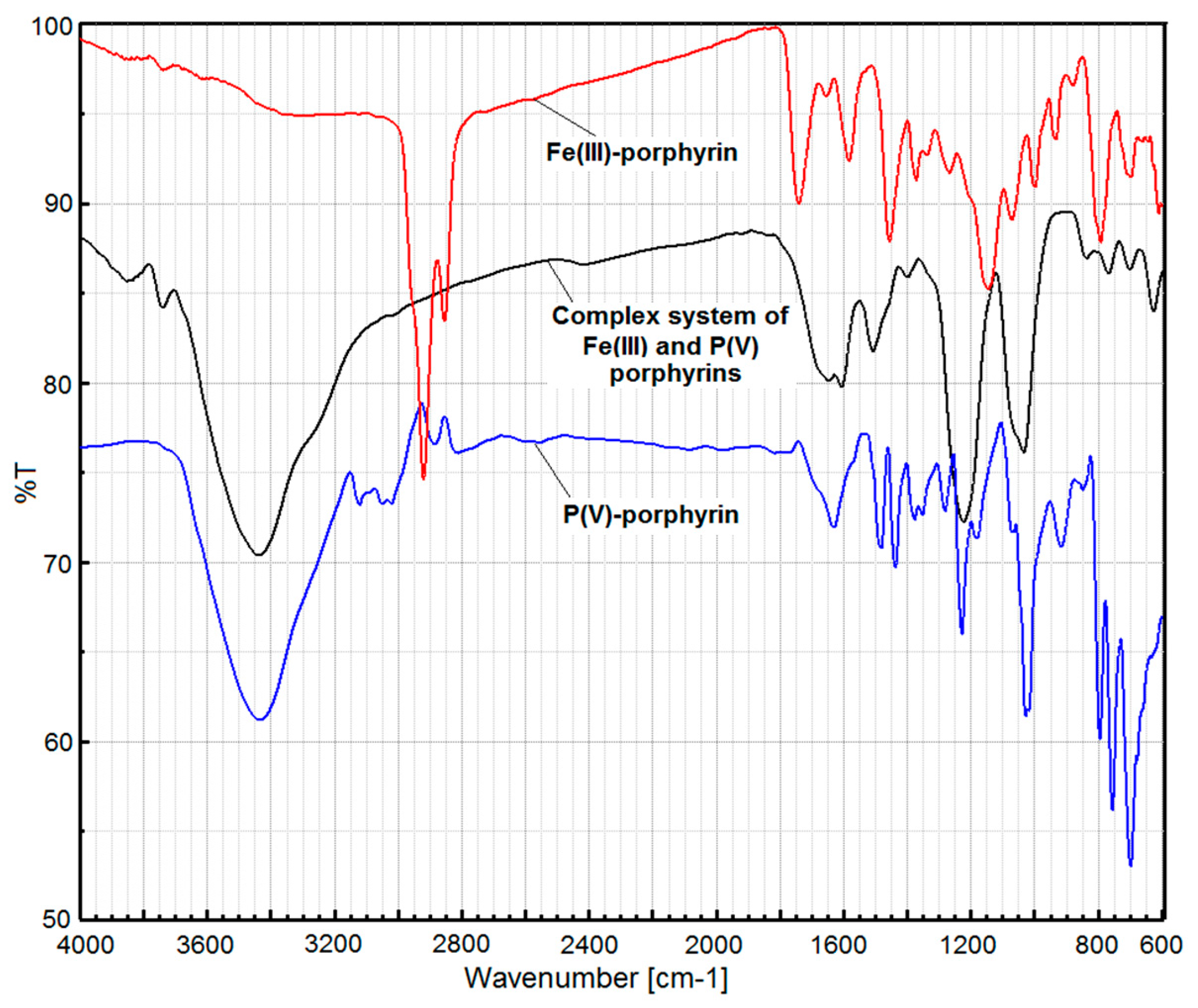
2.3.3. The FT-IR spectrum of compound 3
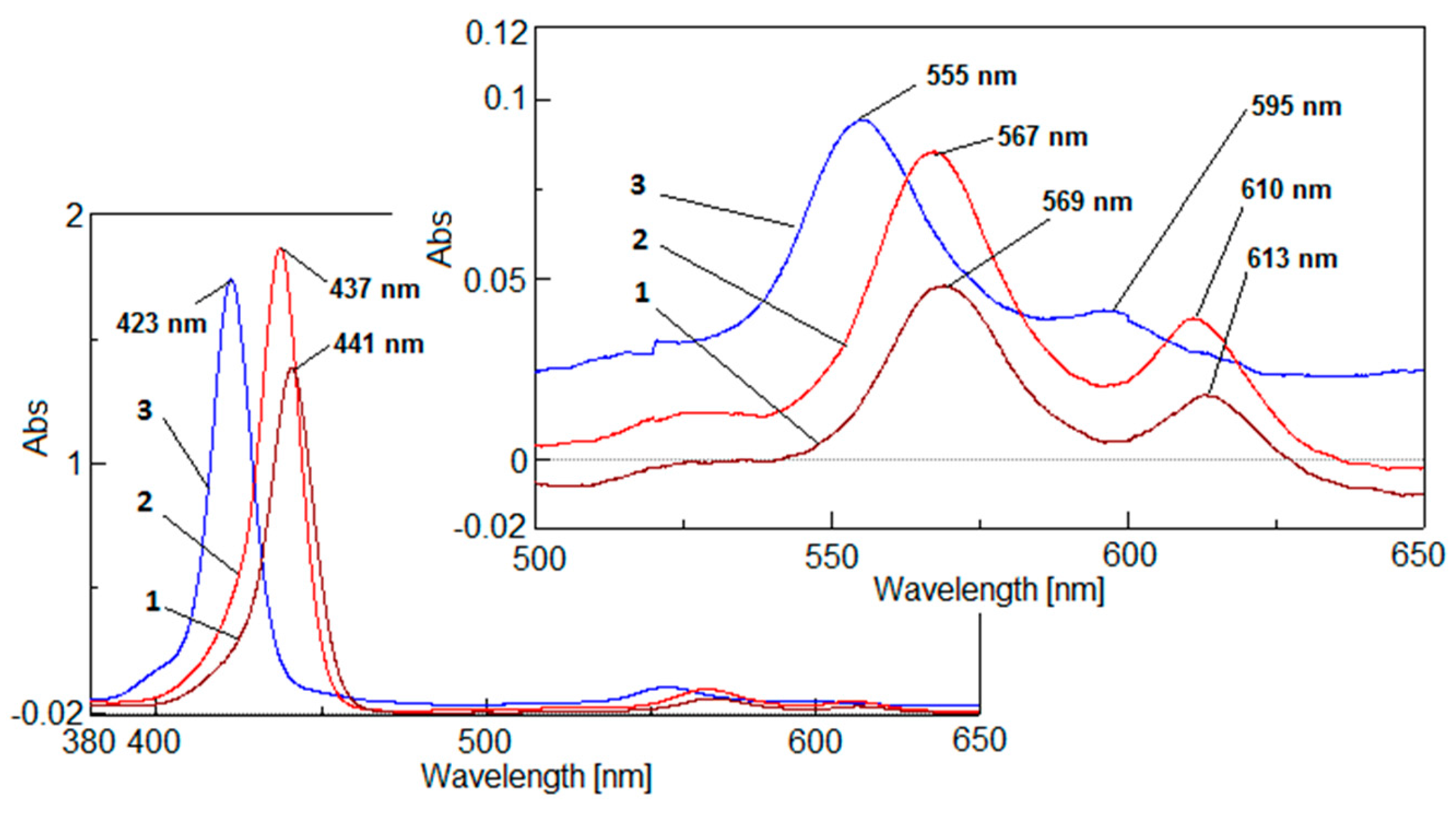
2.4. UV-Vis Analysis
2.4.1. The UV-Vis Study Regarding the Behavior of the Compound 2 in Solvents of Different Polarity
2.4.2. UV-Vis Monitoring of the Compound 3 Generation
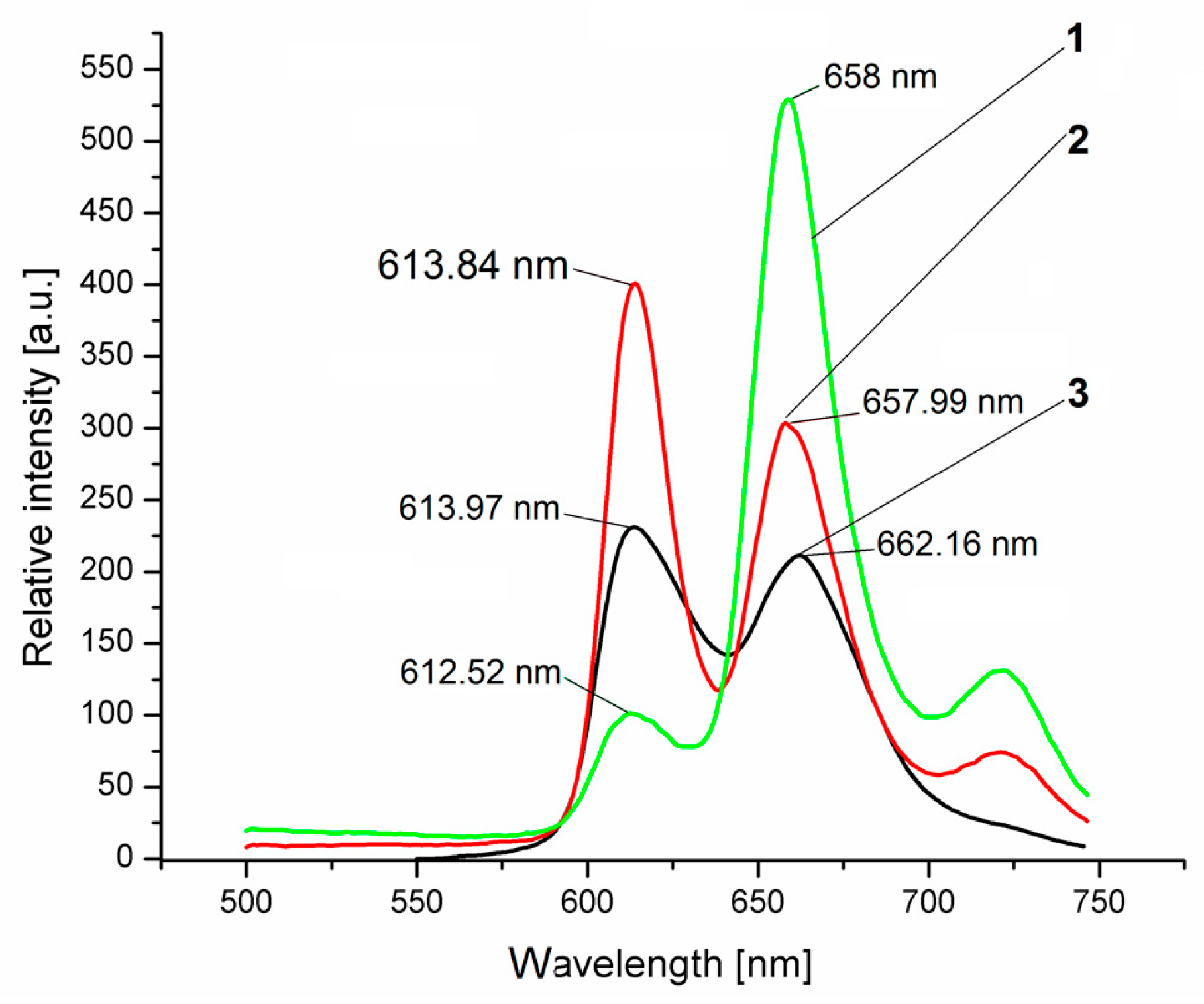
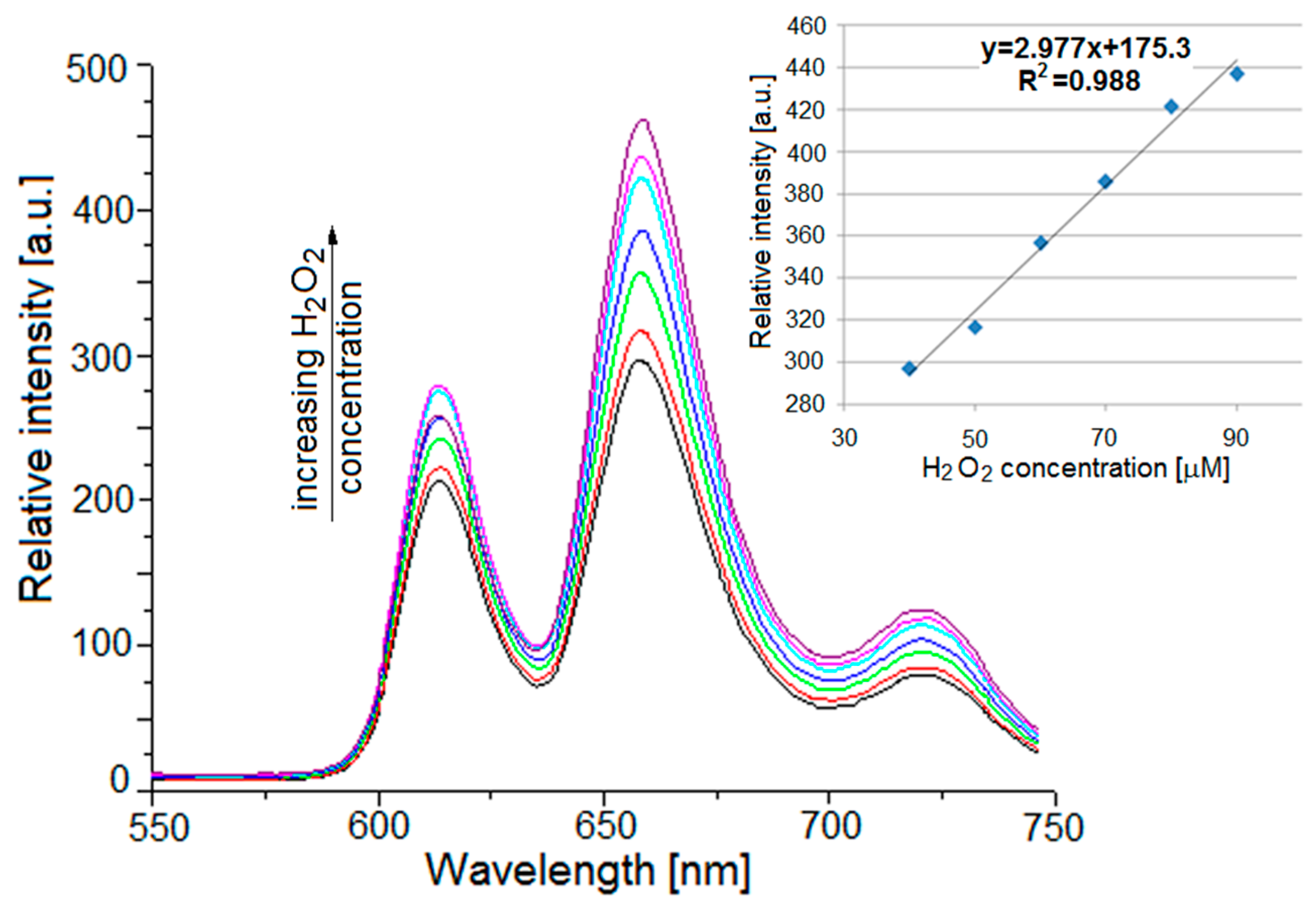
2.5. Fluorescence Properties
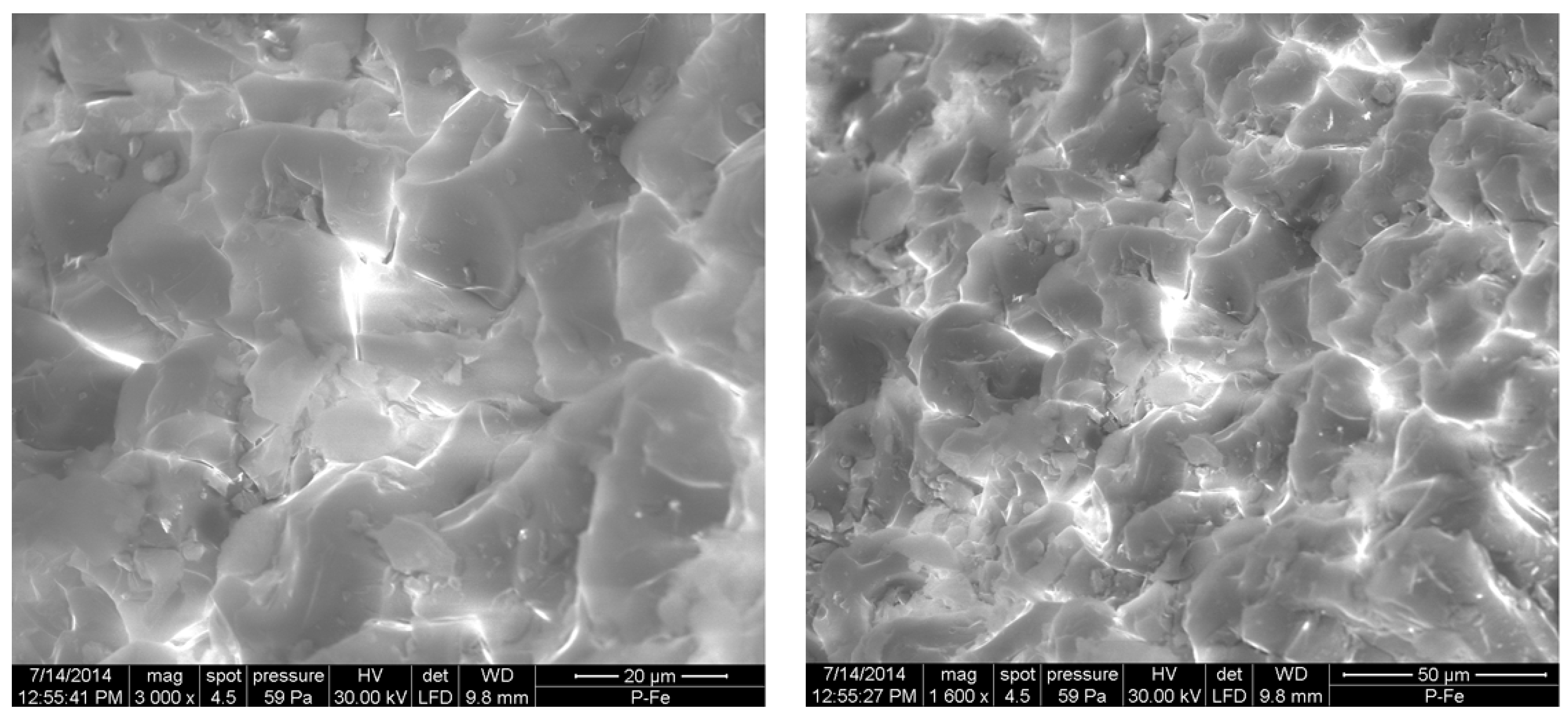
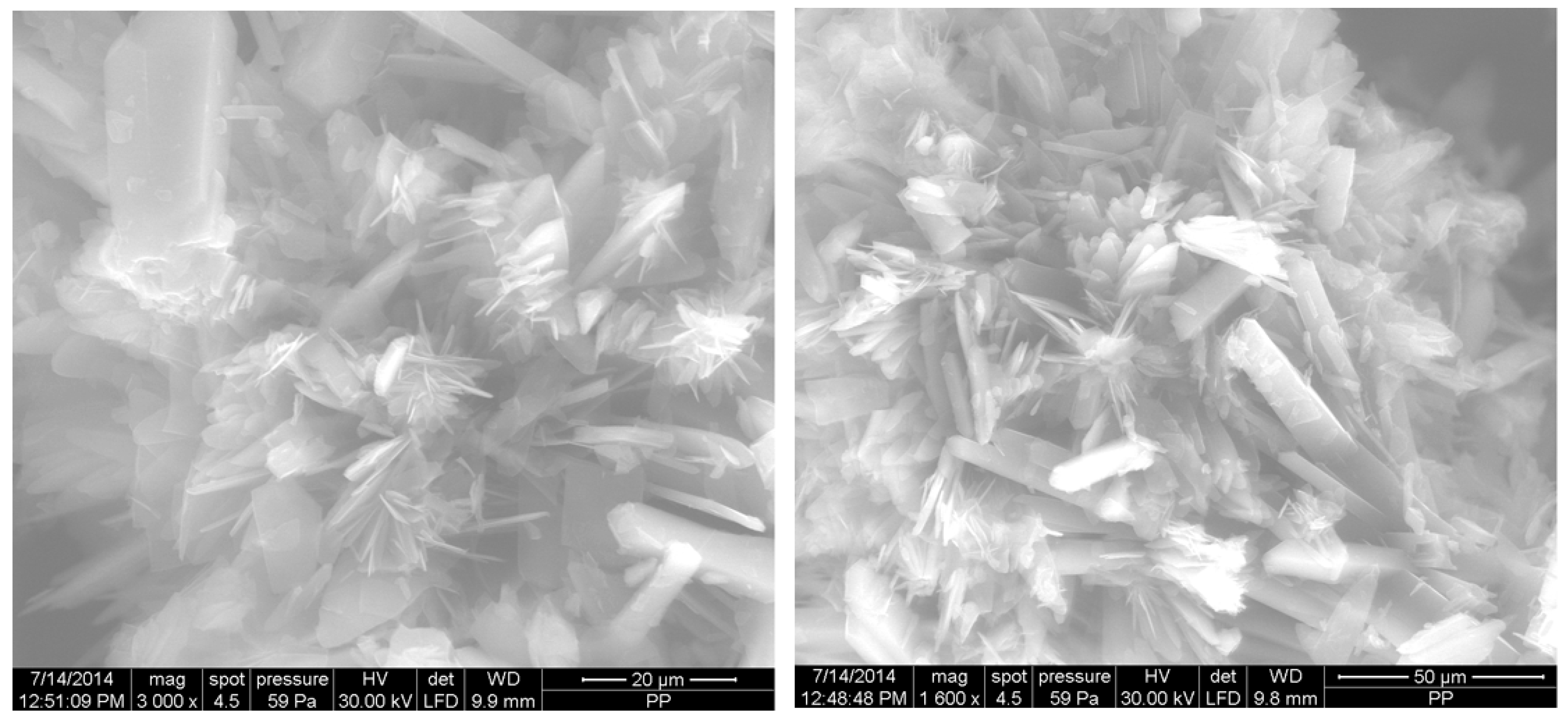
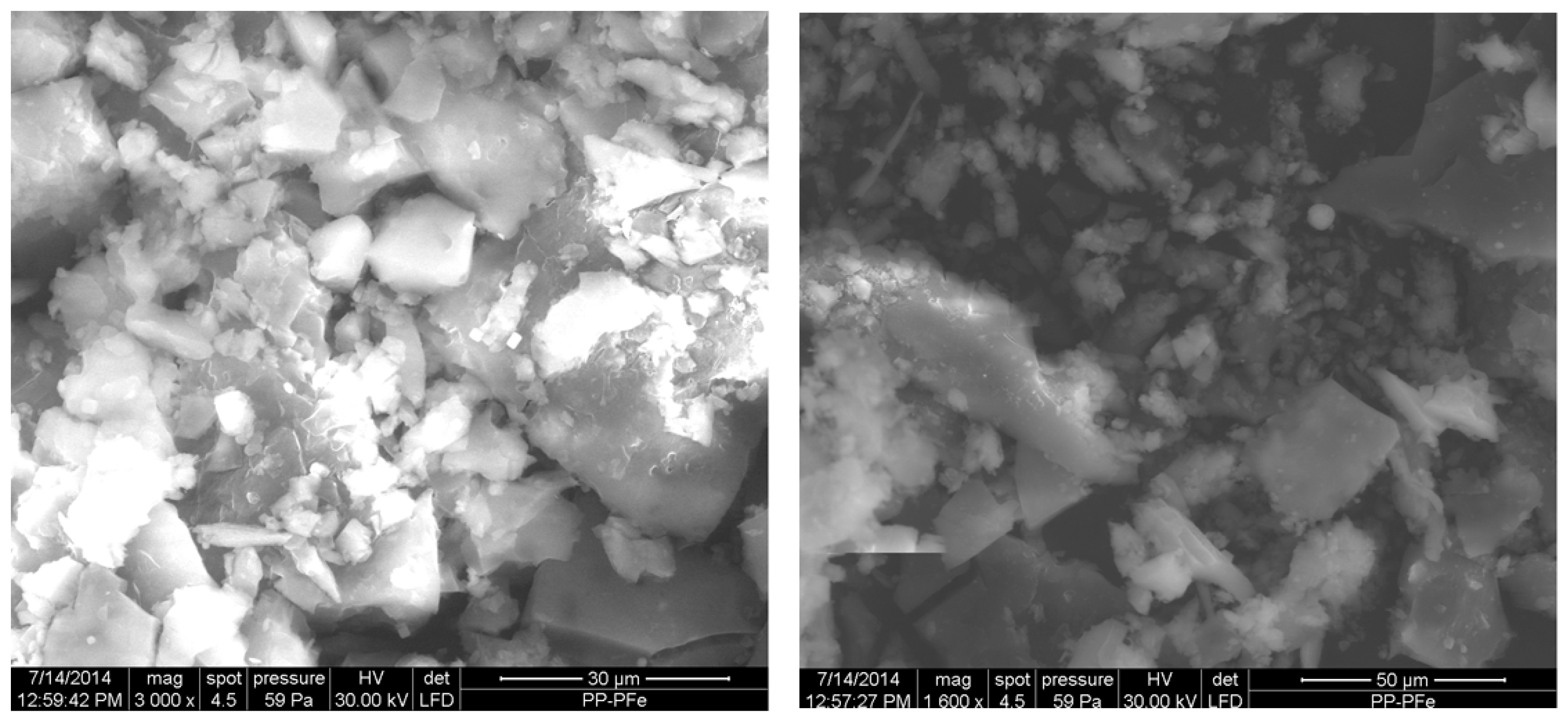
2.6. Microscopic Analysis
3. Materials and Methods
3.1. Materials
3.2. Syntheses
3.2.1. Method for Synthesis of Compound 1
3.2.2. Method for Synthesis of Compound 2
3.2.3. Method for Synthesis of Compound 3
3.2.4. The Main Characteristics of the Heterodimer Complex Compound 3
3.3. Apparatus
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Zhao, H.; Hua, J.; Qin, F.; Zheng, Y.; Zhang, Z.; Cao, W. Ratiometric oxygen sensing using the tunable ratio of phosphorescence to fluorescence emissions from gadolinium porphyrin and porphyrin. J. Lumin. 2017, 183, 452–457. [Google Scholar] [CrossRef]
- Filatov, M.A.; Heinrich, E.; Busko, D.; Ilieva, I.Z.; Landfester, K.; Baluschev, S. Reversible oxygen addition on a triplet sensitizer molecule: Protection from excited state depopulation. Phys. Chem. Chem. Phys. 2015, 17, 6501–6510. [Google Scholar] [CrossRef] [PubMed]
- Wiskur, S.L.; Ait-Haddou, H.; Lavigne, J.J.; Anslyn, E.V. Teaching Old Indicators New Tricks. Acc. Chem. Res. 2001, 34, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Drain, C.; Varotto, M.; Radivojevic, I. Self-organized porphyrinic materials. Chem. Rev. 2009, 9, 1630–1658. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.B.; Gu, W.; Huang, H.X.; Wang, J.B.; Shen, W.X.; Lv, Y.Y.; Shen, J. A porphyrin-based fluorescent probe for optical detection of toxic Cd2+ ion in aqueous solution and living cells. Dyes Pigments 2017, 143, 427–435. [Google Scholar] [CrossRef]
