Spectrum-Effect Relationships between Fingerprints of Caulophyllum robustum Maxim and Inhabited Pro-Inflammation Cytokine Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results of the HPLC Experiment
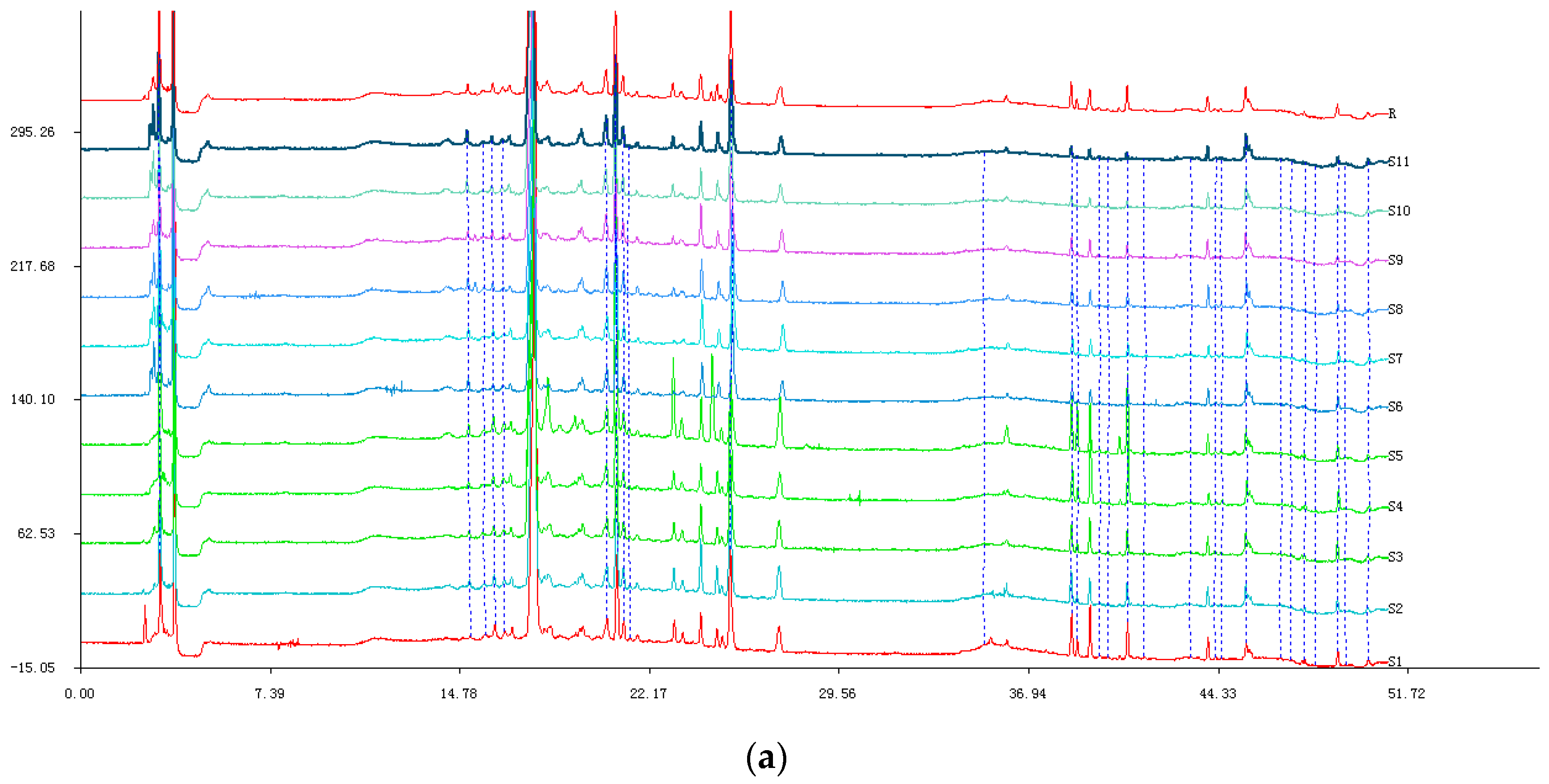
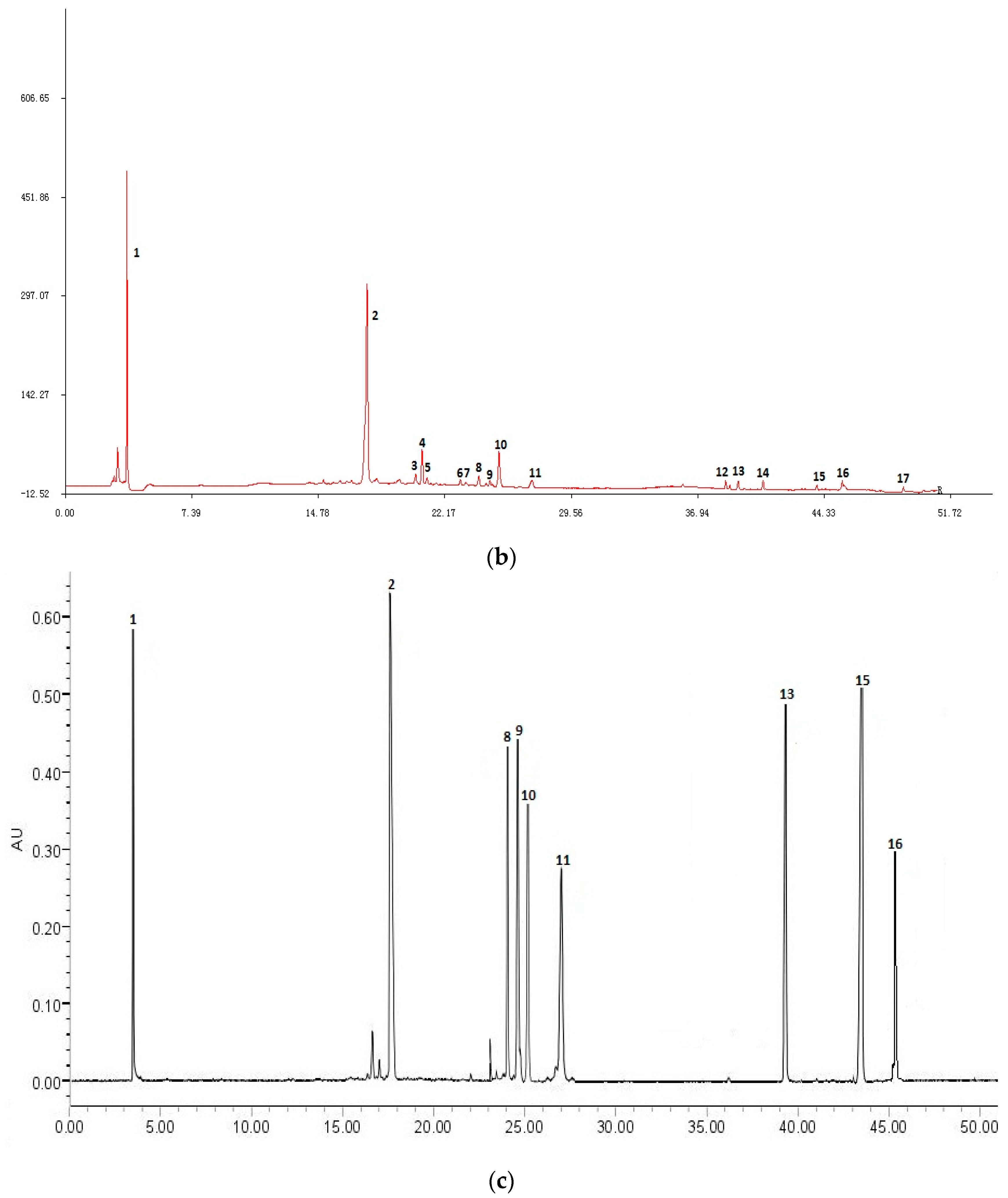
2.1.1. HPLC Experiments
2.1.2. Similarity of Fingerprints
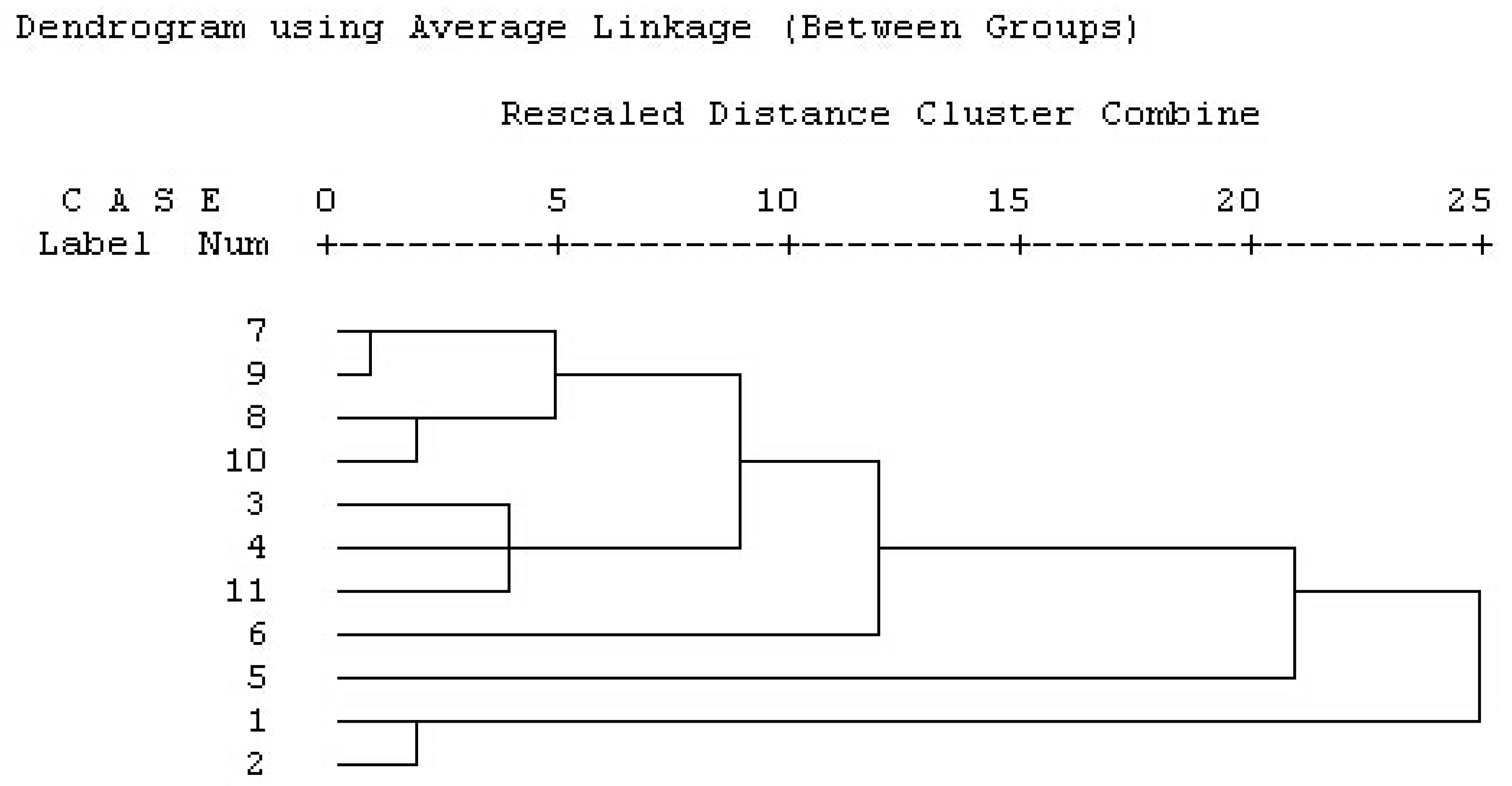
2.1.3. Results of Hierarchical Clustering Analysis (HCA)
2.2. The Results of CIA Pharmacodynamics Experiment
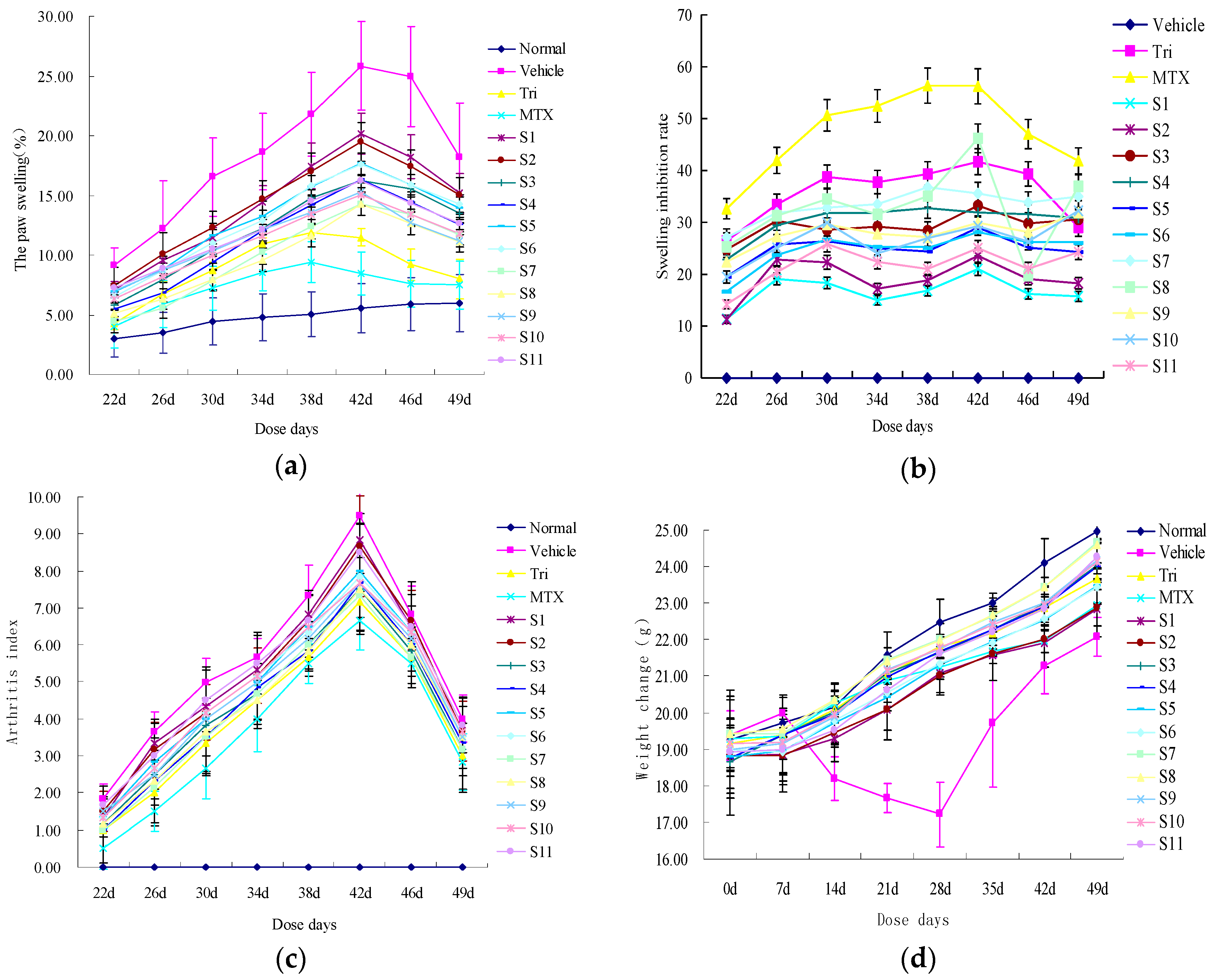
2.2.1. Effect of CRM on Gross Lesions of CIA Mice
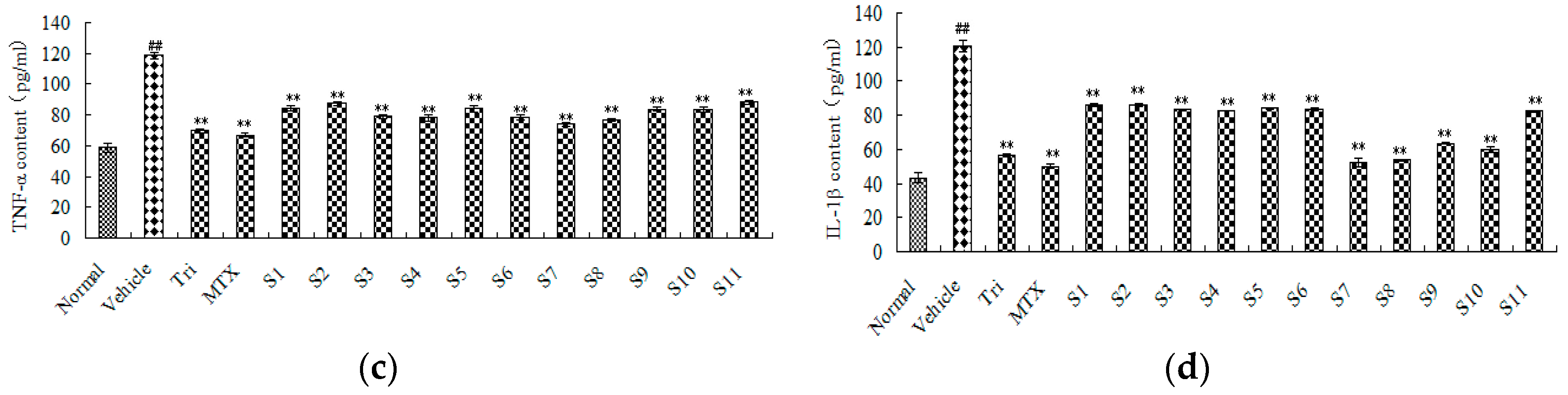
2.2.2. Effect of CRM on Sera Cytokines of CIA Mice
2.3. The Results of the Spectrum-Effect Relationships
2.3.1. Stepwise Multiple Linear Regression
| R2 = 0.879 | p < 0.05 | |
| R2 = 0.983 | ||
| R2 = 0.941 | ||
| R2 = 0.755 |
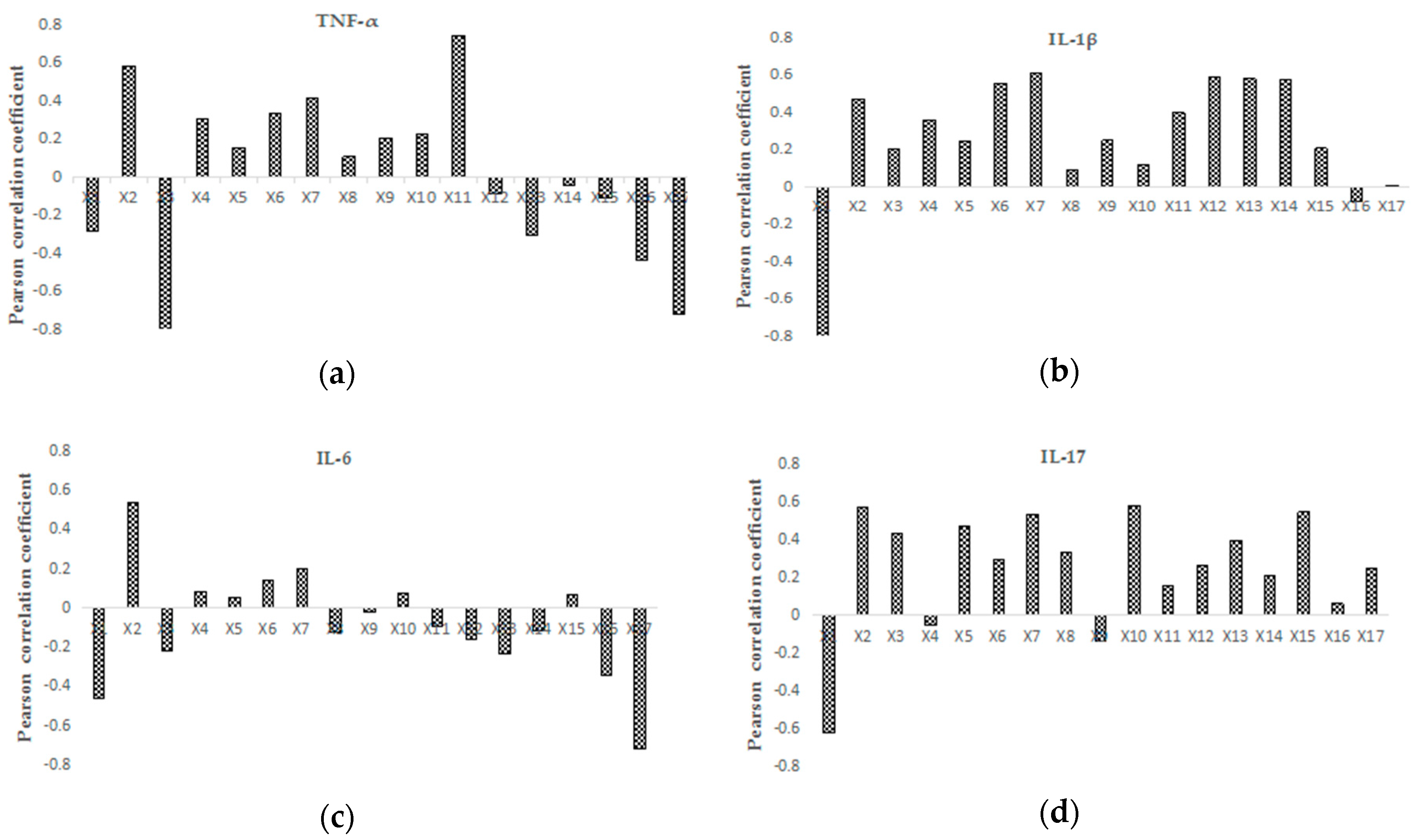
2.3.2. Partial Correlation Analysis
2.3.3. Integration of the Analytical Results
2.3.4. Gray Relational Analysis
2.4. Assignments of the Correlated Peaks
3. Materials and Methods
3.1. Materials and Reagents
3.2. Sample Preparation
3.3. Analysis of HPLC Fingerprints
3.3.1. HPLC Conditions
3.3.2. Validation of Methodology
3.3.3. Similarity of HPLC Analysis
3.3.4. HCA of CRM Fingerprints
3.4. Anti-Inflammatory Effect Experiment
3.4.1. The Establishment of CIA Model, Compound Administration, and Grouping
3.4.2. Morphological Analysis
3.4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
3.5. Analysis of Spectrum-Effect Relationships
3.5.1. Stepwise Multiple Linear Regression Analysis
3.5.2. Partial Correlation Analysis
3.5.3. Gray Relational Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lau, C.S.; Gibofsky, A.; Damjanov, N.; Lula, S.; Marshall, L.; Jones, H.; Emery, P. Down-titration of biologics for the treatment of rheumatoid arthritis: A systematic literature review. Rheumatol. Int. 2017, 37, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Lü, S.W.; Wang, Q.S.; Li, G.Y.; Sun, S.; Guo, Y.Y.; Kuang, H.X. The treatment of rheumatoid arthritis using Chinese medicinal plants: From pharmacology to potential molecular mechanisms. J. Ethnopharmacol. 2015, 176, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.; Panayi, G. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.M.; Xing, H.; Liu, J.J.; Kang, H.X. The study of Chemical composition from Caulophyllum robustum Maxim which belong to Taibai seven medicine. J. Anhui Agric. Sci. 2012, 40, 745–747. [Google Scholar]
- Campbell, I.K.; Hamilton, J.A.; Wicks, I.P. Collagen-induced arthritis in C57BL/6 (H-2b) mice: New insights into an important disease model of rheumatoid arthritis. Eur. J. Immunol. 2000, 30, 1568–1575. [Google Scholar] [CrossRef]
- Song, H.; Qiao, F.; Atkinson, C.; Holers, V.M.; TomLinson, S. Acomplement C3 inhibitor specially targeted to sites of complement activation effectively ameliorates collagen-induced arthritis in DBA/1 J mice. J. Immunol. 2007, 179, 7860–7867. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, R.; Bockermann, R.; Bäcklund, J.; Yamada, H. The molecular pathogenesis of collagen-induced arthritis in mice-a model for rheumatoid arthritis. Ageing Res. Rev. 2002, 1, 135–147. [Google Scholar] [CrossRef]
