Herbicidal Activities of Some Allelochemicals and Their Synergistic Behaviors toward Amaranthus tricolor L.
Abstract
:1. Introduction
2. Results and Discussion
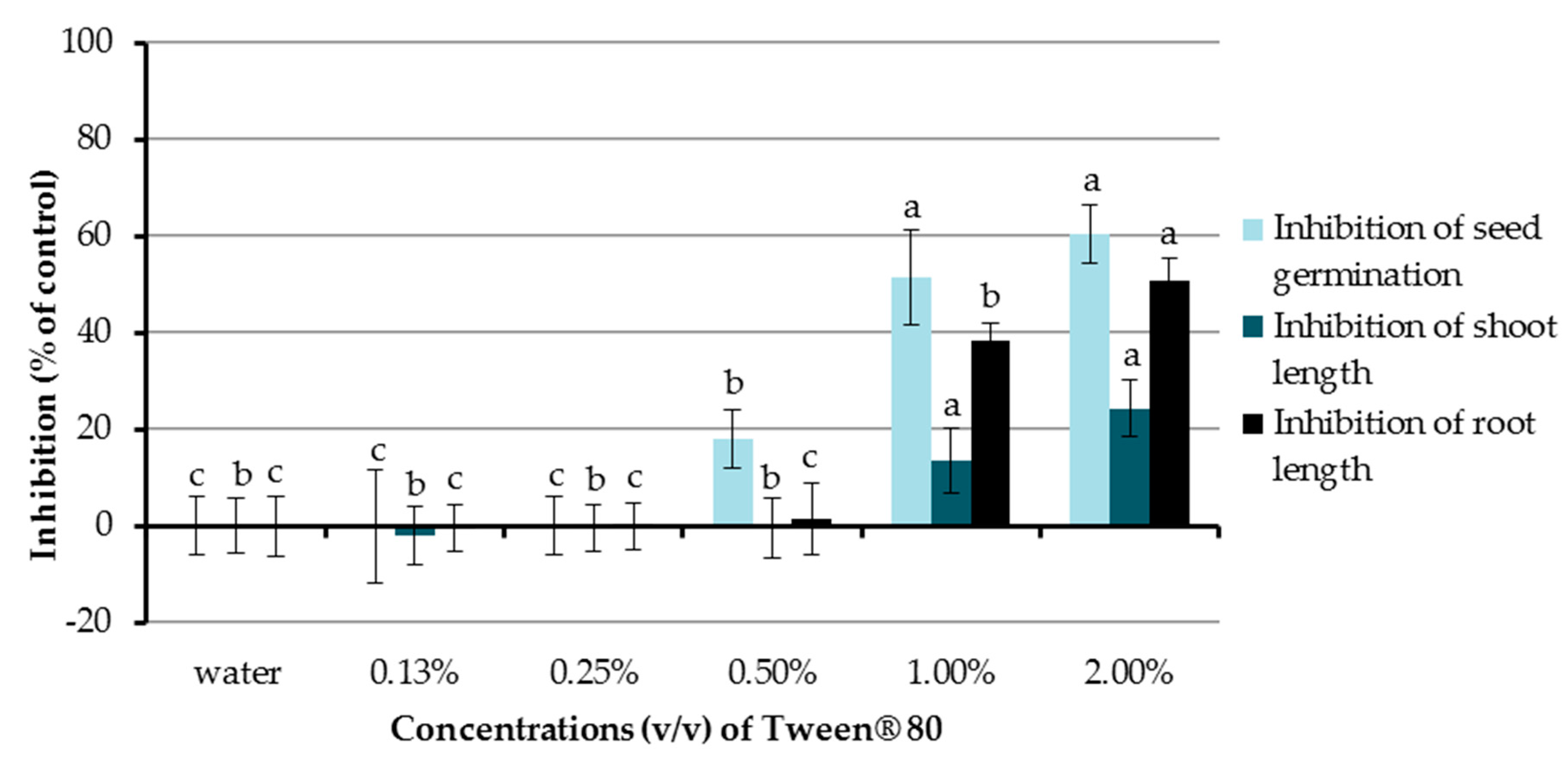
2.1. Effects of Tween® 80 on Germination and Seedling Growth of Chinese Amaranth
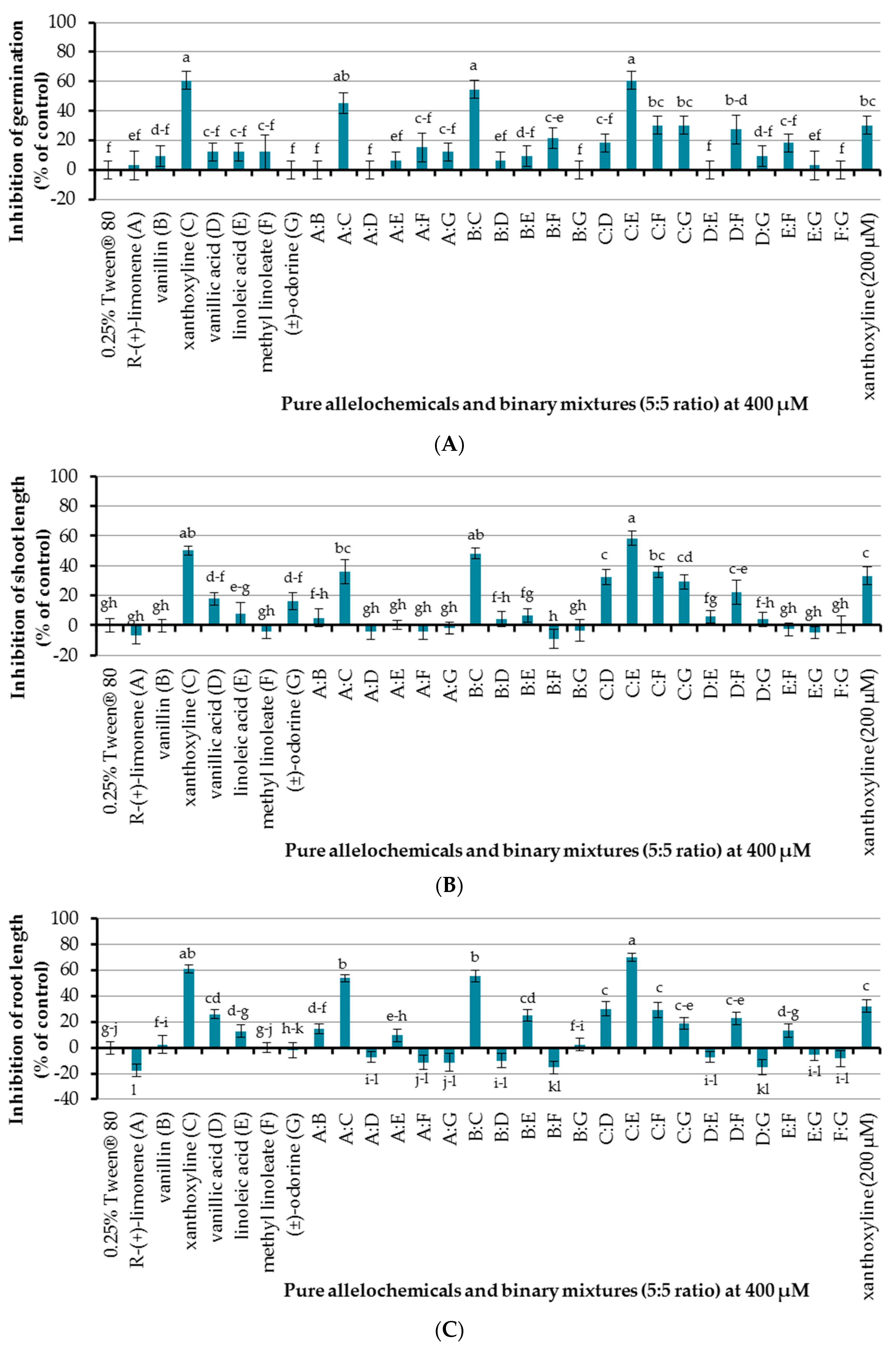
2.2. Allelopathic Effects of Seven Allelochemicals and Their Binary Mixtures on Germination and Seedling Growth of Chinese Amaranth
2.3. Allelopathic Effects of Binary Mixtures (In Different Ratios) of Some Allelochemicals on Germination and Seedling Growth of Chinese Amaranth
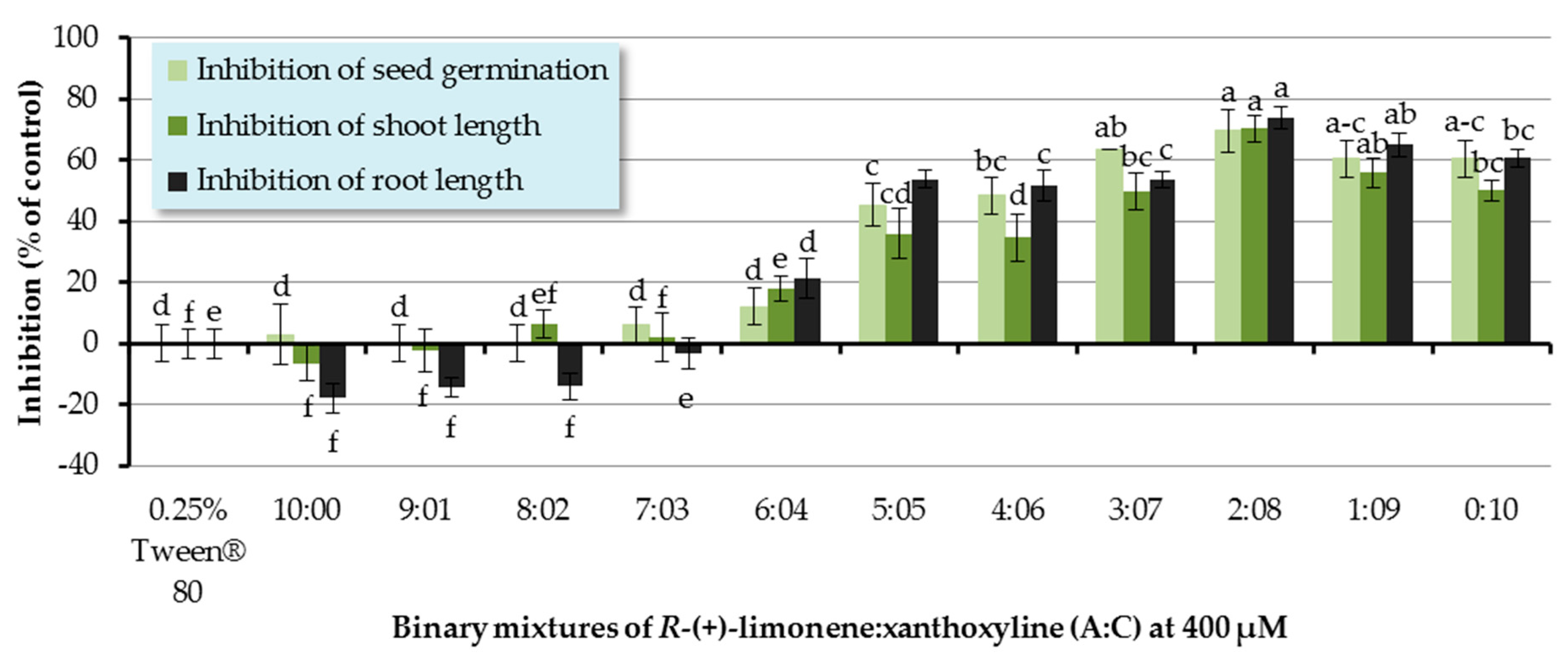
2.3.1. Allelopathic Effects of R-(+)-limonene (A) and Xanthoxyline (C) Mixtures on Germination and Seedling Growth of Chinese Amaranth
2.3.2. Allelopathic Effects of Vanillin (B) and Xanthoxyline (C) Mixtures on Germination and Seedling Growth of Chinese Amaranth
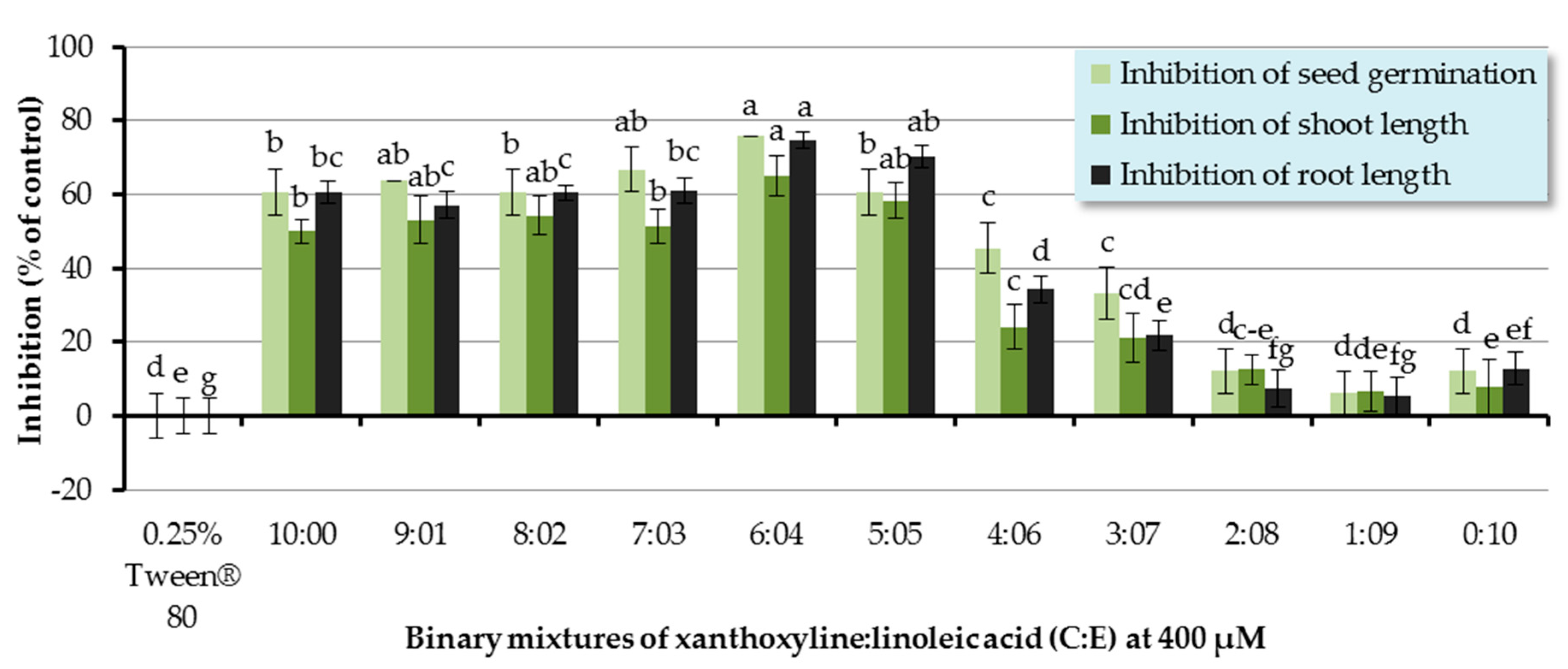
2.3.3. Allelopathic Effects of Xanthoxyline (C) and Linoleic Acid (E) Mixtures on Germination and Seedling Growth of Chinese Amaranth
2.4. Allelopathic Effects of Best Fractions of Binary Mixtures of Some Allelochemicals on Germination and Seedling Growth of Chinese Amaranth
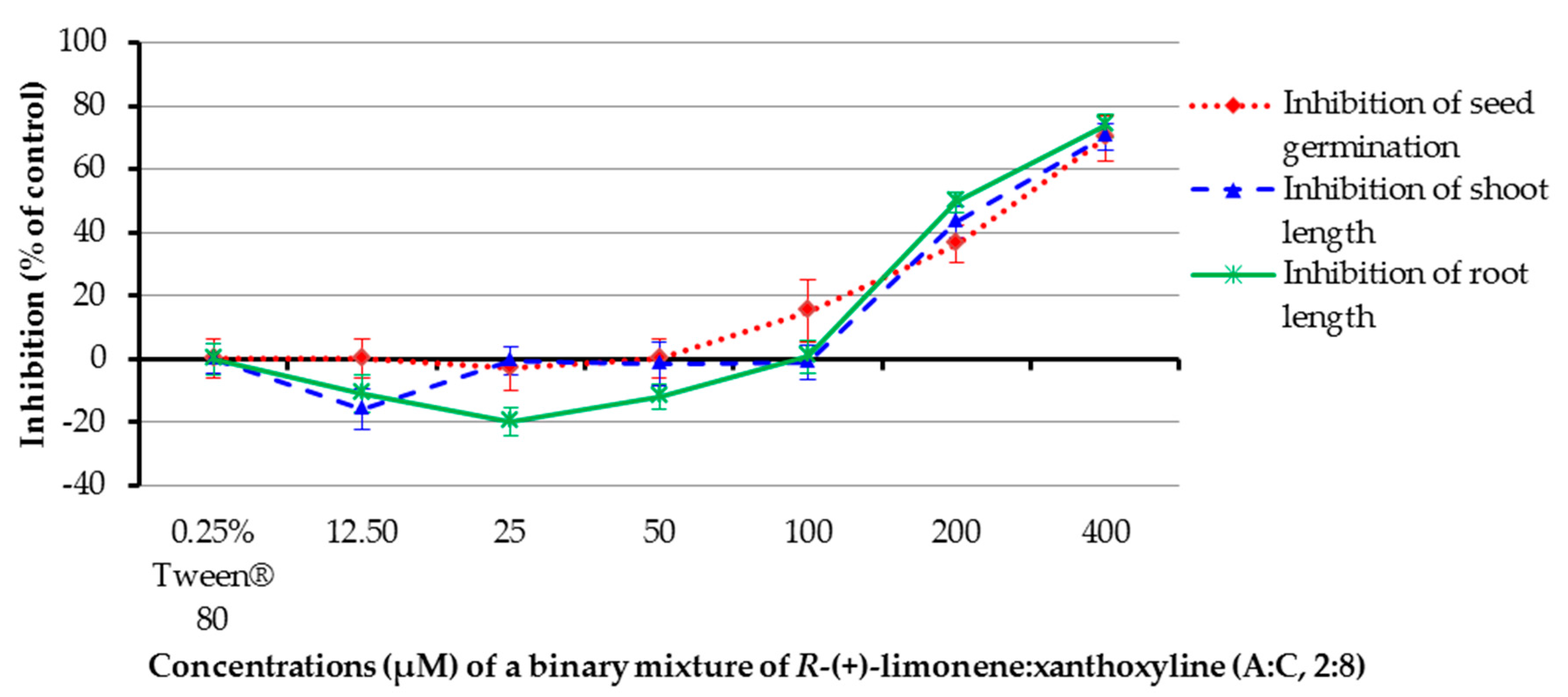
2.4.1. Allelopathic Effects of a Binary Mixture of R-(+)-Limonene:Xanthoxyline (2:8) on Germination and Seedling Growth of Chinese Amaranth
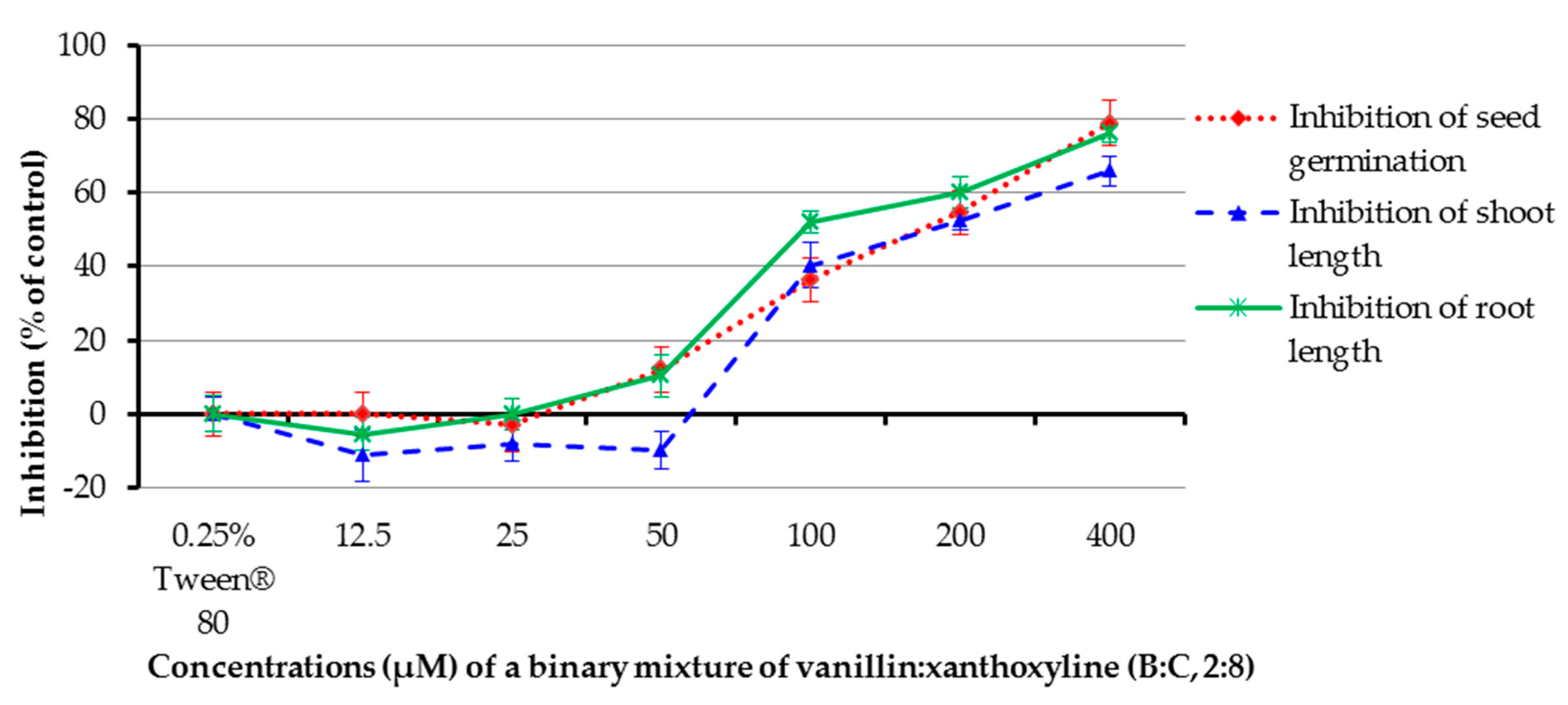
2.4.2. Allelopathic Effects of a Binary Mixture of Vanillin:Xanthoxyline (2:8) on Germination and Seedling Growth of Chinese Amaranth
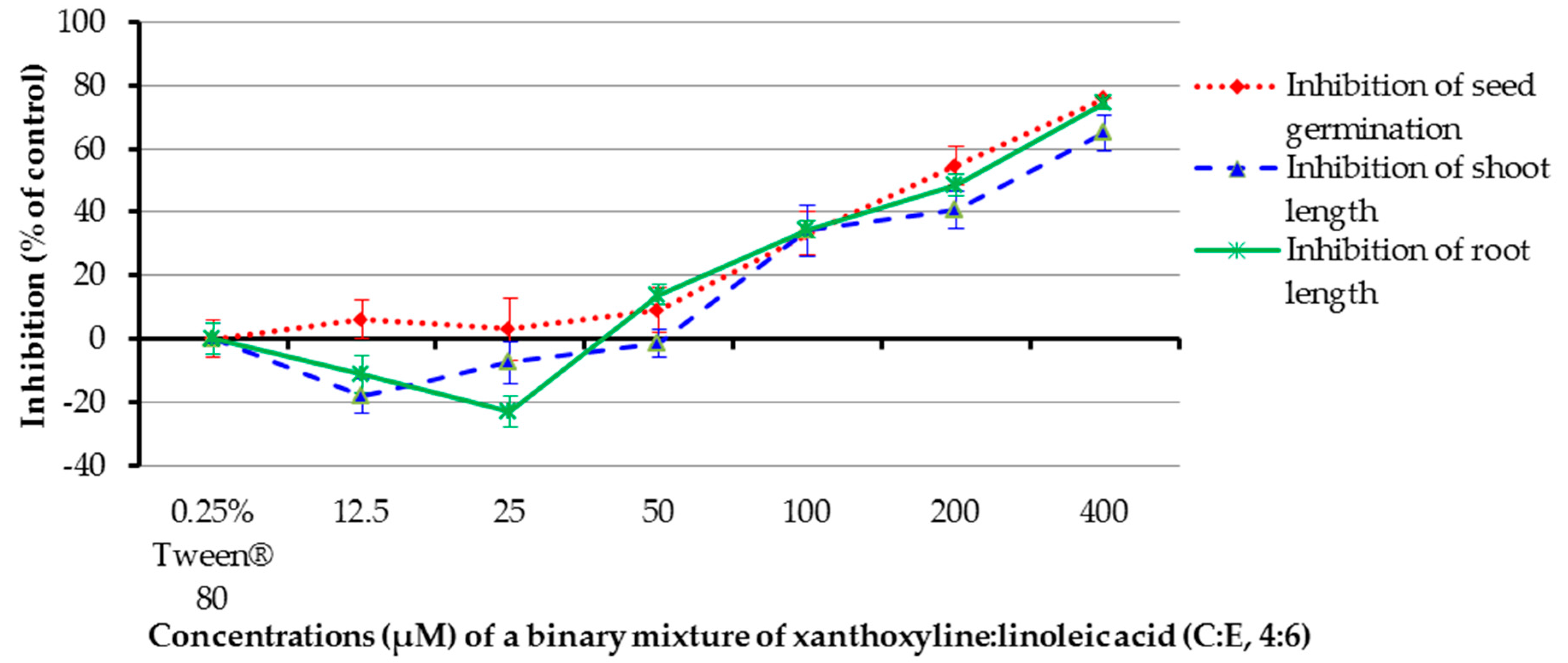
2.4.3. Allelopathic Effects of a Binary Mixture of Xanthoxyline:Linoleic Aicd (4:6) on Germination and Seedling Growth of Chinese Amaranth
3. Experimental
3.1. Chemicals and Instrument
3.2. Isolation of Xanthoxyline (C)
3.3. Isolation of (±)-Odorine (G)
3.4. Preparation of Aqueous Solutions of Tween® 80