- Vlascici, D.; Fagadar-Cosma, E.; Popa, I.; Chiriac, V.; Gil-Agusti, M. A Novel Sensor for Monitoring of Iron(III) Ions Based on Porphyrins. Sensors 2012, 12, 8193–8203. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Zhang, L.N.; Zhu, L.N.; Zhang, R.; Li, X.Z.; Kong, D.M. A water-soluble, cationic bis-porphyrin exhibiting a more sensitive fluorescent response to Cu2+ relative to its monomeric counterpart. Sens. Actuators B 2017, 247, 179–187. [Google Scholar] [CrossRef]
- Cristescu, R.; Popescu, C.; Popescu, A.C.; Mihailescu, I.N.; Ciucu, A.A.; Andronie, A.; Iordache, S.; Stamatin, I.; Fagadar-Cosma, E.; Chrisey, D.B. Functional porphyrin thin films deposited by matrix assisted pulsed laser evaporation. Mater. Sci. Eng. B 2010, 169, 106–110. [Google Scholar] [CrossRef]
- Vlascici, D.; Pruneanu, S.; Olenic, L.; Pogacean, F.; Ostafe, V.; Chiriac, V.; Pica, E.M.; Bolundut, L.C.; Nica, L.; Fagadar-Cosma, E. Manganese(III) Porphyrin-based Potentiometric Sensors for Diclofenac Assay in Pharmaceutical Preparations. Sensors 2010, 10, 8850–8864. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.; Sankar, M. Borylated porphyrin and its metal complexes: Synthesis, electrochemistry and deprotection-protection strategy for anion sensing. Sens. Actuators B 2017, 240, 709–717. [Google Scholar] [CrossRef]
- Popescu, M.; Simandan, I.D.; Sava, F.; Velea, A.; Fagadar-Cosma, E. Sensor of Nitrogen Dioxide Based on Single Wall Carbon Nanotubes and Manganese-Porphyrin. Dig. J. Nanomater. Biostruct. 2011, 6, 1253–1256. [Google Scholar]
- Fagadar-Cosma, E.; Vlascici, D.; Fagadar-Cosma, G.; Palade, A.; Lascu, A.; Creanga, I.; Birdeanu, M.; Cristescu, R.; Cernica, I. A Sensitive A3B Porphyrin Nanomaterial for CO2 Detection. Molecules 2014, 19, 21239–21252. [Google Scholar] [CrossRef] [PubMed]
- Sebarchievici, I.; Tăranu, B.O.; Birdeanu, M.; Rus, S.F.; Făgădar-Cosma, E. Electrocatalytic behavior and application of manganese porphyrin/gold nanoparticle-surface modified glassy carbon electrodes. Appl. Surf. Sci. 2016, 390, 131–140. [Google Scholar] [CrossRef]
