Polyphosphates as Inhibitors for Poly(vinyl Chloride) Photodegradation
Abstract
:1. Introduction
2. Results and Discussion
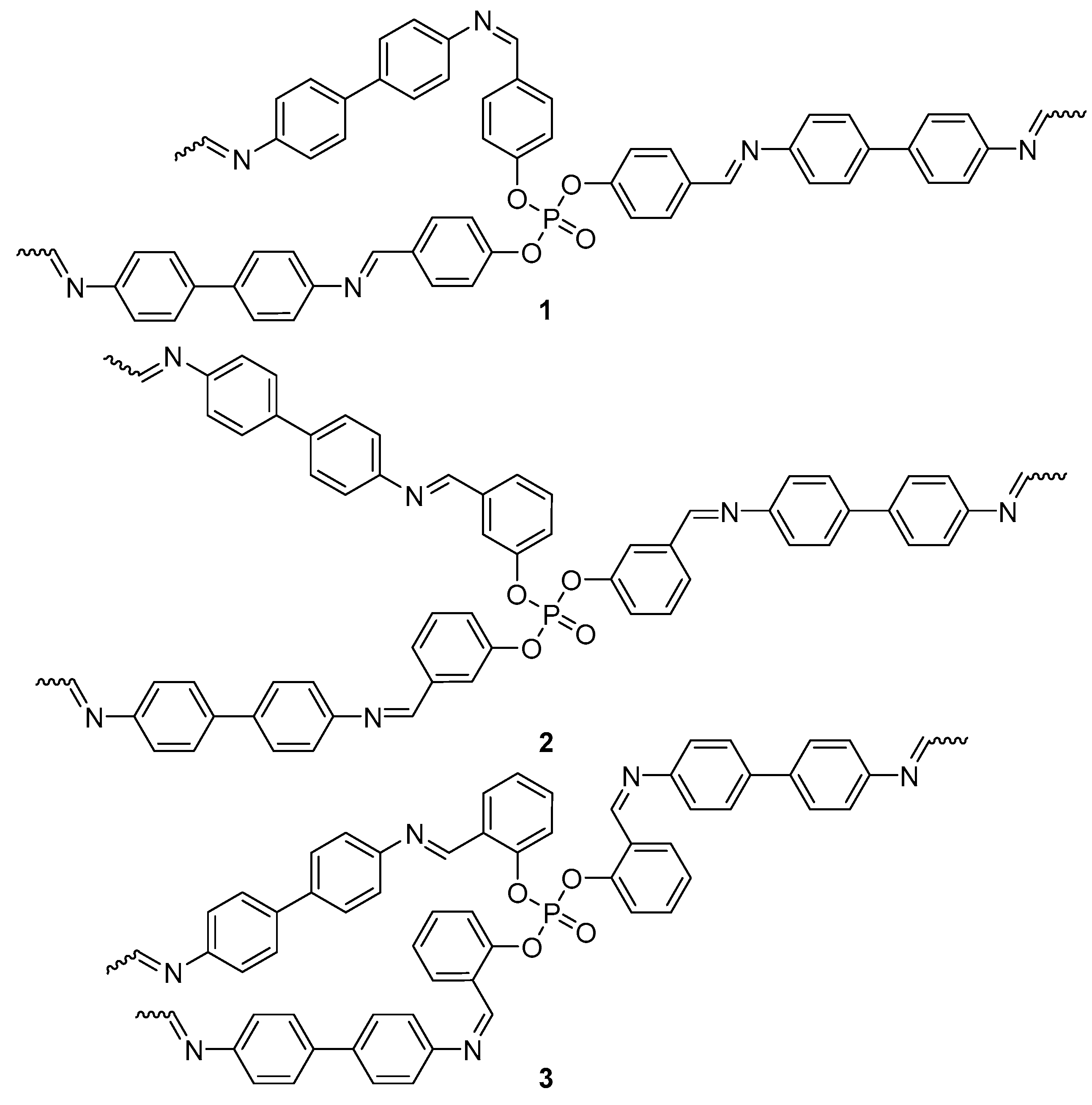
2.1. Synthesis of Polyphosphates 1−3
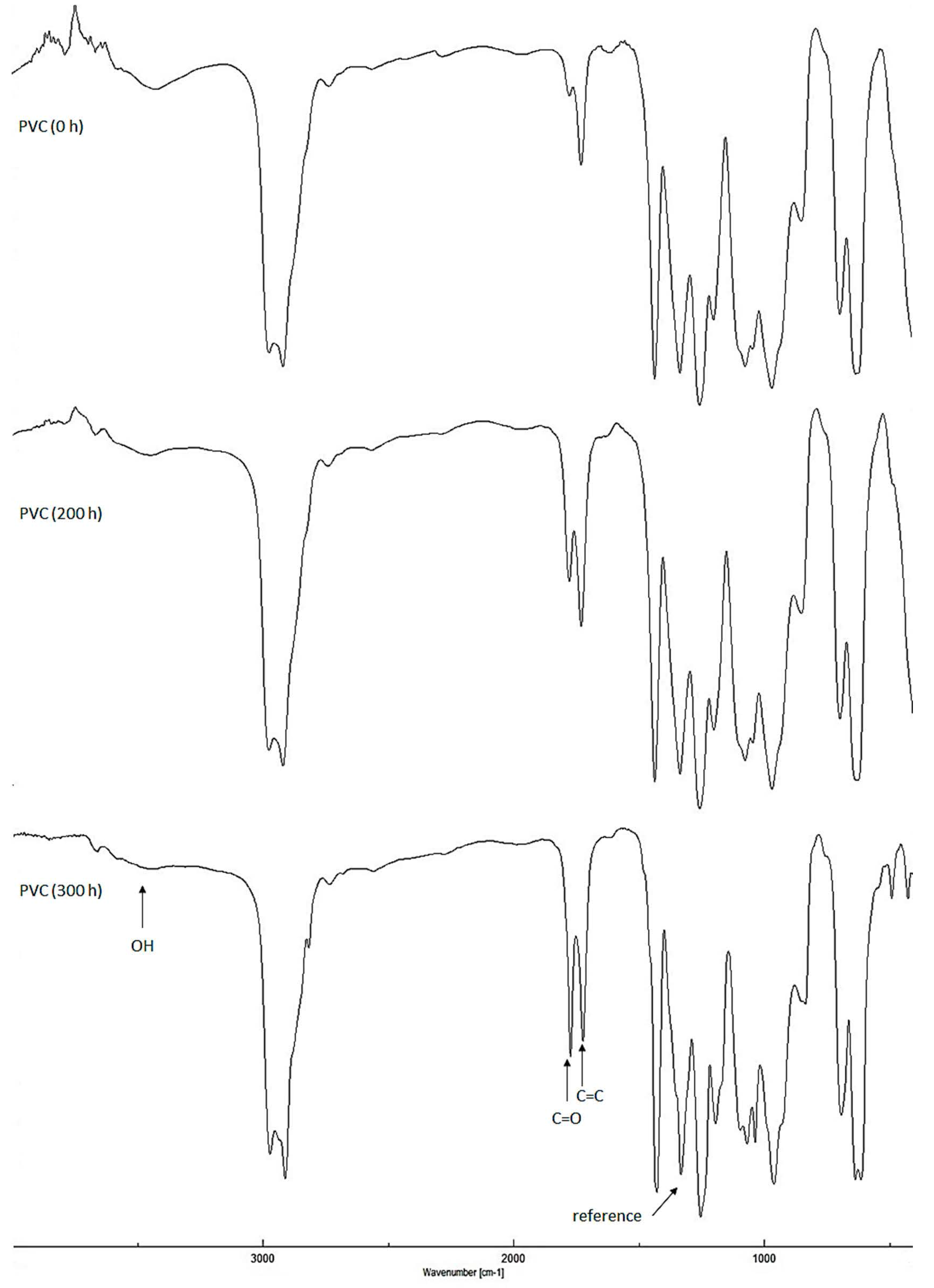
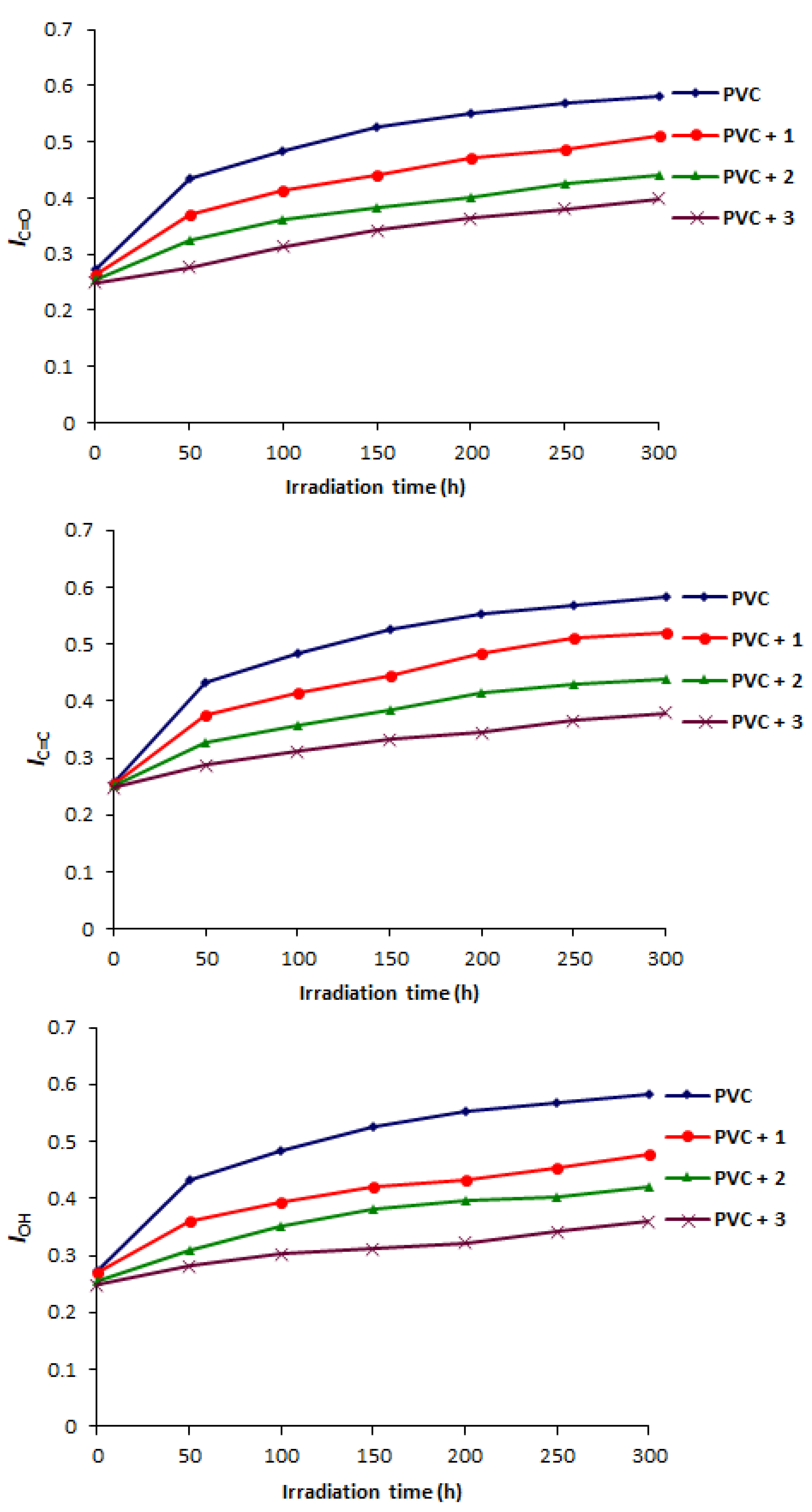
2.2. Determination of PVC Photodegradation by Fourier Transform Infrared (FTIR) Spectrophotometry
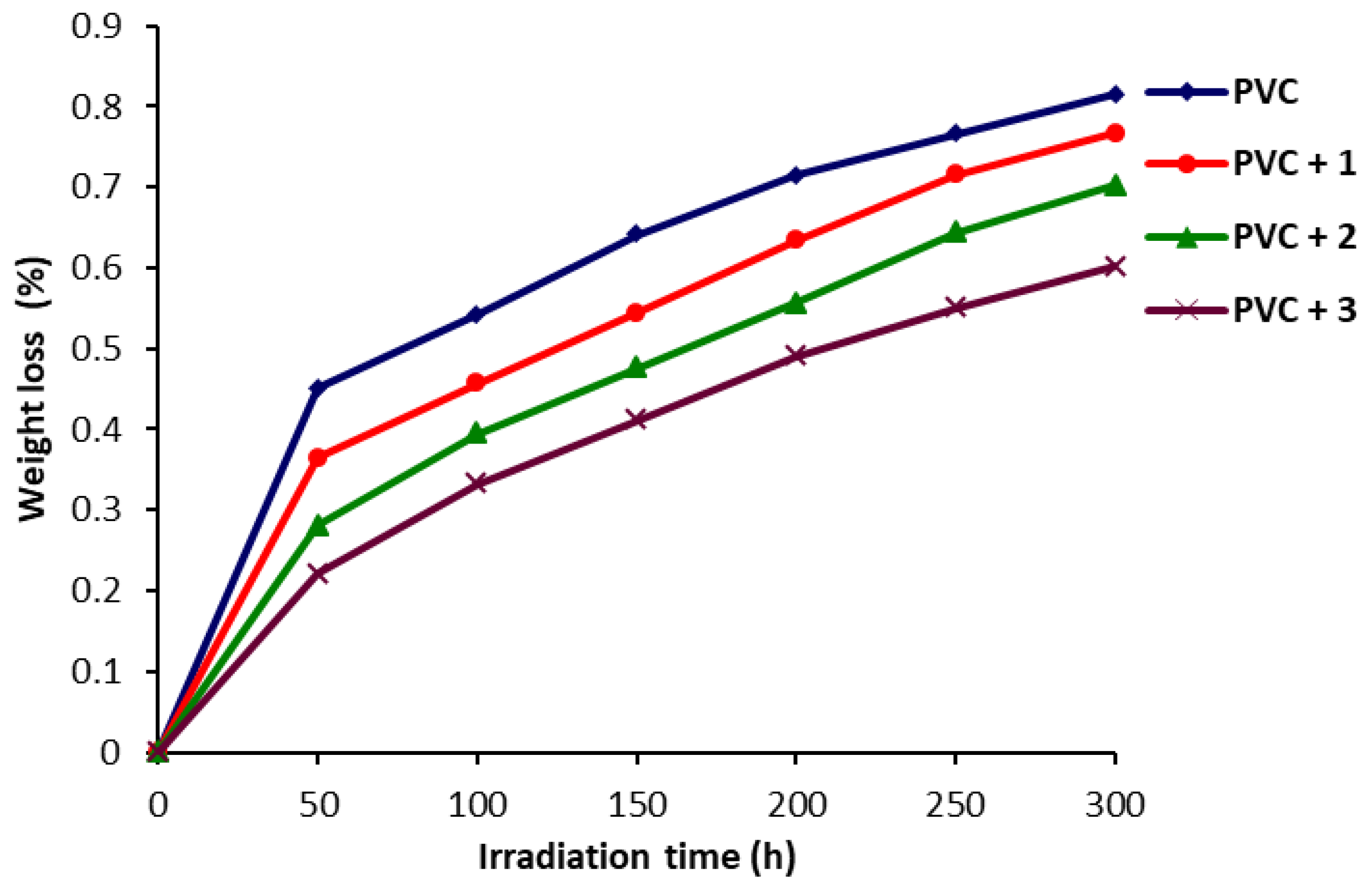
2.3. Determination of PVC Photodegradation by Weight Loss
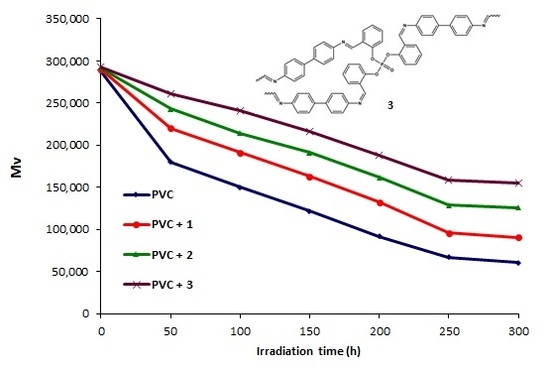
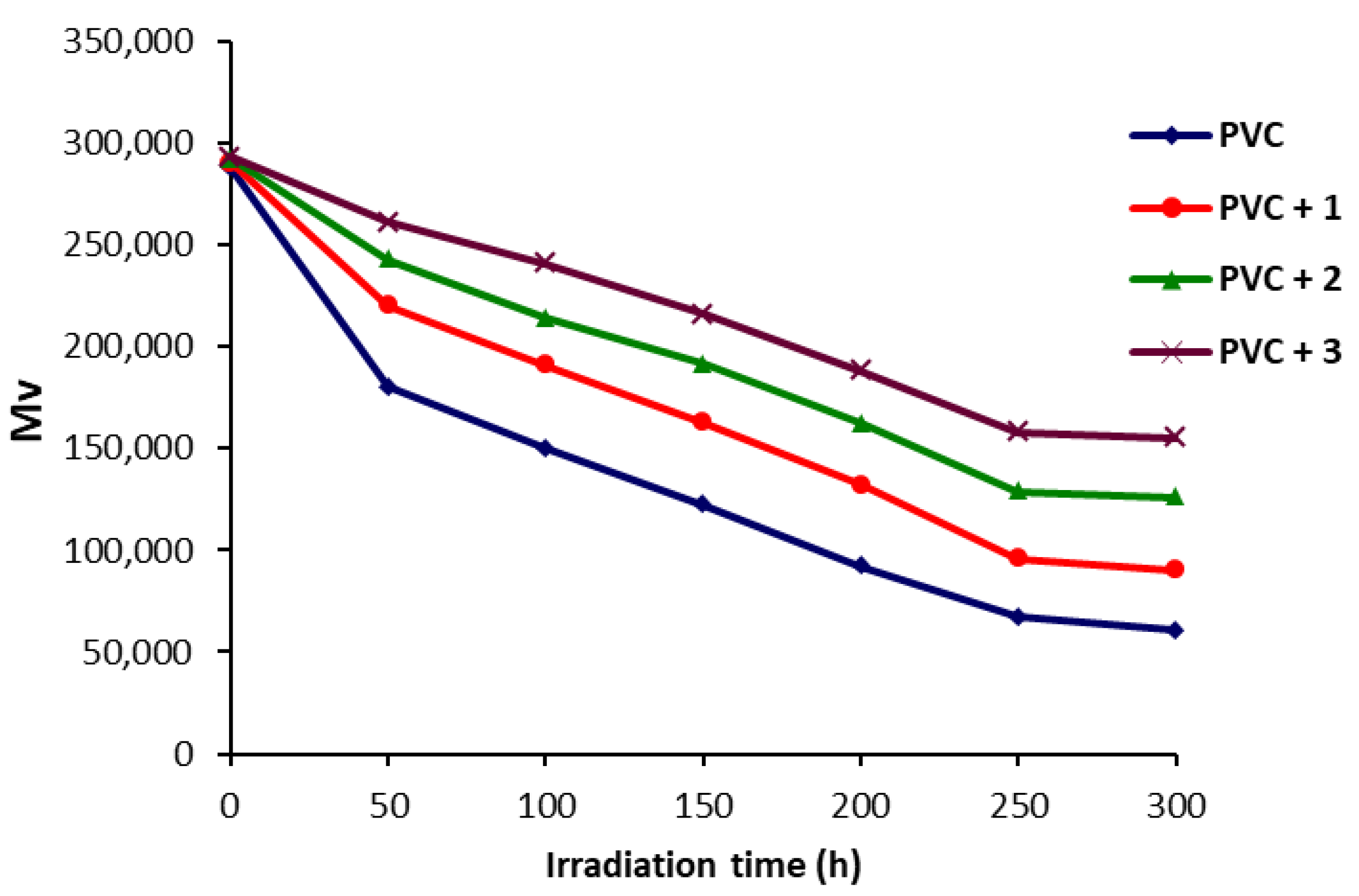
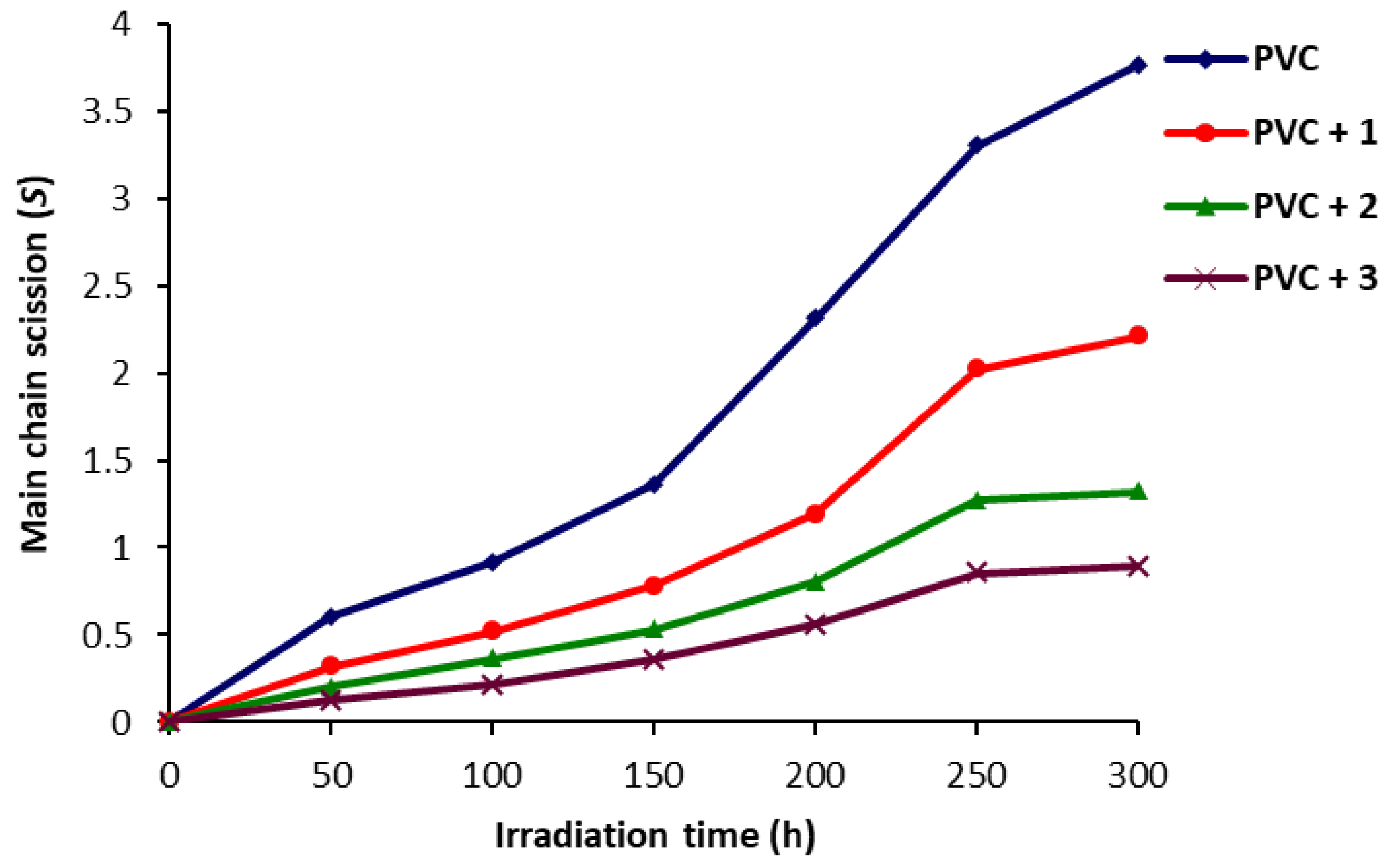
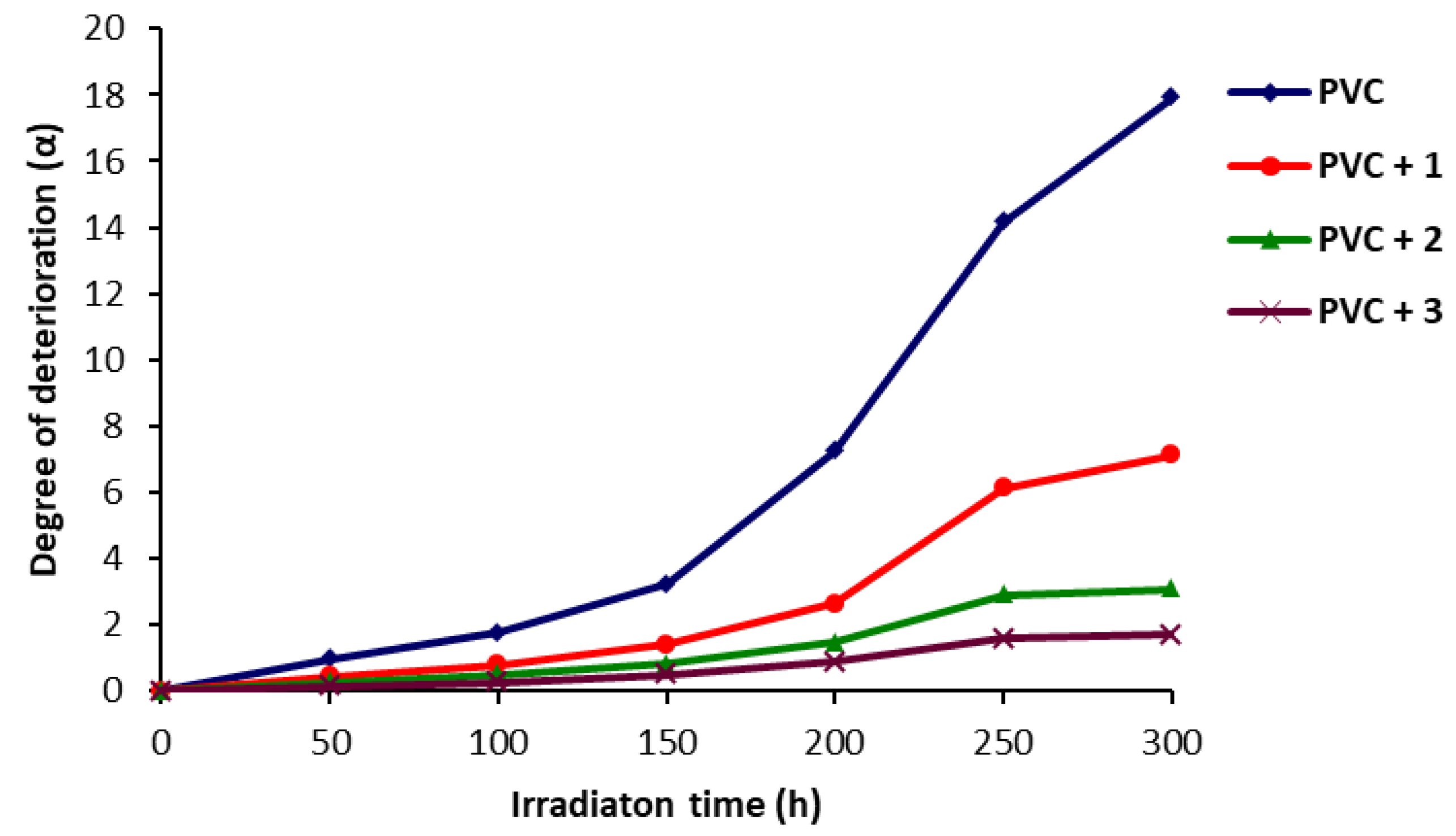
2.4. Determination of PVC Photodegradation by Viscometry Method
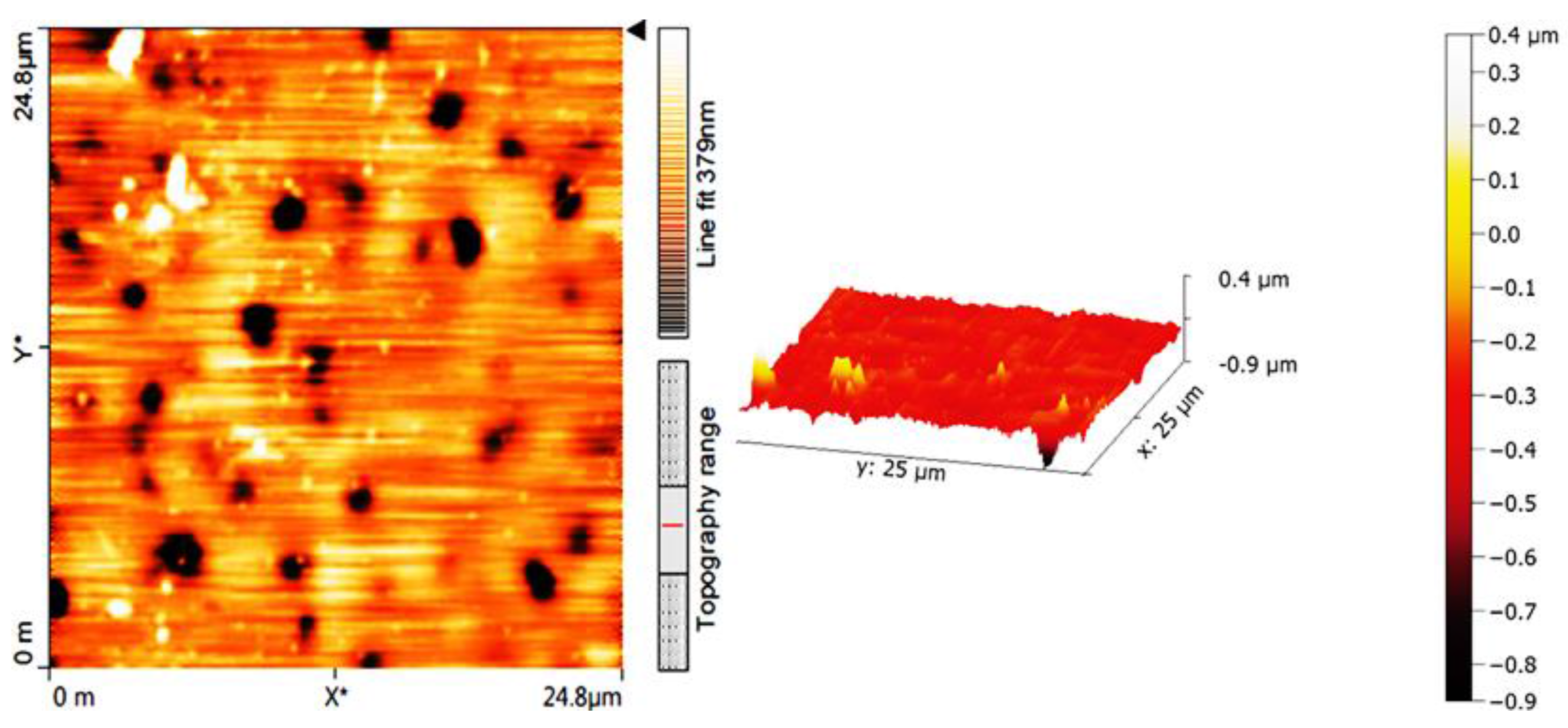
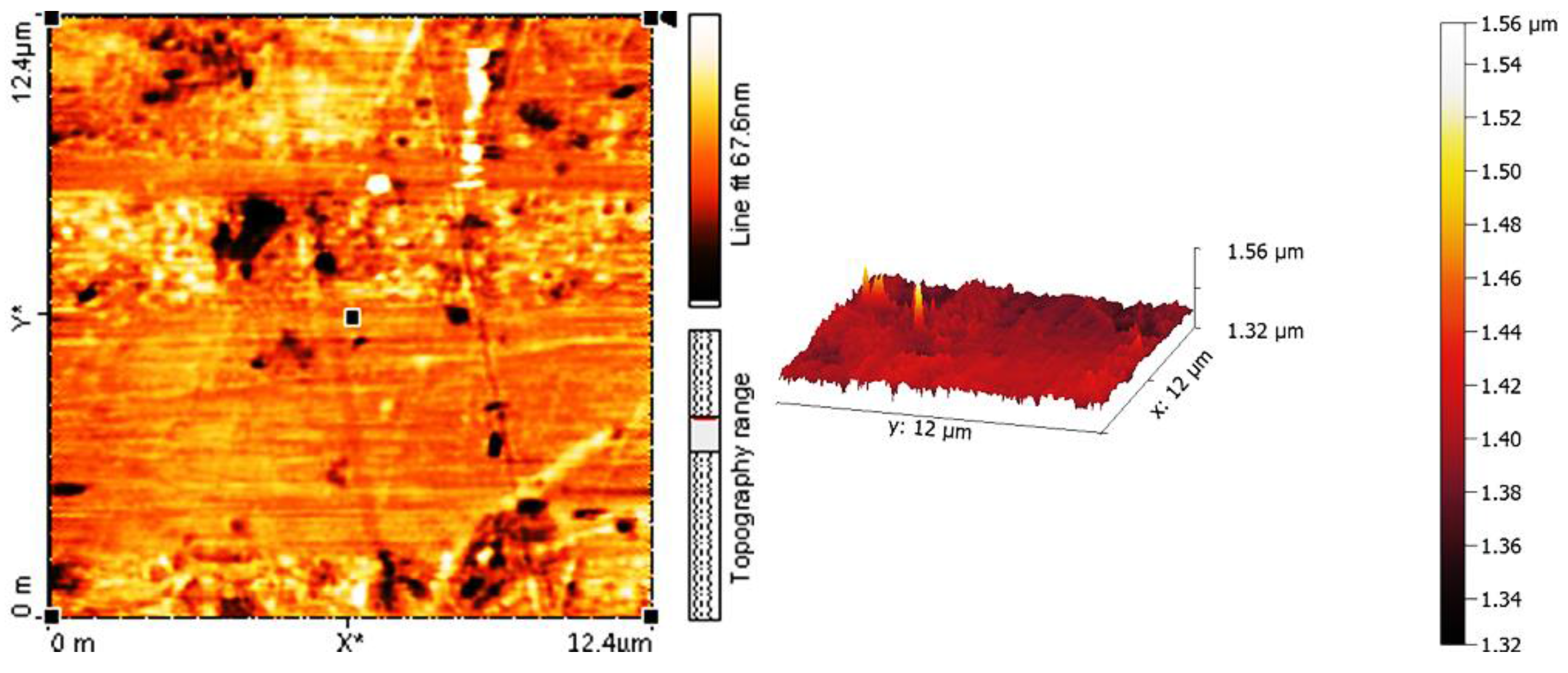
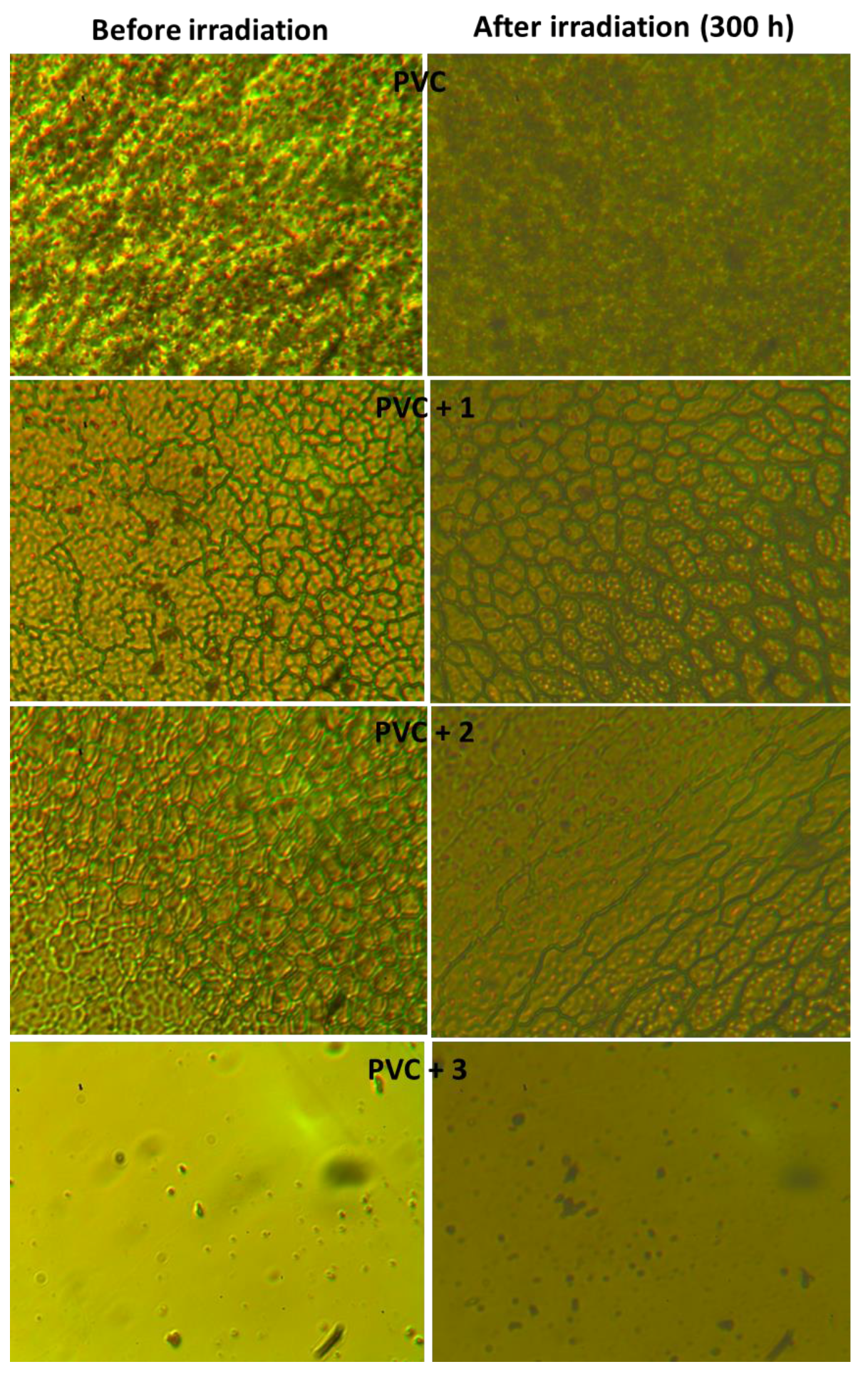
2.5. Determination of PVC Photodegradation by Surface Morphology
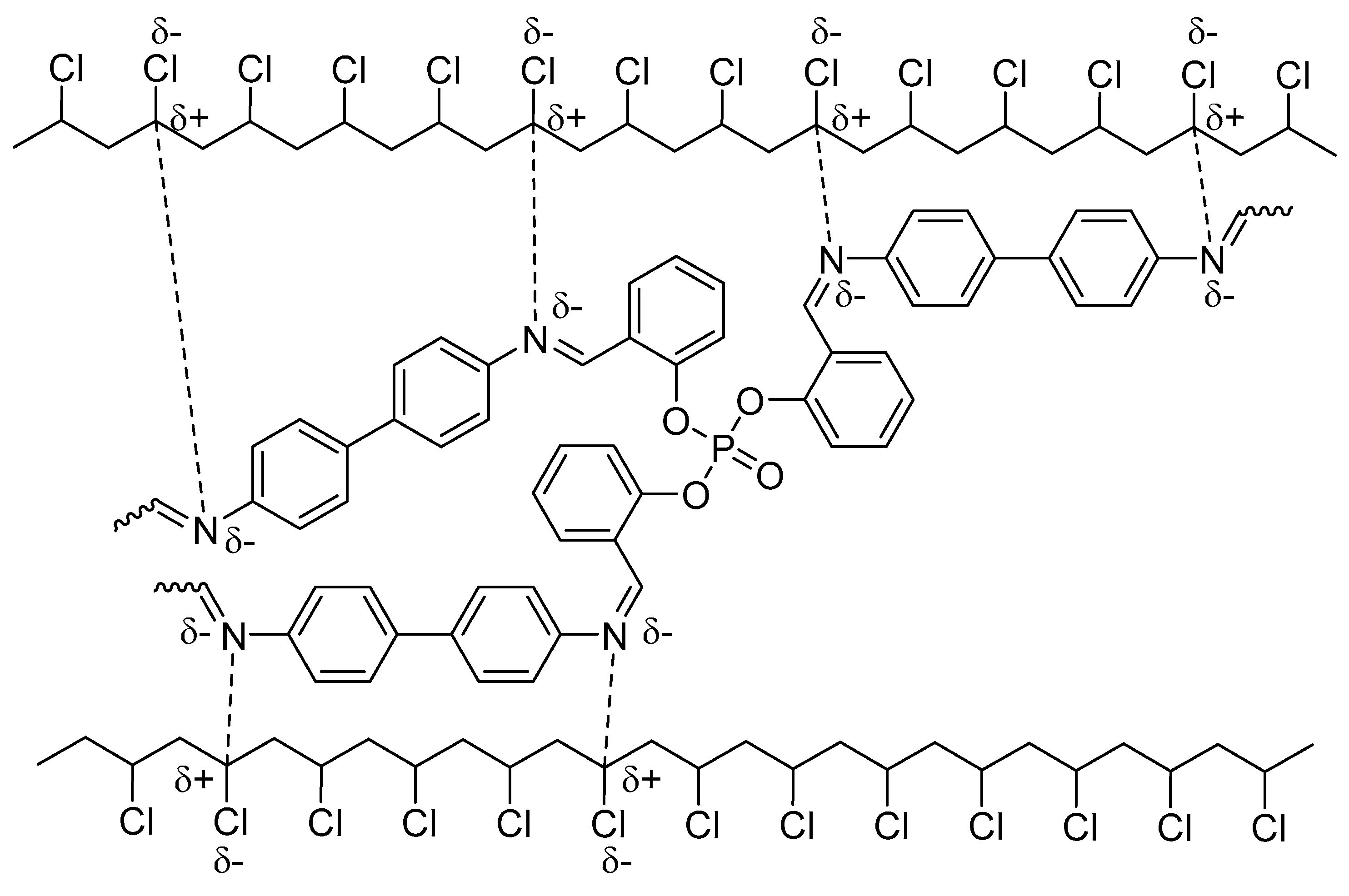
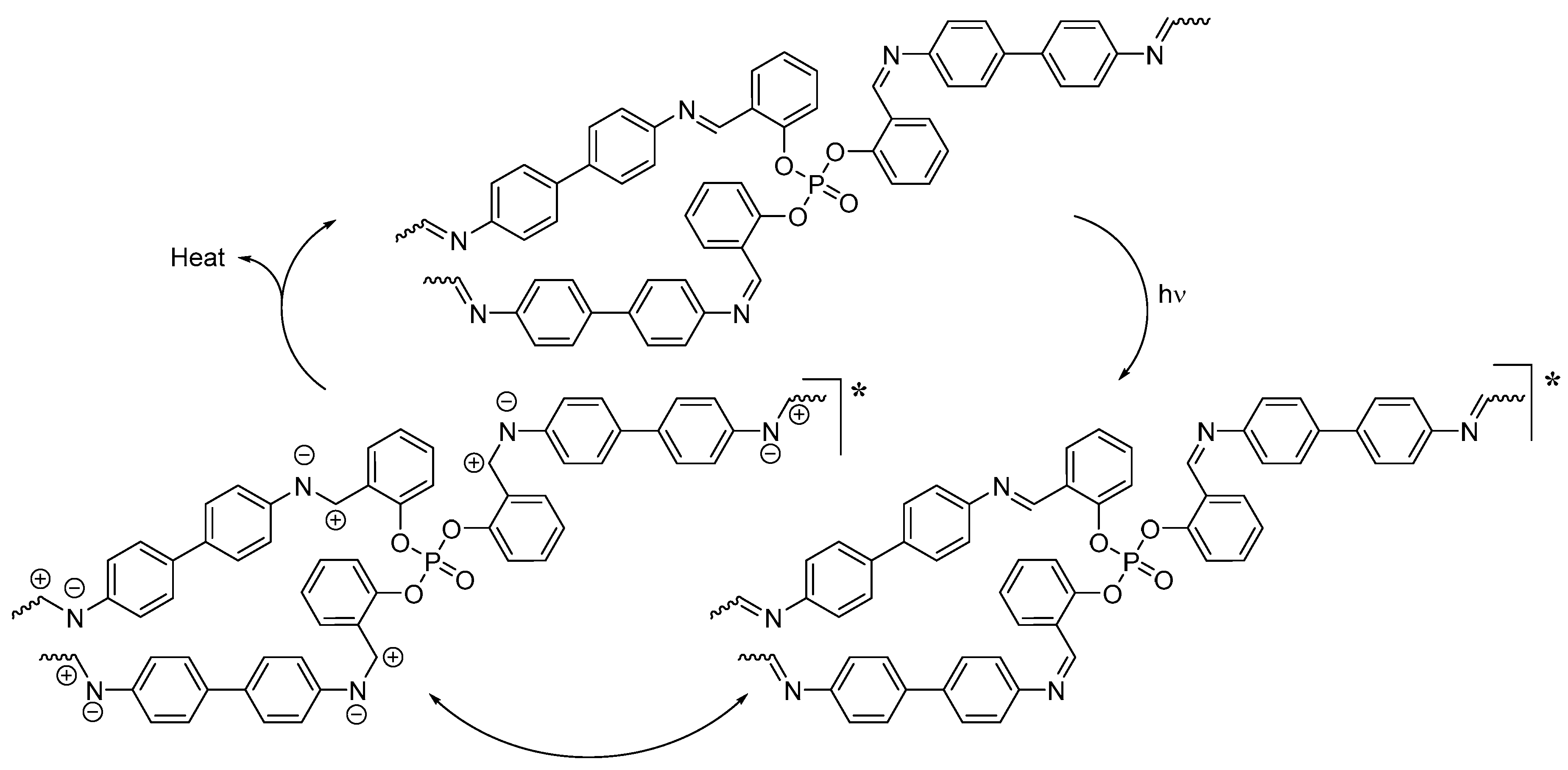
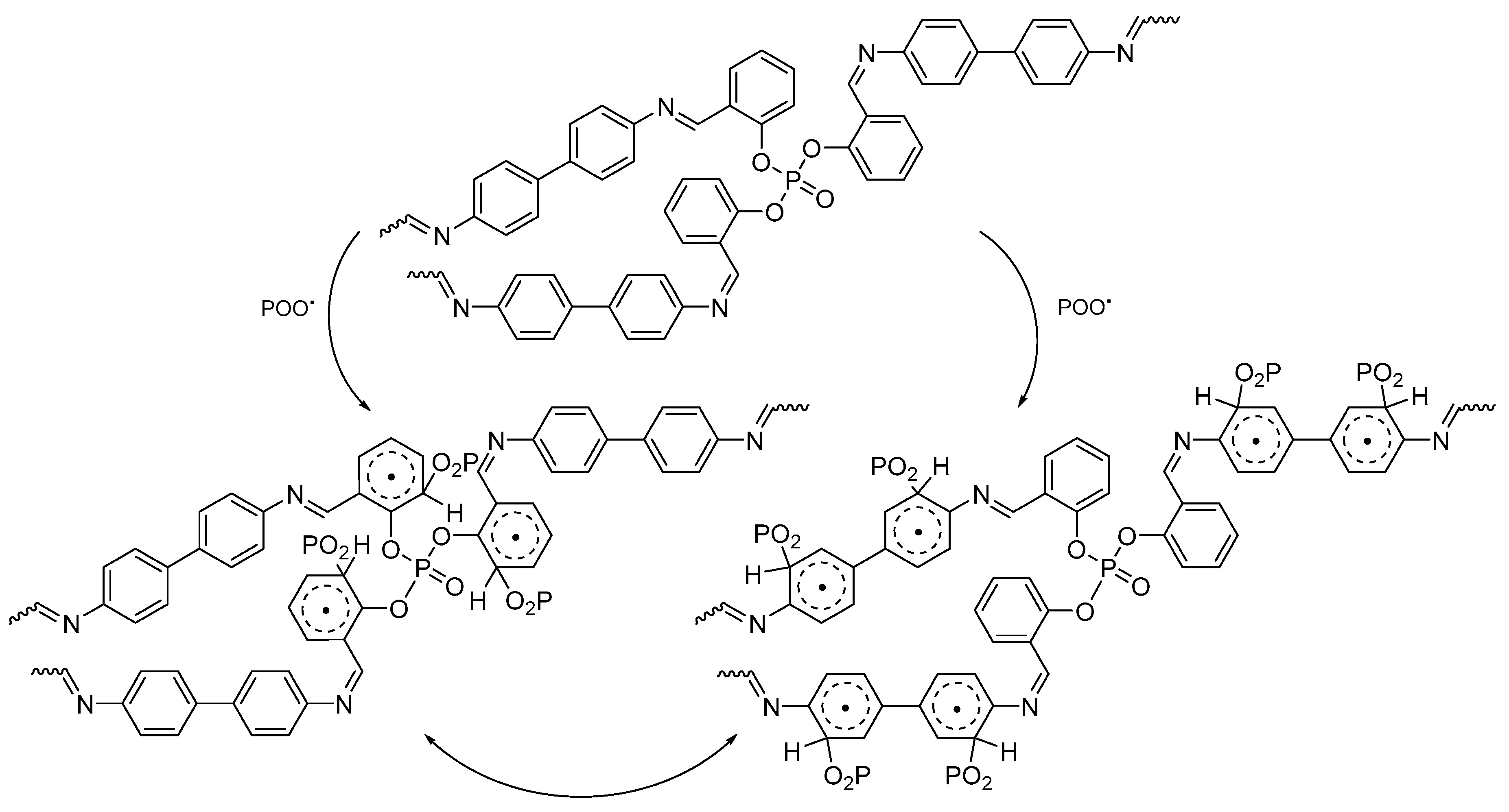
2.6. Suggested Mechanisms of PVC Photostabilization in the Presence of Polyphosphate