3.5. Preparation of Aqueous Solutions of Seven Allelochemicals and Their Binary Mixtures (5:5 Mole Ratio)
3.6. Preparation of Aqueous Solutions of R-(+)-Limonene:Xanthoxyline (A:C, 2:8), Vanillin:Xanthoxyline (B:C, 2:8), and Xanthoxyline:Linoleic Acid (C:E, 4:6) at 400, 200, 100, 50, 25, and 12.5 μM
3.7. Tested Plant
3.8. Seed Germination and Seedling Growth Bioassay
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Tu, M.; Hurd, C.; Randall, J.M. Tools & techniques for use in natural areas. In Weed Control Methods Handbook; The Nature Conservancy: Arlington County, VA, USA, 2001; p. 533. [Google Scholar]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as bioherbicides—Present and perspectives. In Herbicides-Current Research and Case Studies in Use; Intech: Rijeka, Croatia, 2013; p. 662. [Google Scholar]
- Barnard, C.; Padgitt, M.; Uri, N.D. Pesticide use and its measurement. Int. Pest Control 1997, 39, 161–164. [Google Scholar]
- Batish, D.R.; Kaur, M.; Singh, H.P.; Kohli, R.K. Phytotoxicity of a medicinal plant, Anisomeles indica, against Phalaris minor and its potential use as natural herbicide in wheat fields. Crop Prot. 2007, 26, 948–952. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Akcin, T.A.; Mete, E.; Akcin, A.; Aydin, T.; Kilic, H. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan.(Asteraceae). Ind. Crops Prod. 2009, 29, 562–570. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic interactions and allelochemicals: New possibilities for sustainable weed management. Crit. Rev. Plant Sci. 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Duke, S.O.; Romagni, J.G.; Dayan, F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000, 19, 583–589. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Acadamic Press: Orlando, FL, USA, 1984; p. 422. [Google Scholar]
