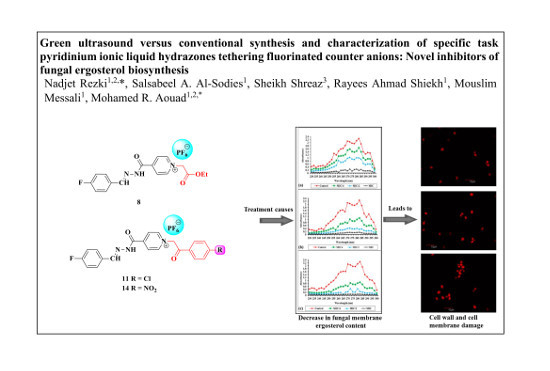
Green Ultrasound versus Conventional Synthesis and Characterization of Specific Task Pyridinium Ionic Liquid Hydrazones Tethering Fluorinated Counter Anions: Novel Inhibitors of Fungal Ergosterol Biosynthesis
Abstract
:1. Introduction
2. Results and Discussion
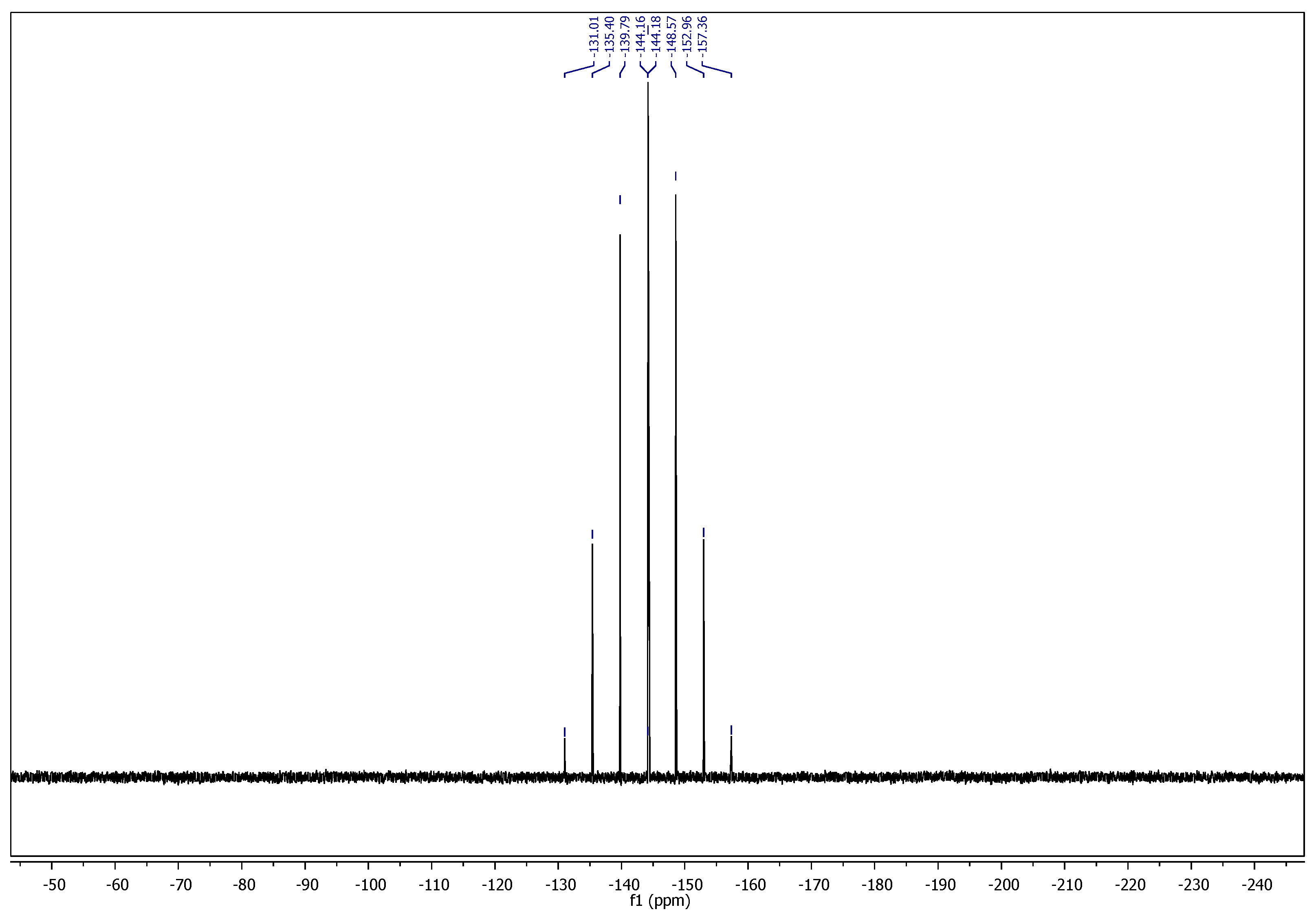
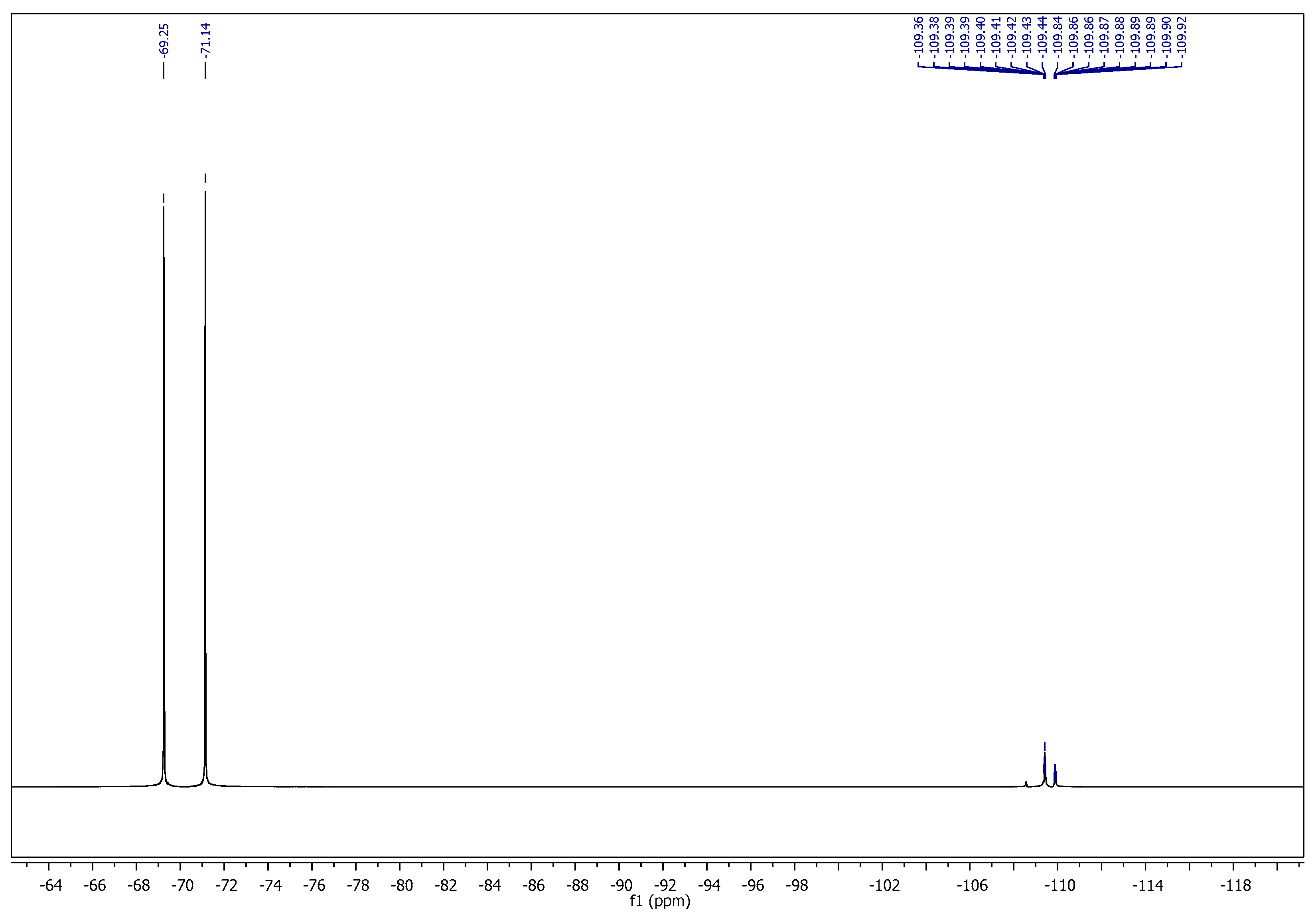
2.1. Chemistry
2.2. Antifungal Screening
2.2.1. Determination of MIC90
2.2.2. Effect of the Synthesized Compounds on Membrane Ergosterol Content
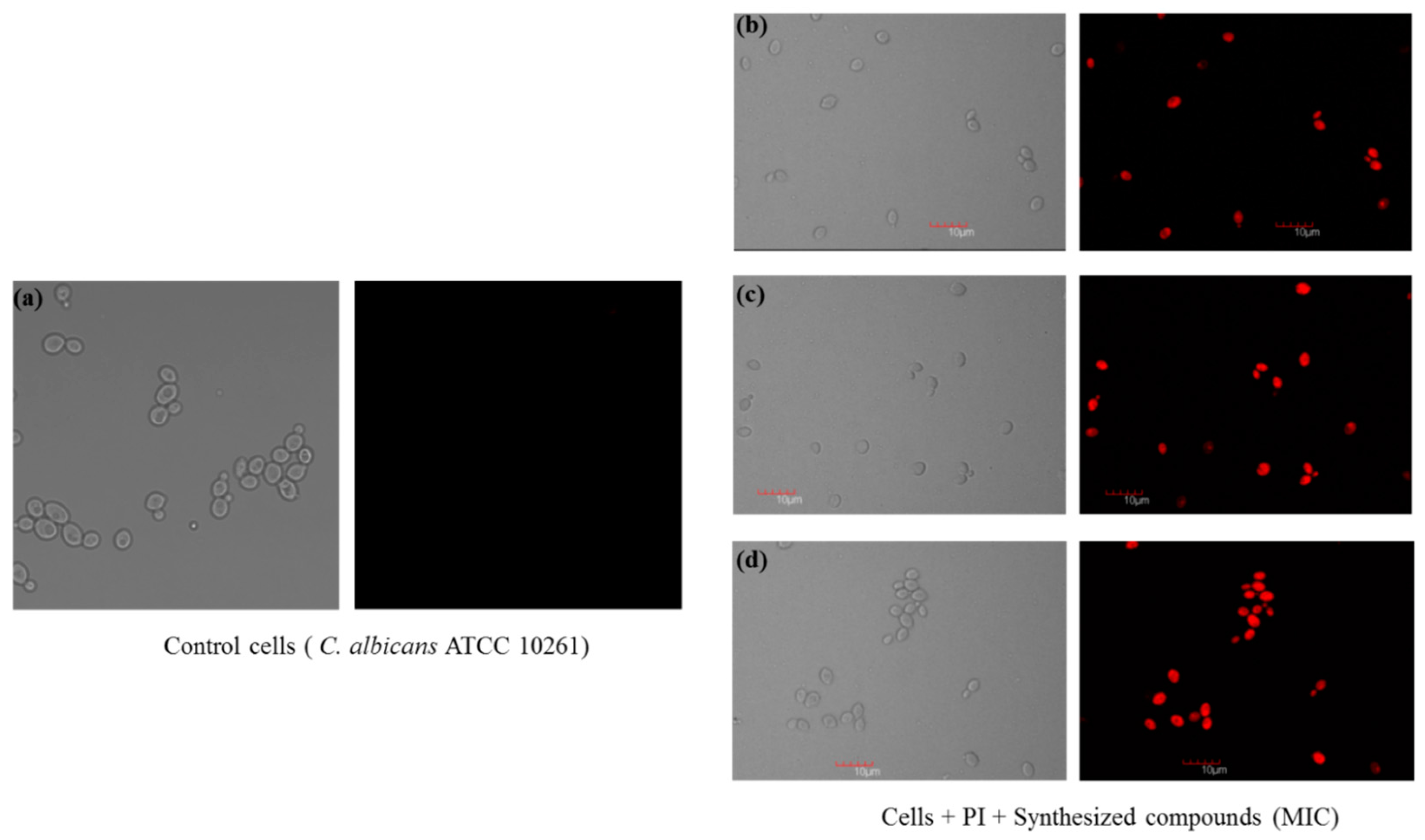
2.2.3. Confocal Scanning Laser Microscopy (CSLM)
3. Experimental Section
3.1. Chemistry
3.2. General Alkylation Procedure for the Synthesis of Halogenated Specific Task ILs Tagged Hydrazones 2–7
3.2.1. Conventional Method (CM)
3.2.2. Ultrasound Method (US)
3.3. General Metathesis Procedure for the Synthesis of Fluorinated Specific Task ILs 8–25
3.3.1. Conventional Method (CM)
3.3.2. Ultrasound Method (US)
3.4. Antifungal Screening
3.4.1. Growth Conditions, Species Identification and Determination of MIC90
3.4.2. Ergosterol Extraction and Estimation Assay
3.4.3. Confocal Scanning Laser Microscopy (CSLM)
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Conflicts of Interest
References
- Paluchowska, P.; Tokarczyk, M.; Bogusz, B.; Skiba, I.; Budak, A. Molecular epidemiology of Candida albicans and Candida glabrata strains isolated from intensive care unit patients in Poland. Mem. Inst. Oswaldo Cruz. 2014, 109, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Rathor, N.; Khillan, V.; Sarin, S.K. Nosocomial candiduria in chronic liver disease patients at a hepatobilliary center. Indian J. Crit. Care Med. 2014, 18, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Garbino, J.; Pittet, D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 2003, 3, 685–702. [Google Scholar] [CrossRef]
- Abi-Said, D.; Anaissie, E.; Uzun, I.; Raad, O.; Pinzcowski, H.; Vartivarian, S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 1997, 24, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.E. Pseudomonas aeruginosa, Candida albicans, and device-related nosocomial infections: Implications, trends, and potential approaches for control. J. Ind. Microbiol. Biotechnol. 