- Zhao, H.; Zang, L.; Wang, L.; Qin, F.; Zhang, Z.; Cao, W. Luminescence ratiometric oxygen sensor based on gadolinium labeled porphyrin and filter paper. Sens. Actuators B 2015, 215, 405–411. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Sebarchievici, I.; Lascu, A.; Creanga, I.; Palade, A.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, G. Optical and electrochemical behavior of new nano-sized complexes based on gold-colloid and Co-porphyrin derivative in the presence of H2O2. J. Alloys Compd. 2016, 686, 896–904. [Google Scholar] [CrossRef]
- Tanaka, Y.; Saito, S.; Mori, S.; Aratani, N.; Shinokubo, H.; Shibata, N.; Higuchi, Y.; Yoon, Z.S.; Kim, K.S.; Noh, S.B.; et al. Metalation of Expanded Porphyrins: A Chemical Trigger Used To Produce Molecular Twisting and Möbius Aromaticity. Angew. Chem. Int. Ed. 2008, 47, 614–617. [Google Scholar] [CrossRef]
- Dehghani, H.; Shaterian, M. Synthesis of new ionic intermediate sitting-atop complexes of free base meso-tetraarylporphyrin and phosphorus(V) chloride under solvent free conditions. Inorg. Chim. Acta 2009, 362, 2868–2871. [Google Scholar] [CrossRef]
- Dehghani, H.; Shaterian, M. Synthesis under solvent free conditions and photoluminescence study of ionic intermediate sitting-atop complexes of meso-tetraarylporphyrins and phosphorus oxychloride. Inorg. Chim. Acta 2009, 362, 5151–5154. [Google Scholar] [CrossRef]
- Hirakawa, K.; Fukunaga, N.; Nishimura, Y.; Arai, T.; Okazaki, S. Photosensitized protein damage by dimethoxyphosphorus(V) tetraphenylporphyrin. Bioorg. Med. Chem. Lett. 2013, 23, 2704–2707. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.A.; Ebrahim, M.M.; Petitdemange, R.; Vaz, G.M.; Paszko, E.; Sergeeva, N.N.; Senge, M.O. Lead structures for applications in photodynamic therapy. 5. Synthesis and biological evaluation of water soluble phosphorus (V) 5,10,15,20-tetraalkylporphyrins for PDT. Photodiagn. Photodyn. Ther. 2014, 11, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.T.; Liu, I.C.; Cheng, P.C.; Lin, C.C.; Chen, J.H.; Wang, S.S.; Zeng, W.F. Structure of (meso-5,10,15,20-tetraphenylporphyrinato)dichlorophosphorus (V) chloride. J. Chem. Crystallogr. 1995, 25, 231–235. [Google Scholar] [CrossRef]