- Macías, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, J.C.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Charoenying, P.; Teerarak, M.; Laosinwattana, C. An allelopathic substance isolated from Zanthoxylum limonella Alston fruit. Sci. Hortic. 2010, 125, 411–416. [Google Scholar] [CrossRef]
- Laosinwattana, C.; Teerarak, M.; Charoenying, P. Effects of Aglaia odorata granules on the seedling growth of major maize weeds and the influence of soil type on the granule residue’s efficacy. Weed Biol. Manag. 2012, 12, 117–122. [Google Scholar] [CrossRef]
- Teerarak, M.; Charoenying, P.; Laosinwattana, C. Physiological and cellular mechanisms of natural herbicide resource from Aglaia odorata Lour. on bioassay plants. Acta Physiol. Plant. 2012, 34, 1277–1285. [Google Scholar] [CrossRef]
- Ribeiro, J.P.N.; Lima, M.I.S. Allelopathic effects of orange (Citrus sinensis L.) peel essential oil. Acta Bot. Bras. 2012, 26, 256–259. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Pratley, J.E.; An, M.; Luckett, D.J.; Lemerle, D. Metabolomics differentiation of canola genotypes: Toward an understanding of canola allelochemicals. Front. Plant Sci. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Dos Santos, V.H.M.; Daneluzzi, G.S.; Silva, L.P.; Silva, R.M.G. Evaluation of allelopathic potential of leaf extract of Kielmeyera coriacea on Lactuca sativa L. Biosci. J. 2015, 31, 259–267. [Google Scholar] [CrossRef]
- Einhellig, F.A. Interactions involving allelopathy in cropping systems. Agron. J. 1996, 88, 886–893. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Salam, M.A.; Suenaga, K. Isolation and identification of potent allelopathic substances in a traditional Bangladeshi rice cultivar Kartikshail. Plant Prod. Sci. 2011, 14, 128–134. [Google Scholar] [CrossRef]
- Kole, R.K.; Karmakar, P.R.; Poi, R.; Mazumdar, D. Allelopathic inhibition of teak leaf extract: A potential pre-emergent herbicide. J. Crop Weed 2011, 7, 101–109. [Google Scholar]