3. Experimental
3.1. General
3.2. Preparation of PVC Films
3.3. Determination of PVC Photodegradation by FTIR Spectrophotometry
3.4. Determination of PVC Photodegradation by Weight Loss
3.5. Determination of PVC Photodegradation by the Viscometry Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Dahl, J.A.; Maddux, B.L.S.; Hutchison, J.E. Toward greener nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef] [PubMed]
- Rabek, J.F. Comprehensive Chemical Kinetic; Degradation of Polymers; Bamford, C.H., Tipper, C.H.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1975; Volume 1. [Google Scholar]
- Andrady, A.L.; Torikai, A.; Fueki, K. Photodegradation of rigid PVC formulations. I. Wavelength sensitivity of light induced yellowing by monochromatic light. J. Appl. Polym. Sci. 1989, 37, 935–946. [Google Scholar] [CrossRef]
- Yousif, E.; Ahmed, A.; Abood, R.; Jaber, N.; Noaman, R.; Yusop, R. Poly(vinyl chloride) derivatives as stabilizers against photodegradation. J. Taibah Univ. Sci. 2015, 9, 203–212. [Google Scholar] [CrossRef]
- Rabie, S.T.; Khalil, A.M.; Nada, A.A. Diamide derivatives as photostabilizers for plasticized poly(vinyl chloride). J. Vinyl Addit. Technol. 2008, 14, 191–196. [Google Scholar] [CrossRef]
- Anton-Prinet, C.; Mur, G.; Gay, M.; Audouin, L.; Verdu, J. Photoageing of rigid PVC III. Influence of exposure conditions on the thickness distribution of photoproducts. Polym. Degrad. Stab. 1998, 60, 283–289. [Google Scholar] [CrossRef]
- Torikai, A.; Hasegawa, H. Accelerated photodegradation of poly(vinyl chloride). Polym. Degrad. Stab. 1999, 63, 441–445. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, C.; Pavlinek, V.; Saha, P.; Wang, H. Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl. Surf. Sci. 2006, 252, 4154–4160. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Zhang, L.; Xu, S.; Zhou, Q.; Zhu, Y.; Qu, X. Main factors in preparation of antibacterial particles/PVC composite. China Particuol. 2004, 2, 226–229. [Google Scholar] [CrossRef]
- Birmingham, J.N. The effect of surface oxidation and titanium dioxide on exterior PVC color retention. J. Vinyl Addit. Technol. 1995, 1, 84–87. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015, 510390. [Google Scholar] [CrossRef]