- Song, L.R.; Hong, X.; Ding, X.L.; Zang, Z.Y. Modern Zhong Yao Da Ci Dian: Volume One; People’s Medical Publishing House: Beijing, China, 2001; Volume 934, ISBN 9787117042260. [Google Scholar]
- Xia, Y.G.; Li, G.Y.; Liang, J.; Yang, B.Y.; Lü, S.W.; Kuang, H.X. Genus Caulophyllum: An Overview of Chemistry and Bioactivity. Evid.-Based Complement. Altern. Med. 2014, 2014, 684508. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jung, J.C.; Ali, Z.; Khan, I.A.; Oh, S. Anti-Inflammatory Effect of Triterpene Saponins Isolated from Blue Cohosh (Caulophyllum thalictroides). Évid.-Based Complement. Altern. Med. 2012, 2012, 798192. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Ma, Y.M.; Xing, H.; Liu, J.J.; Kang, Y.X. Studies on triterpene saponins and their biological activity of Caulophyllum robustum. J. Shaannxi Univ. Sci. Technol. 2013, 31, 62–69. [Google Scholar]
- Lü, S.W.; Su, H.; Yu, F.M.; Guo, Y.Y.; Kuang, H.X. Therapeutic Effect and Mechanism of Caulophyllum robustum Maxim Extract on Adjuvant Arthritis Rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 2017, 28, 164–171. [Google Scholar]
- Xiao, L.W.; Bing, R.L.; Chien, K.C.; Jun, R.W.; Shoei, S.L. Four new fluorenone alkaloids and one new dihydroazafluoranthene alkaloid from Caulophyllum robustum Maxim. Fitoterapia 2011, 82, 793–797. [Google Scholar] [CrossRef]
- Qin, K.M.; Zheng, L.J.; Shen, B.J.; Zhang, X.H.; Li, H.; Di, L.Q.; Xu, Z.S.; Cai, C. Application of spectrum-effect relationship in Chinese medicine research and related thinking. China J. Chin. Mater. Med. 2013, 38, 26–31. [Google Scholar] [CrossRef]
- Huo, S.X.; Kang, Y.T.; Peng, X.M.; Gao, L.; Yan, M. Spectrum-effect relationship of extract from rhizome of Alpinia officinarum on promotion of melanogenesis. Chin. Tradit. Herb. Drugs 2013, 44, 995–1002. [Google Scholar] [CrossRef]
- Liu, N.; Li, J.; Li, B.G. Application of multivariate statistical analysis and thinking in quality control of Chinese medicine. China J. Chin. Mater. Med. 2014, 39, 4268–4271. [Google Scholar]
- Shen, Y.W.; Liu, J.J. Application of the Gray Relevancy Method to College Teachers’ Research Performance Assessment of Humanities and Social Sciences. Sci. Technol. Manag. Res. 2010, 30, 237–240. [Google Scholar]
- Lü, S.W.; Dong, S.Y.; Guo, Y.Y.; Sun, S.; Kuang, H.X. Advance in Application of Data Analysis Technique in Spectrum-effect Relationship of Traditional Chinese Medicines. Chin. J. Exp. Tradit. Méd. Formulae 2015, 21, 226–230. [Google Scholar] [CrossRef]
- Han, Y.P.; Lei, Z.H.; Zhao, D. Influence Factors and Countermeasures of Quality of Traditional Chinese Medicine. Mod. Agric. Sci. Technol. 2015, 24, 113–114. [Google Scholar]
- Sun, M.Y.; Wu, W.J.; Xu, H.L.; Liu, X.; Xia, S.; Shao, Q.X. Methotrexate inhibited the development of chllagen-induced arthritis in mice by decreasing the ratio of Th17/Treg cells. J. Jiangsu Univ. (Med. Ed.) 2012, 4, 291–294. [Google Scholar]