2005, 32, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Laniado-Laborin, R.; Cabrales-Vargas, M.N. Amphotericin: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.P.; DeFeiter, P.W.; VanderKuy, P.H.; VanMook, W.N. Long QTc interval and torsade de pointes caused by fluconazole. Ann. Pharmacother. 2006, 40, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Itoga, M.; Aoki, S.; Suzuki, A.; Yoshida, Y.; Fujinami, Y.; Masuko, M. Toward resolving anxiety about the accelerated corrosive wear of steel lubricated with the fluorine-containing ionic liquids at elevated temperature. Tribol. Int. 2016, 93, 640–650. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Muñoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. Performance of solid state supercapacitors based on polymer electrolytes containing different ionic liquids. J. Power Sources 2016, 326, 560–568. [Google Scholar] [CrossRef]
- Andreeva, N.A.; Chaban, V.V. Amino-functionalized ionic liquids as carbon dioxide scavengers. Ab initio thermodynamics for chemisorption. J. Chem. Thermodyn. 2016, 103, 1–6. [Google Scholar] [CrossRef]
- Savest, N.; Plamus, T.; Tarasova, E.; Viirsalu, M.; Krasnou, I.; Gudkova, V.; Küppar, K.; Krumme, A. The effect of ionic liquids on the conductivity of electrospun polyacrylonitrile membranes. J. Electrost. 2016, 83, 63–68. [Google Scholar] [CrossRef]
- Zhai, W.; Zhu, H.; Wang, L.; Liu, X.; Yang, H. Study of PVDF-HFP/PMMA blended micro-porous gel polymer electrolyte incorporating ionic liquid [BMIM]BF4 for Lithium ion batteries. Electrochim. Acta 2014, 133, 623–630. [Google Scholar] [CrossRef]
- Al-Aqmar, D.M.; Abdelkader, H.I.; AbouKana, M.T.H. Optical, photo-physical properties and photostability of pyrromethene (PM-597) in ionic liquids as benign green-solvents. J. Lumin. 2015, 161, 221–228. [Google Scholar] [CrossRef]
- Poole, C.F.; Lenca, N. Green sample-preparation methods using room temperature ionic liquids for the chromatographic analysis of organic compounds. Trends Anal. Chem. 2015, 71, 144–156. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Faustino, V.F.M.; Monda, D.; Coutinho, J.A.P.; Freire, M.G. Improving the extraction and purification of immunoglobulin G bythe use of ionic liquids as adjuvants in aqueous biphasic systems. J. Biotechnol. 