- Młodzianowska, A.; Latos-Grażyński, L.; Szterenberg, L. Phosphorus Complexes of N-Fused Porphyrin and Its Reduced Derivatives: New Isomers of Porphyrin Stabilized via Coordination. Inorg. Chem. 2008, 47, 6364–6374. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, M.C.; Burrell, A.K.; Boyd, P.D.W.; Brothers, P.J.; Rickard, C.E.F. Synthesis, structure and properties of ferrocene functionalized porphyrins. J. Porph. Phthal. 2002, 6, 737–747. [Google Scholar] [CrossRef]
- Giribabu, L.; Kandhadi, J.; Kanaparthi, R.K. Phosphorus(V)corrole-Porphyrin Based Hetero Trimers: Synthesis, Spectroscopy and Photochemistry. J. Fluoresc. 2013. [Google Scholar] [CrossRef] [PubMed]
- Roales, J.; Pedrosa, J.M.; Guillén, M.G.; Lopes-Costa, T.; Pinto, S.M.A.; Calvete, M.J.F.; Pereira, M.M. Optical detection of amine vapors using ZnTriadporphyrin thin films. Sens. Actuators B 2015, 210, 28–35. [Google Scholar] [CrossRef]
- Zagami, R.; Castriciano, M.A.; Romeo, A.; Trapani, M.; Pedicini, R.; Scolaro, L.M. Tuning supramolecular chirality in nano and mesoscopicporphyrin J aggregates. Dyes Pigments 2017, 142, 255–261. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Fagadar-Cosma, G.; Vasile, M.; Enache, C. Synthesis, spectroscopic and self-assembling characterization of novel photoactive mixed aryl-substituted porphyrin. Curr. Org. Chem. 2012, 16, 931–941. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Tudose, R.; Armeanu, I.; Mosoarca, E.; Vlascici, D.; Costisor, O. UV-vis and fluorescence spectra of meso-tetraphenylporphyrin and meso-tetrakis-(4-methoxyphenyl) porphyrin in THF and THF-water systems. The influence of pH. Rev. Chim. Bucharest 2007, 58, 451–455. [Google Scholar]