- Pedrol, N.; González, L.; Reigosa, M.J. Allelopathy and abiotic stress. In Allelopathy; Springer: Berlin, Germany, 2006; pp. 171–209. [Google Scholar]
- Szakiel, A.; Henry, M. Synergism of lyoniside and triterpenic acids in allelopathic potential of Vaccinium myrtillus L. Planta Med. 2008, 74, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Chaimovitsh, D.; Shachter, A.; Abu-Abied, M.; Rubin, B.; Sadot, E.; Dudai, N. Herbicidal activity of monoterpenes is associated with disruption of microtubule functionality and membrane integrity. Weed Sci. 2017, 65, 19–30. [Google Scholar] [CrossRef]
- Vaid, S.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Phytotoxicity of limonene against Amaranthus viridis L. Bioscan 2011, 6, 163–165. [Google Scholar]
- Wright, C.; Chhetri, B.K.; Setzer, W.N. Chemical composition and phytotoxicity of the essential oil of Encelia farinosa growing in the sonoran desert. Am. J. Essent. Oils Nat. Prod. 2013, 1, 18–22. [Google Scholar]
- Chuah, T.S.; Tan, P.K.; Ismail, B.S. Effects of adjuvants and soil microbes on the phytotoxic activity of coumarin in combination with p-vanillin on goosegrass (Eleusine indica L.) seedling emergence and growth. S. Afr. J. Bot. 2013, 84, 128–133. [Google Scholar] [CrossRef]
- Isfahan, M.N.; Shariati, M. The effect of some allelochemicals on seed germination of Coronilla varia L. seeds. Am. Eurasian J. Agric. Environ. Sci. 2007, 2, 534–538. [Google Scholar]
- Khang, D.T.; Anh, L.H.; Ha, T.; Tuyen, P.T.; Quan, N.; Minh, L.; Quan, N.T.; Minh, T.N.; Xuan, T.D.; Khanh, T.D. Allelopathic activity of dehulled rice and its allelochemicals on weed germination. Int. Lett. Nat. Sci. 2016, 58, 1–20. [Google Scholar] [CrossRef]
- Nasr, M.; Shariati, M. The use of allelochemicals to delay germination of Astragalus cycluphyllus seeds. J. Agron. 2005, 4, 147–150. [Google Scholar]