2016, 236, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L. Develop ionic liquid drugs. Update regulation to spur research into drugs that the body absorbs more easily and that could reach market more quickly, urge. Nature 2015, 528, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Shirkhanloo, H.; Ghazaghi, M.; Mousavi, H.Z. Cadmium determination in human biological samples based on trioctylmethyl ammonium thiosalicylate as a task specific ionic liquid by dispersive liquid–liquid microextraction method. J. Mol. Liq. 2016, 218, 478–483. [Google Scholar] [CrossRef]
- Hernoux-Villière, A.; Lévêque, J.; Kärkkäinen, J.; Papaiconomou, N.; Lajunen, M.; Lassi, U. Task-specific ionic liquid for the depolymerisation of starch-based industrial waste into high reducing sugars. Catal. Today 2014, 223, 11–17. [Google Scholar] [CrossRef]
- Da, Y.; Yuan, W.; Xin, T.; Nie, Y.; Ye, Y.; Yan, Y.; Liang, L.; Chen, Z. Synthesis and biological evaluation of new fluorine substituted derivatives as angiotensin II receptor antagonists with anti-hypertension and anti-tumor effects. Bioorg. Med. Chem. 2012, 20, 7101–7111. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.L. Fluorine in medicinal chemistry: Recent therapeutic applications of fluorinated small molecules. J. Fluor. Chem. 2006, 127, 1013–1029. [Google Scholar] [CrossRef]
- Kirk, K.L. Selective fluorination in drug design and development: An overview of biochemical rationales. Curr. Top. Med. Chem. 2006, 6, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Unzner, T.A.; Magauer, T. Carbon–fluorine bond activation for the synthesis of functionalized molecules. Tetrahedron Lett. 2015, 56, 877–883. [Google Scholar] [CrossRef]
- Václavík, J.; Chernykh, Y.; Jurásek, B.; Beier, P. Nucleophilic tetrafluoroethylation of carbonyl compounds with fluorinated sulfones. J. Fluorine Chem. 2015, 169, 24–31. [Google Scholar] [CrossRef]
- Rezki, N.; Al-Sodies, S.A.; Aouad, M.R.; Bardaweel, S.; Messali, M.; ElAshry, E.S.H. An eco-friendly ultrasound-assisted synthesis ofnovel fluorinated pyridinium salts-based hydrazones and antimicrobial and antitumor screening. Int. J. Mol. Sci. 2016, 17, 766–785. [Google Scholar] [CrossRef] [PubMed]