- Zheng, H.M.; Jin, S. Effects of Glucosidorum Tripterygll Totorum Tablet on Serum HMGB1 and IL-17 in Rats with Collagen-induced Arthritis. Chin. J. Exp. Tradit. Med. Formulae 2013, 15, 247–250. [Google Scholar]
- Nagy, G.; Clark, J.M.; Buzas, E.; Gorman, C.; Pasztoi, M.; Koncz, A.; Falus, A.; Cope, A.P. Nitric oxide production of T lymphocytes is increased in rheumatoid arthritis. Immunol. Lett. 2008, 118, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Or, T.C.; Ho, M.H.; Lan, A.S. Scientific Basis of Botanical Medicine as Alternative Remedies for Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2013, 44, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Kugyelka, R.; Kohl, Z.; Olasz, K.; Ranch, T.A.; Glant, T.T.; Boldizsar, F. Enigma of IL-17 and Th17 Cells in Rheumatoid Arthritis and in Autoimmune Animal Models of Arthritis. Mediat. Inflamm. 2016, 2016, 6145810. [Google Scholar] [CrossRef] [PubMed]
- Mclnnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y. Studies on Chemical Constituents of Active Fraction from Caulophyllum robustum for Anti-Rheumatism. Master’s Thesis, Heilongjiang University of Chinese Medcine, Harbin, China, 2006. [Google Scholar]
- Xia, Y.G.; Li, G.Y.; Liang, J.; Ortori, C.A.; Yang, B.Y.; Kuang, H.X.; Barrett, D.A. A strategy for characterization of triterpene saponins in Caulophyllum robustum hairy roots by liquid chromatography with electrospray ionization quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2014, 100, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Lü, S.W.; Li, G.Y.; Zuo, Y.M.; Kang, H.X. Determination the content of total saponins from Caulophyllum robustum Maxim. Acta Tradit. Chin. Med. 2005, 33, 14–15. [Google Scholar]
- Li, G.Y.; Xu, N.; Lü, S.W.; Kang, H.X. Saponin constituents from roots and rhizomes of Caulophyllum robustum. Chin. Tradit. Herb. Drugs 2015, 46, 1431–1436. [Google Scholar]
- Aminin, D.L.; Agafonova, I.G.; Gnedoi, S.N.; Strigina, L.I.; Anisimov, M.M. The effect of pH on biological activity of plant cytotoxin Cauloside C. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999, 122, 45–51. [Google Scholar] [CrossRef]
- Anisimov, M.M.; Shentsova, E.B.; Shcheglov, V.V.; Shulmilov, Y.N.; Rasskazov, V.A.; Striqina, L.I.; Chetyrina, N.S.; Elyakov, G.B. Mechanism of cytotoxic action of some triterpene glycosides. Toxicon 1978, 16, 207–218. [Google Scholar] [CrossRef]
- Küpeli, E.; Kosar, M.; Yesilada, E.; Hüsnü, K.; Baser, C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002, 72, 645–657. [Google Scholar] [CrossRef]
- Yamashita, R.; Fujiwara, Y.; Ikari, K.; Hamada, K.; Otomo, A.; Yasuda, K.; Noda, M.; Kaburagi, Y. Extracellular proteome of human hepatoma cell, HepG2 analyzed using two-dimensional liquid chromatography coupled with tandem mass spectrometry. Mol. Cell. Biochem. 2007, 298, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sheheglov, V.V.; Anisimov, M.M.; PoPov, A.M.; Kiseleva, M.I.; Sebko, I.G. Effect of trierpene glyeosides on plasma membrane permeability for UV-absorbing substances in Saccharomyces carlsbergensis yeast cells. Antibiotiki 1979, 24, 49–55. [Google Scholar]