- Reigosa, M.J.; Souto, X.C.; Gonz, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Ghareib, H.R.A.; Abdelhamed, M.S.; Ibrahim, O.H. Antioxidative effects of the acetone fraction and vanillic acid from Chenopodium murale on tomato plants. Weed Biol. Manag. 2010, 10, 64–72. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N.; Triska, J. Exudation of allelopathic substances in buckwheat (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.R.M.; Girish, R.; Karthik, N.; Rajendran, R.; Mahendran, V.S. Allelopathic effects of phenolics and terpenoids extracted from Gmelina arborea on germination of black gram (Vigna mungo) and green gram (Vigna radiata). Allelopath. J. 2009, 23, 323–332. [Google Scholar]
- Stupnicka-Rodzynkiewicz, E.; Dabkowska, T.; Stoklosa, A.; Hura, T.; Dubert, F.; Lepiarczyk, A. The effect of selected phenolic compounds on the initial growth of four weed species. J. Plant Dis. Prot. 2006, 20, 479–486. [Google Scholar]
- Zhang, T.T.; Zheng, C.Y.; Hu, W.; Xu, W.W.; Wang, H.F. The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. J. Appl. Phycol. 2010, 22, 71–77. [Google Scholar] [CrossRef]
- An, Z.; Wang, Z.; Li, F.; Tian, Z.; Hu, H. Allelopathic inhibition on red tide microalgae Skeletonema costatum by five macroalgal extracts. Front. Environ. Sci. Eng. China 2008, 2, 297–305. [Google Scholar] [CrossRef]
- Ni, L.; Jie, X.; Wang, P.; Li, S.; Wang, G.; Li, Y.; Li, Y.; Acharya, K. Effect of linoleic acid sustained-release microspheres on Microcystis aeruginosa antioxidant enzymes activity and microcystins production and release. Chemosphere 2015, 121, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lavoie, M.; Fan, X.; Tan, H.; Liu, G.; Xu, P.; Fu, Z.; Paerl, H.W.; Qian, H. Allelopathic interactions of linoleic acid and nitric oxide increase the competitive ability of Microcystis aeruginosa. ISME J. 2017, 11, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Uzun, Y.; Türker, M.; Kaya, A.; Demirel, K. Allelopathic potential of macrofungi on germinating maize (Zea mays L.) grain. Afr. J. Biotechnol. 2010, 9, 1016–1023. [Google Scholar]