- Shreaz, S.; Sheikh, R.A.; Rimple, B.; Hashmi, A.A.; Nikhat, M.; Khan, L.A. Anticandidal activity of cinnamaldehyde, its ligand and Ni(II) complex: Effect of increase in ring and side chain. Microb. Pathog. 2010, 49, 75. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, R.A.; Shreaz, S.; Malik, M.A.; Khan, L.A.; Hashmi, A.A. Spectroscopic elucidation of new metal hetroscorpionates: A novel class of antifungal and antibacterial agents. J. Chem. Pharm. Res. 2010, 2, 133–146. [Google Scholar]
- Sheikh, R.A.; Shreaz, S.; Sharma, G.S.; Khan, L.A.; Hashmi, A.A. Synthesis, characterization and antimicrobial screening of a novel organylborate ligand, potassium hydro (phthalyl)(salicylyl) borate and its Co (II), Ni (II), and Cu (II) complexes. J. Saud. Chem. Soc. 2012, 16, 353–361. [Google Scholar] [CrossRef]
- Sheikh, R.A.; Wani, M.Y.; Shreaz, S.; Hashmi, A.A. Synthesis, characterization and biological screening of some Schiff base macrocyclic ligand based transition metal complexes as antifungal agents. Arab. J. Chem. 2016, 9, S743–S751. [Google Scholar] [CrossRef]
- Sharma, G.S.; Sheikh, R.A.; Shreaz, S.; Hashmi, A.A.; Khan, L.A. Synthesis, characterization and antimicrobial activity of potassium hydro (benzoyl)(phthalyl) borate and Its Cobalt (II), Nickel (II), and Copper (II) Complexes. Chin. J. Chem. 2009, 27, 1300–1306. [Google Scholar] [CrossRef]
- Shiekh, R.A.; Shreaz, S.; Khan, L.A.; Hashmi, A.A. Development and characterization of bioactive macrocyclic metal complexes, use as a potential drug. J. Chem. Pharm. Res. 2010, 2, 172–185. [Google Scholar]
- Messali, M.; Aouad, M.R.; Ali, A.A.-S.; Rezki, N.; Ben Hadda, T.; Hammouti, B. Synthesis, characterization, and POM analysis of novel bioactive imidazolium-based ionic liquids. Med. Chem. Res. 2015, 24, 1387–1395. [Google Scholar] [CrossRef]
- Messali, M.; Aouad, M.R.; El-Sayed, W.S.; Ali, A.A.-S.; Ben Hadda, T.; Hammouti, B. New eco-Friendly 1-alkyl-3-(4-phenoxybutyl) imidazolium-based ionic liquids derivatives: A green ultrasound-assisted synthesis, characterization, antibacterial activity and POM analyses. Molecules 2014, 19, 11741–11759. [Google Scholar] [CrossRef] [PubMed]
- Messali, M.; Almtiri, M.N.; Abderrahman, B.; Salghi, R.; Aouad, M.R.; Alshahateet, S.F.; Ali, A.A.-S. New pyridazinium-based ionic liquids: An eco-friendly ultrasound-assisted synthesis, characterization and biological activity. S. Afr. J. Chem. 2015, 68, 219–225. [Google Scholar]