- Anisimov, M.M.; Ivanova, A.S.; Popov, A.M.; Kiseleva, M.I.; Sebko, I.G. Effect of trierpene glyeosides and polyene antibiotics on cell membrane permeability for K+ ions and UV-absorbing substances. Prikl. Biokhim. Mikrobiol. 1981, 17, 890–895. [Google Scholar] [PubMed]
- Anisimov, M.M.; Shcheglov, V.V.; Kiseleva, M.I. Effect of trierpene glyeosides on the plasma membrane permeability for amino acids in Saccharomyces carlsbergensis yeast cells. Antibiltiki 1978, 23, 66–69. [Google Scholar]
- Tian, L.T.; Ma, L.; Zhe, N.S. Survey of Pharmacology of Aleanolic Acid. China J. Chin. Mater. Med. 2002, 27, 884–901. [Google Scholar]
- Kapi1, A.; Sharma, S. Effect of Oleanolic Acid on completment in Adjuvant and Carrageenan-induced Inflammation in Rats. J. Pharm. Pharmacol. 1995, 47, 585–587. [Google Scholar]
- Kim, Y.P.; Lee, E.B.; Kim, S.Y.; Li, D.; Ban, H.S.; Lim, S.S.; Shin, K.H.; Ohuchi, K. Inhibition of rostaglandin E2 production by platycodin D isolated from the root of Platycodon gradiflorum. Planta Med. 2001, 67, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Park, S.M.; Zhao, R.; Lee, C.; Chun, W.; Son, Y.; Kim, S.H.; Jung, J.Y.; Jegal, K.H.; Cho, I.J.; et al. Hederagenin, a major component of Clematis mandshurica Ruprecht root, attenuates inflammatory responses in RAW 264.7 cells and in mice. Int. Immunopharmacol. 2015, 29, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Guo, T. Studies on the Chemical Constituents of Zanthoxylum armatum DC. and Z. avicenna (Lam.) DC. and Analgesic, Anti-inflammatory Activities of Z. armatum. Master’s Thesis, Fudan University, Shanghai, China, 2011. [Google Scholar]
- Tsypysheva, I.P.; Borisevich, S.S.; Zainullina, L.F.; Makara, N.S.; Koval’skaya, A.V.; Petrova, P.R.; Khursan, S.L.; Vakhitova, Y.V.; Zarudii, F.S. Anti-Inflammatory Activity of Novel 12-N-methylcytisine Derivatives. Anti-Allergy Agents Med. Chem. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.B.; Liu, X.L.; Cheng, R.Q.; Jia, H.X.; Wang, X.Y.; Kong, L.N.; Li, Y.J.; Cui, T.T.; Qu, H.D.; Feng, C.; et al. Influence of β-sitosterol on Gastric Mucosal Side Effect Induced by Aspirin and Its Pharmacological Functions. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 148–152. [Google Scholar]
- Zhang, S.J.; Chen, Q.B. The application of statistical analysis software SPSS (V), correlation analysis and regression analysis. Anim. Husb. Vet. Med. 2003, 35, 16–18. [Google Scholar]
- Wang, M.Z.; Jing, S.L.; He, X.J.; Lv, C.; Li, J.; Luo, D.; Lv, A.P. The association studies about CIA rats foot plantar size and joint. Chin. J. Basic Med. Tradit. Chin. Med. 2013, 19, 1417–1419. [Google Scholar]
Sample Availability: Not available. |

| Sample | Retention Time | ||||||||
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | |
| S1 | 0.135 | 0.651 | 0.754 | 0.768 | 0.778 | 0.850 | 0.862 | 0.888 | 0.911 |
| S2 | 0.134 | 0.650 | 0.753 | 0.767 | 0.777 | 0.849 | 0.862 | 0.888 | 0.911 |
| S3 | 0.134 | 0.650 | 0.754 | 0.768 | 0.778 | 0.850 | 0.861 | 0.888 | 0.912 |
| S4 | 0.133 | 0.649 | 0.752 | 0.766 | 0.777 | 0.848 | 0.860 | 0.888 | 0.911 |
| S5 | 0.134 | 0.649 | 0.752 | 0.766 | 0.777 | 0.847 | 0.859 | 0.888 | 0.903 |
| S6 | 0.134 | 0.648 | 0.752 | 0.766 | 0.777 | 0.848 | 0.860 | 0.888 | 0.911 |