- Annarao, S.; Sidhu, O.P.; Roy, R.; Tuli, R.; Khetrapal, C.L. Lipid profiling of developing Jatropha curcas L. seeds using 1H NMR spectroscopy. Bioresour. Technol. 2008, 99, 9032–9035. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Valencia, V.A.; Us-Vázquez, R.A.; Larqué-Saavedra, F.A.; Barahona-Pérez, L.F. Naturally occurring fatty acid methyl esters and ethyl esters in the green microalga Chlamydomonas reinhardtii. Ann. Microbiol. 2012, 62, 865–870. [Google Scholar] [CrossRef]
- Perez, E.; Martin, D.F. Critical micelle concentrations of allelopathic substances produced by Nannochloris oculata which affect a red tide organism, Gymnodinium breve. Cytobios 2001, 106, 163–170. [Google Scholar] [PubMed]
- Perez, E.; Sawyers, W.G.; Martin, D.F. Identification of allelopathic substances produced by Nannochloris oculata that affect a red tide organism, Gymnodinium breve. Biomed. Lett. 1997, 56, 7–14. [Google Scholar]
- Arora, K.; Batish, D.; Kohli, R.; Singh, H. Allelopathic impact of essential oil of Tagetes minuta on common agricultural and wastedland weeds. Innovare J. Agric. Sci. 2017, 5, 1–4. [Google Scholar]
- Deka, S.; Rao, S. Allelopathic effects on herbaceous weed plant seeds germination. Int. J. Phytopharm. 2015, 6, 225–228. [Google Scholar]
- Hossain, M.K.; Anwar, S.; Nandi, R. Allelopathic effects of Mikania cordata on forest and agricultural crops in Bangladesh. J. For. Res. 2016, 27, 155–159. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Zhou, J. N deposition affects allelopathic potential of Amaranthus retroflexus with different distribution regions. An. Acad. Bras. Cienc. 2017, 89, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Cheng, Z.H.; Meng, H.W. Transcriptomic insights into the allelopathic effects of the garlic allelochemical diallyl disulfide on tomato roots. Sci. Rep. 2016, 6, 38902. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, C.A.S.; Cardoso, M.D.G.; de Carvalho, M.L.M.; Figueiredo, A.C.S.; Nelson, D.L.; de Oliveira, C.M.; Gomes, M.D.S.; de Andrade, J.; de Souza, J.A.; de Albuquerque, L.R.M. Chemical composition and allelopathic activity of Parthenium hysterophorus and Ambrosia polystachya weeds essential oils. Am. J. Plant Sci. 2014, 5, 1248–1257. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Choopayak, C.; Woranoot, K.; Naree, P.; Kongbangkerd, A.; Wongkrajang, K.; Buaruaeng, R. Phytotoxic effects of Piper betle L. extracts on germination of Eclipta prostrata L. and Chloris barbata Sw. weeds. NU. Int. J. Sci. 2016, 12, 11–24. [Google Scholar]
- De Oliveira, C.M.; das Graças Cardoso, M.; da Silva Figueiredo, A.C.; de Carvalho, M.L.M.; de Miranda, C.A.S.F.; Albuquerque, L.R.M.; Nelson, D.L.; de Souza Gomes, M.; Silva, L.F.; de Andrade Santiago, J. Chemical composition and allelopathic activity of the essential oil from Callistemon viminalis (myrtaceae) blossoms on lettuce (Lactuca sativa L.) seedlings. Am. J. Plant Sci. 2014, 5, 3551–3557. [Google Scholar] [CrossRef]
- Dilipkumar, M.; Chuah, T.S. Is combination ratio an important factor to determine synergistic activity of allelopathic crop extract and herbicide? Int. J. Agric. Biol. 2013, 15, 259–265. [Google Scholar]
- Hegab, M.M.; Khodary, S.E.A.; Hammouda, O.; Ghareib, H.R. Autotoxicity of chard and its allelopathic potentiality on germination and some metabolic activities associated with growth of wheat seedlings. Afr. J. Biotechnol. 2008, 7, 884–892. [Google Scholar]
- Wang, H.Q.; Wu, Z.B.; Zhang, S.H.; Cheng, S.P.; He, F.; Liang, W. Relationship between the allelopathic activity and molecular structure of hydroxyl derivatives of benzoic acid and their effects on cyanobacterium Microcystis aeruginosa. Allelopath. J. 2008, 22, 205–211. [Google Scholar]