- Mak, C.A.; Pericas, M.A.; Fagadar-Cosma, E. Functionalization of A3B-type porphyrin with Fe3O4 MNPs. Supramolecular assemblies, gas sensor and catalytic applications. Catal. Today 2017. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Fagadar-Cosma, G. [(p-Tolyl)Dichlorophosphine and Di(p-Tolyl)-Chlorophosphine – Sources of New Organophosphorus(III) And (V) Compounds. Rev. Roum. Chim. 2003, 48, 211–217. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, S.; Seol, H.; You, J.M.; Jeong, E.S.; Kim, S.K.; Seo, K.S.; Jeon, S. Determination of hydrogen peroxide on modified glassy carbon electrode by polytetrakis(2-aminophenyl)porphyrin nanowire. Bull. Korean Chem. Soc. 2009, 30, 2979–2983. [Google Scholar] [CrossRef]
- Kaçar, C.; Dalkiran, B.; Erden, P.E.; Kiliç, E. An amperometric hydrogen peroxide biosensor based on Co3O4 nanoparticles and multiwalled carbon nanotube modified glassy carbon electrode. Appl. Surf. Sci. 2014, 311, 139–146. [Google Scholar] [CrossRef]
- Filatov, M.A.; Senge, M.O. Molecular devices based on reversible singlet oxygen binding in optical and photomedical applications. Mol. Syst. Des. Eng. 2016, 1, 258–272. [Google Scholar] [CrossRef]
- Moylan, C.; Scanlan, E.M.; Senge, M.O. Chemical synthesis and medicinal applications of glycoporphyrins. Curr. Med. Chem. 2015, 22, 2238–2348. [Google Scholar] [CrossRef]
- Rogers, L.; Sergeeva, N.N.; Paszko, E.; Vaz, G.M.F.; Senge, M.O. Lead structures for applications in photodynamic therapy. 6. Temoporfin anti-inflammatory conjugates to target the tumor microenvironment for in vitro PDT. PLoS ONE 2015, 10, e0125372. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Stafford, S. Getting it right: 3D cell cultures for the assessment of photosensitizers for photodynamic therapy. Future Med. Chem. 2015, 7, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Kiran, P.P.; Reddy, D.R.; Maiya, B.G.; Rao, D.N. Third-order nonlinearity and optical limiting studies in phosphorus (V) porphyrins with charge transfer states. Opt. Mat. 2002, 21, 565–568. [Google Scholar] [CrossRef]
- Matsumoto, J.; Shinbara, T.; Tanimura, S.; Matsumoto, T.; Shiragami, T.; Yokoi, H.; Nosaka, Y.; Okazaki, S.; Hirakawa, K.; Yasuda, M. Water-soluble phosphorus porphyrins with high activity for visible light-assisted inactivation of Saccharomyces cerevisiae. J. Photochem. Photobiol. A Chem. 2011, 218, 178–184. [Google Scholar] [CrossRef]