| S7 | 0.133 | 0.646 | 0.750 | 0.764 | 0.774 | 0.845 | 0.857 | 0.886 | 0.910 |
| S8 | 0.132 | 0.645 | 0.749 | 0.763 | 0.774 | 0.845 | 0.859 | 0.885 | 0.909 |
| S9 | 0.132 | 0.645 | 0.749 | 0.763 | 0.773 | 0.845 | 0.856 | 0.885 | 0.909 |
| S10 | 0.132 | 0.645 | 0.749 | 0.763 | 0.774 | 0.845 | 0.856 | 0.885 | 0.909 |
| S11 | 0.132 | 0.645 | 0.750 | 0.764 | 0.775 | 0.846 | 0.857 | 0.887 | 0.910 |
| 0.133 | 0.647 | 0.751 | 0.765 | 0.776 | 0.847 | 0.795 | 0.887 | 0.910 | |
| RSD% | 0.721 | 0.352 | 0.232 | 0.260 | 0.217 | 0.235 | 0.273 | 0.164 | 0.260 |
| Sample | Retention Time | ||||||||
| X10 | X11 | X12 | X13 | X14 | X15 | X16 | X17 | ||
| S1 | 0.930 | 1.000 | 1.419 | 1.446 | 1.499 | 1.614 | 1.669 | 1.800 | |
| S2 | 0.930 | 1.000 | 1.418 | 1.445 | 1.498 | 1.614 | 1.668 | 1.799 | |
| S3 | 0.931 | 1.000 | 1.419 | 1.445 | 1.498 | 1.614 | 1.668 | 1.800 | |
| S4 | 0.930 | 1.000 | 1.418 | 1.445 | 1.498 | 1.614 | 1.668 | 1.799 | |
| S5 | 0.930 | 1.000 | 1.417 | 1.443 | 1.497 | 1.612 | 1.666 | 1.797 | |
| S6 | 0.930 | 1.000 | 1.418 | 1.444 | 1.497 | 1.613 | 1.667 | 1.799 | |
| S7 | 0.929 | 1.000 | 1.413 | 1.439 | 1.492 | 1.607 | 1.661 | 1.792 | |
| S8 | 0.929 | 1.000 | 1.412 | 1.438 | 1.491 | 1.606 | 1.660 | 1.791 | |
| S9 | 0.929 | 1.000 | 1.412 | 1.438 | 1.491 | 1.606 | 1.660 | 1.791 | |
| S10 | 0.928 | 1.000 | 1.413 | 1.439 | 1.492 | 1.607 | 1.661 | 1.792 | |
| S11 | 0.929 | 1.000 | 1.416 | 1.442 | 1.495 | 1.611 | 1.665 | 1.796 | |
| 0.930 | 1.000 | 1.416 | 1.443 | 1.496 | 1.611 | 1.665 | 1.796 | ||
| RSD% | 0.001 | 0.000 | 0.210 | 0.219 | 0.222 | 0.215 | 0.214 | 0.210 | |
| Sample | Peak Area | ||||||||
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | |
| S1 | 9.456 | 27.201 | 1.565 | 2.515 | 0.938 | 0.943 | 0.943 | 1.181 | 0.607 |
| S2 | 7.074 | 21.749 | 1.319 | 2.144 | 0.900 | 1.346 | 0.767 | 1.346 | 0.702 |
| S3 | 6.824 | 13.654 | 1.684 | 1.757 | 0.961 | 1.157 | 0.879 | 1.257 | 0.533 |
| S4 | 8.613 | 19.969 | 1.899 | 2.195 | 0.974 | 0.906 | 0.948 | 1.236 | 0.769 |
| S5 | 4.969 | 9.526 | 1.034 | 3.895 | 0.584 | 1.501 | 0.658 | 0.771 | 1.312 |
| S6 | 10.942 | 21.792 | 1.645 | 1.931 | 0.922 | 0.600 | 0.599 | 1.083 | 0.553 |
| S7 | 11.652 | 18.294 | 1.930 | 1.893 | 1.157 | 0.411 | 0.463 | 1.326 | 0.803 |
| S8 | 12.413 | 18.635 | 1.626 | 1.874 | 0.881 | 0.374 | 0.756 | 1.487 | 0.781 |
| S9 | 13.809 | 22.756 | 1.492 | 2.207 | 1.027 | 0.443 | 0.577 | 1.290 | 0.764 |
| S10 | 15.058 | 25.958 | 2.264 | 2.615 | 1.262 | 0.977 | 0.959 | 1.725 | 0.629 |
| S11 | 11.053 | 23.134 | 2.245 | 2.842 | 1.350 | 0.706 | 1.265 | 1.502 | 1.003 |
| 10.157 | 20.040 | 1.695 | 2.373 | 1.002 | 0.842 | 0.763 | 1.296 | 0.910 | |
| RSD% | 29.17 | 24.75 | 20.84 | 24.89 | 19.73 | 43.43 | 33.25 | 18.14 | 0.003 |
| Sample | Peak Area | ||||||||
| X10 | X11 | X12 | X13 | X14 | X15 | X16 | X17 | ||
| S1 | 3.312 | 1.000 | 0.780 | 0.940 | 0.776 | 0.588 | 0.716 | 0.405 | |
| S2 | 3.402 | 1.000 | 0.461 | 0.387 | 0.411 | 0.399 | 0.758 | 0.375 | |
| S3 | 2.646 | 1.000 | 0.534 | 0.577 | 0.415 | 0.430 | 0.845 | 0.399 | |
| S4 | 3.276 | 1.000 | 0.721 | 1.259 | 1.201 | 0.491 | 0.845 | 0.571 | |