- Chatel, G.; MacFarlane, D.R. Ionic liquids and ultrasound in combination: Synergies and challenges. Chem. Soc. Rev. 2014, 43, 8132–8149. [Google Scholar] [CrossRef] [PubMed]
- Marullo, S.; D’Anna, F.; Rizzo, C.; Noto, R. The ultrasounds–ionic liquids synergy on the copper catalyzed azide–alkyne cycloaddition between phenylacetylene and 4-azidoquinoline. Ultrason. Sonochem. 2015, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Messali, M. Conventional vs. ultrasound and microwave assisted synthesis: Some new environmentally friendly functionalized picolinium-based ionic liquids with potential antibacterial activity. Acta Pharm. 2015, 65, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Rezki, N.; Al-Yahyawi, A.M.; Bardaweel, S.K.; Al-Blewi, F.F.; Aouad, M.R. Synthesis of novel 2,5-disubstituted-1,3,4-thiadiazoles clubbed 1,2,4-triazole, 1,3,4-thiadiazole, 1,3,4-oxadiazole and/or schiff base as potential antimicrobial and antiproliferative agents. Molecules 2015, 20, 16048–16067. [Google Scholar] [CrossRef] [PubMed]
- Czub, J.; Baginski, M. Comparative molecular dynamics study of lipid membranes containing cholesterol and ergosterol. Biophys. J. 2006, 90, 2368–2382. [Google Scholar] [CrossRef] [PubMed]
- de Kroon, A.I.; Rijken, P.J.; De Smet, C.H. Checks and balances in membrane phospholipid class and acyl chain homeostasis, the yeast perspective. Prog. Lipid Res. 2013, 52, 374–394. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [PubMed]
- National Committee on Clinical Laboratory Standards (NCCLS). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard M27-A2; National Committee on Clinical Laboratory Standards: Wayne, PA, USA, 2002. [Google Scholar]
- Shreaz, S.; Sheikh, R.A.; Bhatia, R.; Neelofar, K.; Imran, S.; Hashmi, A.A.; Manzoor, N.; Basir, S.F.; Khan, L.A. Antifungal activity of α-methyl trans cinnamaldehyde, its ligand and metal complexes: Promising growth and ergosterol inhibitors. Biometals 2011, 24, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Influences of cinnamicaldehydes on H+ extrusion activity and ultrastructure of Candida. J. Med. Microbiol. 