- Yousif, E.; Hameed, A.; Rasheed, R.; Mansoor, H.; Farina, Y.; Graisa, A.; Salih, N.; Salimon, J. Synthesis and photostability study of some modified poly(vinyl chloride) containing pendant benzothiazole and benzimidozole ring. Int. J. Chem. 2010, 2, 65–80. [Google Scholar] [CrossRef]
- Yousif, E.; Al-Amiery, A.A.; Kadihum, A.; Kadhum, A.H.; Mohamad, A. Photostabilizing efficiency of PVC in the presence of Schiff bases as photostabilizers. Molecules 2015, 20, 19886–19899. [Google Scholar] [CrossRef] [PubMed]
- Tomi, I.H.R.; Ali, G.Q.; Jawad, A.H.; Yousef, E. Synthesis and characterization of gallic acid derivatives and their utilized as organic photo-stabilizers for poly(vinyl chloride). J. Polym. Res. 2007, 24, 119. [Google Scholar] [CrossRef]
- Zhao, Y.; Dan, Y. Preparation and characterization of a high molecular weight UV-stabilizer based on a derivative of 2,4-dihydroxybenzophenone and its application in polymer materials. J. Appl. Polym. Sci. 2006, 102, 2203–2211. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohammed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Yousif, E.; Hameed, A.; Salih, N.; Salimon, J.; Abdullah, B. New photostabilizers for polystyrene based on 2,3-dihydro-(5-mercapto-1,3,4-oxadiazol-2-yl)-phenyl-2-(substituted)-1,3,4-oxazepine-4,7-dione compounds. SpringerPlus 2013, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- Bojinov, V.B.; Grabchev, I.K. Novel functionalized 2-(2-hydroxyphenyl)-benzotriazole-benzo[de]isoquinoline-1,3-dione fluorescent UV absorbers: Synthesis and photostabilizing efficiency. J. Photochem. Photobiol. A Chem. 2005, 172, 308–315. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdul Naby, A.S.; Mohamed, R.R. N-Phenyl-3-substituted-5-pyrazolone derivatives as organic stabilizer for rigid PVC against photodegradation. J. Appl. Polym. Sci. 2005, 101, 1543–1555. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef]
- Corbridge, D.E.C. Phosphorus: Chemistry, Biochemistry and Technology, 6th ed.; CRC Press: New York, NY, USA, 2013. [Google Scholar]