| S5 | 1.422 | 1.000 | 0.560 | 0.519 | 0.846 | 0.191 | 0.391 | 0.215 | |
| S6 | 2.847 | 1.000 | 0.808 | 1.378 | 1.172 | 0.546 | 1.182 | 0.612 | |
| S7 | 3.189 | 1.000 | 0.533 | 0.591 | 0.441 | 0.584 | 1.004 | 0.515 | |
| S8 | 3.324 | 1.000 | 0.439 | 0.334 | 0.283 | 0.316 | 1.068 | 0.507 | |
| S9 | 3.795 | 1.000 | 0.434 | 0.385 | 0.387 | 0.716 | 1.176 | 0.438 | |
| S10 | 3.985 | 1.000 | 0.428 | 0.440 | 0.329 | 0.468 | 0.744 | 0.463 | |
| S11 | 3.532 | 1.000 | 0.370 | 0.306 | 0.358 | 0.445 | 0.958 | 0.283 | |
| 3.174 | 1.000 | 0.562 | 0.620 | 0.575 | 0.459 | 0.875 | 0.432 | ||
| RSD% | 20.73 | 0.000 | 26.50 | 59.66 | 58.48 | 30.79 | 25.39 | 26.24 | |
| Sample | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1.000 | 0.908 | 0.980 | 0.916 | 0.968 | 0.917 | 0.942 | 0.959 | 0.964 | 0.951 | 0.948 |
| S2 | 0.908 | 1.000 | 0.915 | 0.862 | 0.904 | 0.891 | 0.902 | 0.905 | 0.903 | 0.909 | 0.891 |
| S3 | 0.980 | 0.915 | 1.000 | 0.928 | 0.977 | 0.933 | 0.959 | 0.967 | 0.968 | 0.954 | 0.947 |
| S4 | 0.916 | 0.862 | 0.928 | 1.000 | 0.920 | 0.906 | 0.919 | 0.921 | 0.916 | 0.908 | 0.908 |
| S5 | 0.968 | 0.904 | 0.977 | 0.920 | 1.000 | 0.936 | 0.971 | 0.983 | 0.974 | 0.948 | 0.944 |
| S6 | 0.917 | 0.891 | 0.933 | 0.906 | 0.936 | 1.000 | 0.953 | 0.952 | 0.946 | 0.942 | 0.941 |
| S7 | 0.942 | 0.902 | 0.959 | 0.919 | 0.971 | 0.953 | 1.000 | 0.982 | 0.969 | 0.941 | 0.937 |
| S8 | 0.959 | 0.905 | 0.967 | 0.921 | 0.983 | 0.952 | 0.982 | 1.000 | 0.980 | 0.957 | 0.948 |
| S9 | 0.964 | 0.903 | 0.968 | 0.916 | 0.974 | 0.946 | 0.969 | 0.980 | 1.000 | 0.964 | 0.950 |
| S10 | 0.951 | 0.909 | 0.954 | 0.908 | 0.948 | 0.942 | 0.941 | 0.957 | 0.964 | 1.000 | 0.956 |
| S11 | 0.948 | 0.891 | 0.947 | 0.908 | 0.944 | 0.941 | 0.937 | 0.948 | 0.950 | 0.956 | 1.000 |
| R | 0.977 | 0.935 | 0.985 | 0.945 | 0.984 | 0.965 | 0.979 | 0.987 | 0.985 | 0.976 | 0.971 |
| The Content of TNF-α | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | N | Student for Alpha = 0.05 | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Student-Newman-Keuls a | Normal | 6 | 58.78 | |||||
| S7 | 6 | 73.80 | ||||||
| S8 | 6 | 76.54 | ||||||
| S4 | 6 | 78.18 | ||||||
| S6 | 6 | 78.34 | ||||||
| S3 | 6 | 78.93 | ||||||
| S10 | 6 | 83.45 | ||||||
| S9 | 6 | 83.91 | ||||||
| S5 | 6 | 84.09 | ||||||
| S1 | 6 | 84.42 | ||||||
| S2 | 6 | 87.51 | ||||||
| S11 | 6 | 88.19 | ||||||
| Vehicle | 6 | 118.67 | ||||||
| Sig. | 1.00 | 1.00 | 0.107 | 0.783 | 0.510 | 1.00 | ||
| The Content of IL-1β | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | N | Student for Alpha = 0.05 | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| Student-Newman-Keuls a | Normal | 6 | 43.39 | |||||||
| S7 | 6 | 50.32 | ||||||||
| S8 | 6 | 53.61 | ||||||||
| S10 | 6 | 60.11 | ||||||||
| S9 | 6 | 63.65 | ||||||||
| S11 | 6 | 82.40 | ||||||||
| S4 | 6 | 82.62 | ||||||||
| S3 | 6 | 83.32 | ||||||||
| S6 | 6 | 83.59 | ||||||||
| S5 | 6 | 83.99 | 83.99 | |||||||
| S1 | 6 | 85.95 | ||||||||
| S2 | 6 | 85.99 | ||||||||
| Vehicle | 6 | 120.49 | ||||||||
| Sig. | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.395 | 0.072 | 1.00 | ||