2013, 62, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Shreaz, S.; Bhatia, R.; Ahmad, S.I.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Anticandidal activity of curcumin and methyl cinnamaldehyde. Fitoterapia 2012, 83, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, N.P.; Alam, M.; Shreaz, S.; Hashmi, A.A. Synthesis, characterization and biological studies of oil based tin polymer. J. Inorg. Organomet. Polym. Mat. 2009, 19, 459–574. [Google Scholar] [CrossRef]
- Bharathi, N.P.; Khan, N.U.; Lam, M.A.; Shreaz, S.; Hashmi, A.A. Cadmium incorporated oil based bioactive polymers: Synthesis, characterization and physico-chemical studies. J. Inorg. Organom. Pol. Mat. 2010, 20, 833–840. [Google Scholar] [CrossRef]
- Shreaz, S.; Bhatia, R.; Khan, N.; Maurya, I.K.; Ahmad, S.I.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Cinnamic aldehydes affect hydrolytic enzyme secretion and morphogenesis in oral Candida isolates. Microb. Pathog. 2012, 52, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Shreaz, S.; Manzoor, N.; Khan, L.A.; Rizvi, M.M.A. Anticandidal activity of Cassia fistula and its effect on ergosterol biosynthesis. Pharm. Biol. 2011, 49, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Basir, S.F.; Manzoor, N.; Khan, L.A. Exposure of Candida to p-anisaldehyde inhibits its growth and ergosterol biosynthesis. J. Gen. Appl. Microbiol. 2011, 57, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Neelofar, K.; Shreaz, S.; Rimple, B.; Muralidhar, S.; Nikhat, M.; Khan, L.A. Curcumin as a promising anticandidal of clinical interest. Can. J. Microbiol. 2011, 57, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Vaseem, R.; Sheikh, S.; Jawad, M.B.; Weqar, A.S. In vitro effect of ethanolic extract of Curcuma longa rhizome on growth and sterol synthesis in different Candida isolates. J. Pure Appl. Microbio. 2016, 10, 2231–2239. [Google Scholar]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Basir, S.F.; Manzoor, N.; Khan, L.A. Spice oil cinnamaldehyde exhibits potent anticandidal activity against fluconazole resistant clinical isolates. Fitoterapia 2011, 82, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Vaseem, R.; Shreaz, S.; Siddiqui, W.A. Curcuma longa rhizome extract as a promising source of anticandidal agents. Adv. Sci. Lett. 2014, 20, 1644–1649. [Google Scholar]