- Zhang, T.T.; Hu, W.; Zhang, D. Allelopathic effect of Typha Angustifolia L. on phytoplankton. Adv. Mat. Res. 2012, 383–390, 3724–3728. [Google Scholar] [CrossRef]
- Wang, C.M.; Li, T.C.; Jhan, Y.L.; Weng, J.H.; Chou, C.H. The impact of microbial biotransformation of catechin in enhancing the allelopathic effects of Rhododendron formosanum. PLoS ONE 2013, 8, e85162. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M.; Whitehand, L.C. Adverse synergistic effects between acetic, propionic, butyric and valeric acids on the growth of wheat seedling roots. Soil Biol. Biochem. 1980, 12, 445–446. [Google Scholar] [CrossRef]
- Zhang, T.T.; He, M.; Wu, A.P.; Nie, L.W. Allelopathic effects of submerged macrophyte Charo vulgaris on toxic Microcystis aeruginosa. Allelopath. J. 2009, 23, 391–401. [Google Scholar]
- Zuo, S.; Zhou, S.; Ye, L.; Ding, Y.; Jiang, X. Antialgal effects of five individual allelochemicals and their mixtures in low level pollution conditions. Environ. Sci. Pollut. Res. 2016, 23, 15703–15711. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.G.; de Felippe, A.P.V.; Guimarães, E.F.; Kato, M.J.; Ellena, J.; Doriguetto, A.C. 2-Hydroxy-4, 6-dimethoxyacetophenone from leaves of Peperomia glabella. J. Braz. Chem. Soc. 2006, 17, 1205–1210. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal Activities of Some Allelochemicals and Their Synergistic Behaviors toward Amaranthus tricolor L. Molecules 2017, 22, 1841. https://doi.org/10.3390/molecules22111841
Chotsaeng N, Laosinwattana C, Charoenying P. Herbicidal Activities of Some Allelochemicals and Their Synergistic Behaviors toward Amaranthus tricolor L. Molecules. 2017; 22(11):1841. https://doi.org/10.3390/molecules22111841
Chicago/Turabian StyleChotsaeng, Nawasit, Chamroon Laosinwattana, and Patchanee Charoenying. 2017. "Herbicidal Activities of Some Allelochemicals and Their Synergistic Behaviors toward Amaranthus tricolor L." Molecules 22, no. 11: 1841. https://doi.org/10.3390/molecules22111841