- Baschir, L.; Fagadar-Cosma, E.; Creanga, I.; Palade, A.; Lascu, A.; Birdeanu, M.; Savastru, D.; Savu, V.; Antohe, S.; Velea, A.; et al. UV sensing effect in Langmuir-Blodgett complex films containing a novel synthesized Fe(III) porphyrin. Dig. J. Nanomater. Biostruct. 2014, 9, 847–857. [Google Scholar]
- Mohajer, D.; Dehghani, H. Preparation and spectroscopic characterization of 2:1 molecular complexes of tetracyanoethylene and meso-tetraphenylporphyrins. Bull. Chem. Soc. Jpn. 2000, 73, 1477–1484. [Google Scholar] [CrossRef]
- Mohajer, D.; Rayati, S. Novel 1:2 molecular complexation of free base mesotetraarylporphyrins with σ-acceptor trialkylsilyl chlorides. New J. Chem. 2003, 27, 242–244. [Google Scholar] [CrossRef]
- Dehghani, H.; Mansournia, M.R. Novel sitting-atop complexation between uranyl and meso-tetraarylporphyrins under mild conditions. Polyhedron 2008, 27, 849–853. [Google Scholar] [CrossRef]
- Dehghani, H.; Payam, M.; Mansournia, M.R. Sitting-atop complex formation of free base meso-tetraarylporphyrins with zirconium(IV) chloride. Polyhedron 2008, 27, 2416–2420. [Google Scholar] [CrossRef]
- Dehghani, H.; Fathi, F. Molecular complexation of meso-tetraarylporphyrins with SO2. Dyes Pigments 2008, 77, 323–326. [Google Scholar] [CrossRef]
- Kuoimoto, K.; Segawa, H.; Sbimidzu, T. Selective Synthesis of Unsymmetrical Dialkoxyphosphorus(V)tetraphenylporphine Derivatives by Stepwise Substitution of Axial Position. Tetrahedron Lett. 1992, 33, 6327–6330. [Google Scholar] [CrossRef]
- Abedian, N.; Dehghani, H. Novel molecular complexation between meso-tetraarylporphyrinato magnesium(II) and phosphorus(III) chloride. Inorg. Chem. Commun. 2013, 36, 77–80. [Google Scholar] [CrossRef]
- Thomas, L.C. Interpretation of the Infrared Spectra of Organophosphorus Compounds; Heyden: London, UK, 1975; p. 257. [Google Scholar]