- Shreaz, S.; Shiekh, R.A.; Raja, V.; Wani, W.A.; Behbehani, J.M. Impaired ergosterol biosynthesis mediated fungicidal activity of Co(II) complex with ligand derived from cinnamaldehyde. Chem. Biol. Int. 2016, 247, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Ahmad, S.I.; Irshad, M.; Wani, W.A.; Siddiqi, W.A.; Shreaz, S. Anticandidal activity of ethanolic root extract of Juglans regia (L.): Effect on growth, cell morphology, and key virulence factors. J. Mycol. Med. 2017, 16, 30311–30320. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Compound No. | R | Conventional Method CM | Ultrasound Method US | ||
|---|---|---|---|---|---|
| Time (h) | Yield (%) | Time (h) | Yield (%) | ||
| 2 | CH2COOEt | 20 | 90 | 4 | 94 |
| 3 | p-ClC6H4COCH2 | 24 | 91 | 5 | 94 |
| 4 | p-NO2C6H4COCH2 | 24 | 80 | 5 | 88 |
| 5 | C6H5O(CH2)4 | 24 | 93 | 4 | 96 |
| 6 | C6H5CH2 | 40 | 89 | 6 | 92 |
| 7 | OH(CH2)2 | 48 | 80 | 6 | 88 |
| Compound No. | R | Y | Conventional Method CM | Ultrasound Method US | ||
|---|---|---|---|---|---|---|
| Time (h) | Yield (%) | Time (h) | Yield (%) | |||
| 8 | CH2COOEt | PF6 | 16 | 88 | 4 | 92 |
| 9 | CH2COOEt | BF4 | 16 | 85 | 4 | 90 |
| 10 | CH2COOEt | COOCF3 | 16 | 83 | 4 | 90 |
| 11 | p-ClC6H4COCH2 | PF6 | 16 | 90 | 4 | 94 |
| 12 | p-ClC6H4COCH2 | BF4 | 16 | 86 | 4 | 90 |
| 13 | p-ClC6H4COCH2 | COOCF3 | 16 | 92 | 4 | 96 |
| 14 | p-NO2C6H4COCH2 | PF6 | 16 | 89 | 4 | 92 |
| 15 | p-NO2C6H4COCH2 | BF4 | 16 | 84 | 4 | 92 |
| 16 | p-NO2C6H4COCH2 | COOCF3 | 16 | 91 | 4 | 94 |
| 17 | C6H5O(CH2)4 | PF6 | 16 | 84 | 5 | 92 |
| 18 | C6H5O(CH2)4 | BF4 | 16 | 84 | 5 | 90 |
| 19 | C6H5O(CH2)4 | COOCF3 | 16 | 89 | 5 | 92 |
| 20 | C6H5CH2 | PF6 | 16 | 94 | 5 | 96 |
| 21 | C6H5CH2 | BF4 | 16 | 88 | 5 | 92 |
| 22 | C6H5CH2 | COOCF3 | 16 | 86 | 5 | 90 |
| 23 | OH(CH2)2 | PF6 | 16 | 90 | 5 | 94 |
| 24 | OH(CH2)2 | BF4 | 16 | 83 | 5 | 90 |
| 25 | OH(CH2)2 | COOCF3 | 16 | 87 | 5 | 92 |
| Isolates/Strain | Species (Number of Strains) |
|---|---|
| Sensitive (standard, n = 4) | |
| ATCC 10261 | C. albicans |
| ATCC 90030 | C. glabrata |
| ATCC 750 | C. tropicalis |
| ATCC 6258 | C. krusei |
| Clinical isolates (n = 36) | C. albicans (19), C. tropicalis (5), C. glabrata (8), C. krusei (4) |