| The Content of IL-6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | N | Student for Alpha = 0.05 | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| Student-Newman-Keuls a | Normal | 6 | 92.34 | |||||||
| S8 | 6 | 103.01 | ||||||||
| S7 | 6 | 104.89 | 104.89 | |||||||
| S4 | 6 | 106.37 | 106.37 | 106.37 | ||||||
| S3 | 6 | 109.40 | 109.40 | 109.40 | 109.40 | |||||
| S5 | 6 | 112.13 | 112.13 | 112.13 | 112.13 | 112.13 | ||||
| S10 | 6 | 112.43 | 112.43 | 112.43 | 112.43 | 112.43 | ||||
| S6 | 6 | 113.97 | 113.97 | 113.97 | 113.97 | 113.97 | ||||
| S9 | 6 | 115.68 | 115.68 | 115.68 | 115.68 | |||||
| S1 | 6 | 117.42 | 117.42 | 117.42 | ||||||
| S2 | 6 | 120.36 | 120.36 | |||||||
| S11 | 6 | 122.37 | ||||||||
| Vehicle | 6 | 152.93 | ||||||||
| Sig. | 1.000 | 0.055 | 0.071 | 0.060 | 0.148 | 0.130 | 0.085 | 1.00 | ||
| The Content of IL-17 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | N | Student for Alpha = 0.05 | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Student-Newman-Keuls a | Normal | 6 | 75.15 | |||||
| S8 | 6 | 86.52 | ||||||
| S7 | 6 | 90.33 | 90.33 | |||||
| S5 | 6 | 91.75 | 91.75 | |||||
| S6 | 6 | 91.83 | 91.83 | |||||
| S9 | 6 | 92.93 | 92.93 | |||||
| S10 | 6 | 94.10 | 94.10 | 94.10 | ||||
| S11 | 6 | 97.64 | 97.64 | 97.64 | ||||
| S1 | 6 | 99.20 | 99.20 | 99.20 | ||||
| S2 | 6 | 102.14 | 102.14 | |||||
| S3 | 6 | 104.01 | ||||||
| S4 | 6 | 105.63 | ||||||
| Vehicle | 6 | 129.14 | ||||||
| Sig. | 1.000 | 0.165 | 0.084 | 0.059 | 0.092 | 1.00 | ||
| Correlation Analysis | TNF-α | IL-1β | IL-6 | IL-17 |
|---|---|---|---|---|
| STEPWISE MLR | X2, X6 | X1, X3, X6 | X1, X13 | X1, X2, X10, X15 |
| PCA | X2, X11, X17 | X1, X6, X7, X12, X13, X14 | X2, X17 | X1, X2, X7, X10, X15 |
| in total | X2, X6, X11, X17 | X1, X3, X6, X7, X12, X13, X14 | X1, X2, X13, X17 | X1, X2, X7, X10, X15 |
| TNF-α | IL-1β | IL-6 | IL-17 | |||||
|---|---|---|---|---|---|---|---|---|
| No. | Correlation | No. | Correlation | No. | Correlation | No. | Correlation | |
| 1 | X17 | 0.7878 | X17 | 0.7214 | X8 | 0.9528 | X17 | 0.9221 |
| 2 | X15 | 0.7861 | X15 | 0.7199 | X5 | 0.9506 | X15 | 0.9188 |
| 3 | X12 | 0.7811 | X12 | 0.7144 | X11 | 0.9498 | X12 | 0.9135 |
| 4 | X14 | 0.7783 | X14 | 0.7105 | X7 | 0.9497 | X14 | 0.9094 |
| 5 | X13 | 0.7775 | X13 | 0.7100 | X3 | 0.9476 | X13 | 0.9075 |
| 6 | X9 | 0.7685 | X9 | 0.7042 | X16 | 0.9451 | X9 | 0.8979 |
| 7 | X7 | 0.7680 | X16 | 0.7035 | X6 | 0.9439 | X7 | 0.8958 |
| 8 | X16 | 0.7668 | X7 | 0.7031 | X13 | 0.9420 | X16 | 0.8940 |
| 9 | X6 | 0.7633 | X5 | 0.6980 | X12 | 0.9414 | X6 | 0.8896 |
| 10 | X5 | 0.7607 | X6 | 0.6971 | X14 | 0.9397 | X5 | 0.8858 |
| 11 | X11 | 0.7569 | X11 | 0.6939 | X9 | 0.9395 | X11 | 0.8813 |
| 12 | X8 | 0.7480 | X8 | 0.6873 | X4 | 0.9372 | X8 | 0.8686 |
| 13 | X3 | 0.7328 | X3 | 0.6736 | X15 | 0.9359 | X3 | 0.8477 |
| 14 | X4 | 0.7082 | X4 | 0.6505 | X17 | 0.9317 | X4 | 0.8172 |
| 15 | X10 | 0.6881 | X10 | 0.6355 | X10 | 0.9030 | X10 | 0.7886 |
| 16 | X1 | 0.5820 | X1 | 0.5445 | X1 | 0.7286 | X1 | 0.6531 |
| 17 | X2 | 0.5227 | X2 | 0.4894 | X2 | 0.6380 | X2 | 0.5789 |
| No. | Mass Data | Compound | Aglycones | Formula | Mol. Wt. | |
|---|---|---|---|---|---|---|