- Cunningham, I.D.; Danks, T.N.; O’Connell, K.T.A.; Scott, P.W. Kinetics and mechanism of the hydrogen peroxide oxidation of a pentafluorophenyl-substituted iron(III) porphyrin. J. Chem. Soc. Perkin Trans. 1999, 2, 2133–2139. [Google Scholar] [CrossRef]
- Brausam, A.; Eigler, S.; Jux, N.; van Eldik, R. Mechanistic Investigations of the Reaction of an Iron(III) Octa-Anionic Porphyrin Complex with Hydrogen Peroxide and the Catalyzed Oxidation of Diammonium-2,20-azinobis(3-ethylbenzothiazoline-6-sulfonate). Inorg. Chem. 2009, 48, 7667–7678. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.A.; Bell, A.T. A Study of the Mechanism and Kinetics of Cyclooctene Epoxidation Catalyzed by Iron(III) Tetrakispentafluorophenyl Porphyrin. J. Am. Chem. Soc. 2005, 127, 8635–8643. [Google Scholar] [CrossRef] [PubMed]
- Traylor, T.G.; Xu, F. Mechanisms of Reactions of Iron(III) Porphyrins with Hydrogen Peroxide and Hydroperoxides: Solvent and Solvent Isotope Effects. J. Am. Chem. Soc. 1990, 112, 178–186. [Google Scholar] [CrossRef]
- Hirakawa, K.; Kawanishi, S.; Hirano, T.; Segawa, H. Guanine-specific DNA oxidation photosensitized by the tetraphenylporphyrin phosphorus(V) complex via singlet oxygen generation and electron transfer. J. Photochem. Photobiol. B Biol. 2007, 87, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, D.; Madejska, I.; Habdas, J.; Dudkowiak, A. The spectroscopic characterisation of proline derivatives of tolyl-porphyrins and their iron and cobalt complexes. J. Mol. Struct. 2008, 876, 177–185. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Vlascici, D.; Fagadar-Cosma, G.; Vasile, M.; Bazylak, G. Novel nanomaterials based on 5,10,15,20-tetrakis(3,4-dimethoxyphenyl)-21H,23H-porphyrin entrapped in silica matrices. Mat. Res. Bull. 2009, 44, 2186–2193. [Google Scholar] [CrossRef]
- Carrano, C.J.; Tsutsui, M. Unusual metalloporphyrins. Phosphorus complexes of tetraphenylporphyrine. J. Coord. Chem. 1977, 7, 79–83. [Google Scholar] [CrossRef]