| Test Agents | MIC90 Range (μg/mL) |
|---|---|
| 2 | >1000 |
| 3 | >1000 |
| 4 | >1000 |
| 5 | >1000 |
| 6 | >1000 |
| 7 | 500–1000 |
| 8 | 62.5–250 |
| 9 | 250–500 |
| 10 | 250–500 |
| 11 | 31.25–62.5 |
| 12 | 125–250 |
| 13 | 250–500 |
| 14 | 62.5–125 |
| 15 | 250–500 |
| 16 | 500–1000 |
| 17 | 125–250 |
| 18 | 250–500 |
| 19 | 500–1000 |
| 20 | 125–250 |
| 21 | 250–500 |
| 22 | 500–1000 |
| 23 | 250–500 |
| 24 | 500–1000 |
| 25 | 250–500 |
| Fluconazole (standard drug) | 1–32 |
| % Ergosterol Decrease | ||||
|---|---|---|---|---|
| Test Compound | Concentration | Standard Strains (n = 3) | Clinical Strains (n = 3) | |
| Control | 0 | 0 | ||
| 8 | MIC/4 | 11.6 (± 0.69) | 12.9 (± 1.15) | |
| MIC/2 | 38.3 (± 3.05) | 36.73 (± 2.61) | ||
| MIC | 59.06 (± 4.10) | 55.23 (± 2.92) | ||
| 11 | MIC/4 | 52.43 (± 3.89) | 53.91 (± 3.26) | |
| MIC/2 | 65.66 (± 4.93) | 62.76 (± 4.27) | ||
| MIC | 88.26 (± 2.12) | 84.3 (± 5.00) | ||
| 14 | MIC/4 | 36.13 (± 4.00) | 29.26 (± 1.16) | |
| MIC/2 | 51.16 (± 2.36) | 45.72 (± 1.83) | ||
| MIC | 70.4 (± 2.62) | 71.41 (± 3.16) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezki, N.; Al-Sodies, S.A.; Shreaz, S.; Shiekh, R.A.; Messali, M.; Raja, V.; Aouad, M.R. Green Ultrasound versus Conventional Synthesis and Characterization of Specific Task Pyridinium Ionic Liquid Hydrazones Tethering Fluorinated Counter Anions: Novel Inhibitors of Fungal Ergosterol Biosynthesis. Molecules 2017, 22, 1532. https://doi.org/10.3390/molecules22111532
Rezki N, Al-Sodies SA, Shreaz S, Shiekh RA, Messali M, Raja V, Aouad MR. Green Ultrasound versus Conventional Synthesis and Characterization of Specific Task Pyridinium Ionic Liquid Hydrazones Tethering Fluorinated Counter Anions: Novel Inhibitors of Fungal Ergosterol Biosynthesis. Molecules. 2017; 22(11):1532. https://doi.org/10.3390/molecules22111532
Chicago/Turabian StyleRezki, Nadjet, Salsabeel A. Al-Sodies, Sheikh Shreaz, Rayees Ahmad Shiekh, Mouslim Messali, Vaseem Raja, and Mohamed R. Aouad. 2017. "Green Ultrasound versus Conventional Synthesis and Characterization of Specific Task Pyridinium Ionic Liquid Hydrazones Tethering Fluorinated Counter Anions: Novel Inhibitors of Fungal Ergosterol Biosynthesis" Molecules 22, no. 11: 1532. https://doi.org/10.3390/molecules22111532