| X1 | [M + H]+ | 205.1 | N-methylcytisine | - | C12H16N2O | 204 |
| X2 | [M + H]+ | 343.2 | Magnoflorine | - | C20H24NO4 | 342 |
| X3 | [M + H]+ | 344.1 | Unknown | - | - | 343 |
| X4 | [M − H]− | 1559.7 | Leyemudanoside D | Caulophyllogenin | C71H116O37 | 1560 |
| X5 | [M − H]− | 1251.6 | Leyemudanoside C | Caulophyllogenin | C59H96O28 | 1252 |
| X6 | [M + Na]+ | 1111.5 | Leyemudanoside G | 11-oxo-Hederagenin | C53H84O23 | 1088 |
| X7 | [M − H]− | 1251.6 | Leyemudanoside B | Caulophyllogenin | C59H96O28 | 1252 |
| X8 | [M − H]− | 1251.2 | Cauloside H | Caulophyllogenin | C59H96O28 | 1252 |
| X9 | [M − H]− [M + HCOOH − H]− | 1089.0 1135.1 | Leonticin D | Caulophyllogenin | C53H86O23 | 1090 |
| X10 | [M − H]− | 1235.2 | Cauloside G | Hederagenin | C59H96O27 | 1236 |
| X11 | [M − H]− [M + HCOOH − H]− | 1073.0 1119.1 | Cauloside D | Hederagenin | C53H86O22 | 1074 |
| X12 | [M − H]− [2M − H]− | 618.91 1239.5 | Cauloside B | Caulophyllogenin | C35H56O9 | 620 |
| X13 | [M − H]− [M + Na]+ | 765.07 789.08 | Cauloside C | Hederagenin | C41H66O13 | 766 |
| X14 | [M − H]− | 603.3 | Cauloside A | Hederagenin | C35H56O8 | 604 |
| X15 | [M − H]− | 455.3 | Oleanic acid | - | C30H48O3 | 456 |
| X16 | [M − CH3]− | 399 | β-sitosterol | - | C29H50O | 414 |
| X17 | [M − H]− | 749.1 | Unknown | - | - | 750 |
| Sample No. | Collection Time | Origin |
|---|---|---|
| S1 | September 2013 | Dazhou mountain in Sichuan Province, China |
| S2 | September 2013 | Dazhou mountain in Sichuan Province, China |
| S3 | September 2014 | Mao county in Sichuan Province, China |
| S4 | August 2014 | Mao county in Sichuan Province, China |
| S5 | September 2014 | Anguo city in Hebei Province, China |
| S6 | September 2014 | Anguo city in Hebei Province, China |
| S7 | September 2014 | Suiling Zhangjiawan forest farm in Heilongjiang Province, China |
| S8 | September 2014 | Suiling Zhangjiawan forest farm in Heilongjiang Province, China |
| S9 | September 2013 | Suiling Zhangjiawan forest farm in Heilongjiang Province, China |
| S10 | September 2013 | Suiling Zhangjiawan forest farm in Heilongjiang Province, China |
| S11 | September 2012 | Suiling Zhangjiawan forest farm in Heilongjiang Province, China |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lü, S.; Dong, S.; Xu, D.; Duan, J.; Li, G.; Guo, Y.; Kuang, H.; Wang, Q. Spectrum-Effect Relationships between Fingerprints of Caulophyllum robustum Maxim and Inhabited Pro-Inflammation Cytokine Effects. Molecules 2017, 22, 1826. https://doi.org/10.3390/molecules22111826
Lü S, Dong S, Xu D, Duan J, Li G, Guo Y, Kuang H, Wang Q. Spectrum-Effect Relationships between Fingerprints of Caulophyllum robustum Maxim and Inhabited Pro-Inflammation Cytokine Effects. Molecules. 2017; 22(11):1826. https://doi.org/10.3390/molecules22111826
Chicago/Turabian StyleLü, Shaowa, Shuyu Dong, Dan Xu, Jixin Duan, Guoyu Li, Yuyan Guo, Haixue Kuang, and Qiuhong Wang. 2017. "Spectrum-Effect Relationships between Fingerprints of Caulophyllum robustum Maxim and Inhabited Pro-Inflammation Cytokine Effects" Molecules 22, no. 11: 1826. https://doi.org/10.3390/molecules22111826