- Marrese, C.A.; Carrano, C.J. Synthesis, characterization, and electrochemistry of (5,10,15,20-tetraphenylporphinato)dichlorophosphorus(V) chloride. Inorg. Chem. 1983, 22, 1858–1862. [Google Scholar] [CrossRef]
- Dehghani, H.; Shaterian, M. Synthesis of intermediate sitting-atop complexes (i-SAT) from the reaction between free base meso-tetraarylporphyrins and phosphorus(III) chloride in solvent free media. Polyhedron 2008, 27, 3263–3266. [Google Scholar] [CrossRef]
- Zha, Q.; Rui, X.; Wei, T.; Xie, Y. Recent advances in the design strategies for porphyrin-based coordination polymers. CrystEngComm 2014, 16, 7371–7384. [Google Scholar] [CrossRef]
- Ghosh, M.; Mora, A.K.; Nath, S.; Chandra, A.K.; Hajra, A.; Sinha, S. Photophysics of Soret-excited free base tetraphenylporphyrin and its zinc analog in solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 466–472. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



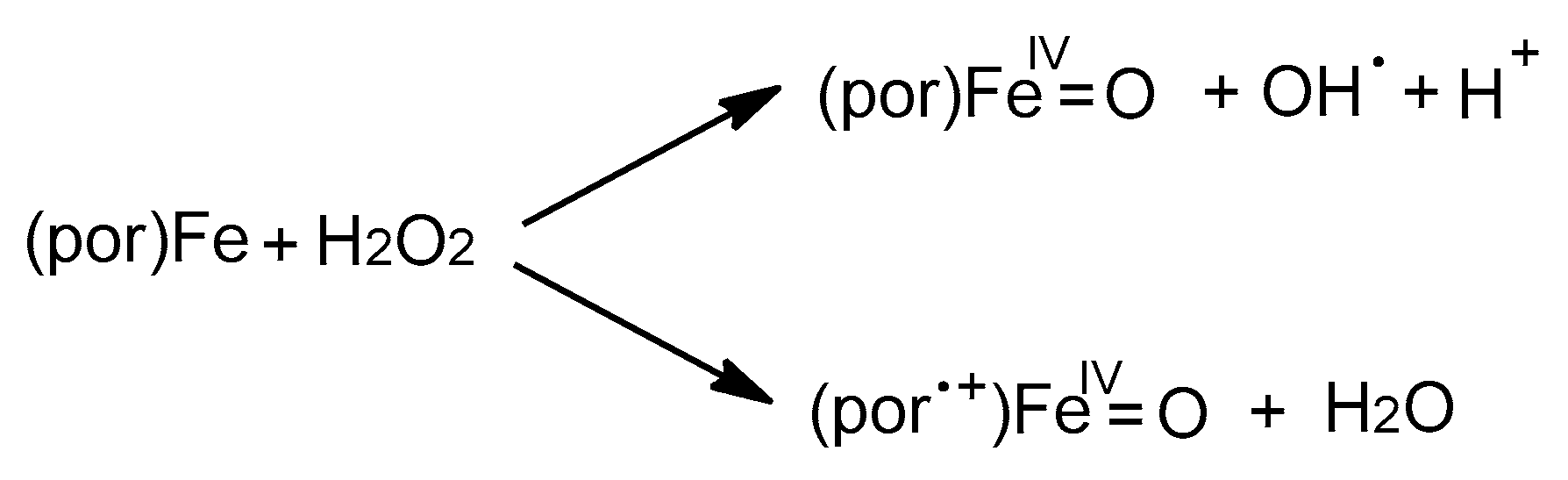
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagadar-Cosma, E.; Badea, V.; Fagadar-Cosma, G.; Palade, A.; Lascu, A.; Fringu, I.; Birdeanu, M. Trace Oxygen Sensitive Material Based on Two Porphyrin Derivatives in a Heterodimeric Complex. Molecules 2017, 22, 1787. https://doi.org/10.3390/molecules22101787
Fagadar-Cosma E, Badea V, Fagadar-Cosma G, Palade A, Lascu A, Fringu I, Birdeanu M. Trace Oxygen Sensitive Material Based on Two Porphyrin Derivatives in a Heterodimeric Complex. Molecules. 2017; 22(10):1787. https://doi.org/10.3390/molecules22101787
Chicago/Turabian StyleFagadar-Cosma, Eugenia, Valentin Badea, Gheorghe Fagadar-Cosma, Anca Palade, Anca Lascu, Ionela Fringu, and Mihaela Birdeanu. 2017. "Trace Oxygen Sensitive Material Based on Two Porphyrin Derivatives in a Heterodimeric Complex" Molecules 22, no. 10: 1787. https://doi.org/10.3390/molecules22101787
APA StyleFagadar-Cosma, E., Badea, V., Fagadar-Cosma, G., Palade, A., Lascu, A., Fringu, I., & Birdeanu, M. (2017). Trace Oxygen Sensitive Material Based on Two Porphyrin Derivatives in a Heterodimeric Complex. Molecules, 22(10), 1787. https://doi.org/10.3390/molecules22101787