- Iliescu, S.; Zubizarreta, L.; Plesu, N.; Macarie, L.; Popa, A.; Ilia, G. Polymers containing phosphorus groups and polyethers: From synthesis to application. Chem. Cent. J. 2012, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.-J. Phosphorus-containing polymers: A great opportunity for the biomedical field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Sun, J.; Wu, B.; Zhou, Q. Synthesis and properties of a phosphorus-containing flame retardant epoxy resin based on bis-phenoxy (3-hydroxy) phenyl phosphine oxide. Polym. Degrad. Stab. 2007, 92, 956–961. [Google Scholar] [CrossRef]
- Petreus, O.; Vlad-Bubulac, T.; Hamciuc, C. Synthesis and characterization of new polyesters with enhanced phosphorus content. Eur. Polym. J. 2015, 41, 2663–2670. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Wang, Y.-Z.; Ban, D.-M.; Yang, B.; Zhao, G.-M. A novel phosphorus-containing polymer as a highly effective flame retardant. Macromol. Mater. Eng. 2004, 289, 703–707. [Google Scholar] [CrossRef]
- Ranganathan, T.; Zilberman, J.; Farris, R.J.; Coughlin, E.B.; Emrick, T. Synthesis and characterization of halogen-free antiflammable polyphosphonates containing 4,4′-bishydroxydeoxybenzoin. Macromolecules 2006, 39, 5974–5975. [Google Scholar] [CrossRef]
- Roy, S.; Maiti, S. Design of multiple flame-retardant polymers. J. Appl. Polym. Sci. 2001, 81, 785–792. [Google Scholar] [CrossRef]
- Gupta, A.S.; Lopina, S.T. Synthesis and characterization of l-tyrosine based novel polyphosphates for potential biomaterial applications. Polymer 2004, 45, 4653–4662. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Akiyoshi, K. Synthesis and characterization of amphiphilic polyphosphates with hydrophilic graft chains and cholesteryl groups as nanocarriers. Biomacromolecules 2006, 7, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Screening and evaluation of poly(3-hydroxybutyrate) with Rhodococcus equi using different carbon sources. Arab. J. Sci. Eng. 2017, 42, 2371–2379. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016, 5, 762. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Al-Zuhairi, A.J.; El-Hiti, G.A.; Alshammari, M.B. Comparison of cyclic and polymeric disulfides as catalysts for the regioselective chlorination of phenols. J. Sulfur Chem. 2015, 36, 74–85. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl)propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; El-Hiti, G.A. Poly(propylene sulfide)-borane: Convenient and versatile reagent for organic synthesis. Tetrahedron 2012, 68, 7834–7839. [Google Scholar] [CrossRef]
- Rabek, J.; Ranby, B. Photodegradation, Photooxidation and Photostabilization of Polymers; John Wiley and Sons: New York, NY, USA, 1975. [Google Scholar]
- Yousif, E.; Salimon, J.; Salih, N.; Jawad, A.; Win, Y.-F. New stabilizers for PVC based on some diorganotin(IV) complexes with benzamidoleucine. Arab. J. Chem. 2016, 9, S1394–S1401. [Google Scholar] [CrossRef]
- Grassie, N.; Scott, G. Polymer Degradation and Stabilisation; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Jawanda, M.; Lai, B.F.L.; Kizhakkedathu, J.N.; Ishihara, K.; Narain, R. Linear and hyperbranched phosphoryl choline based homopolymers for blood biocompatibility. Polym. Chem. 2013, 4, 3140–3146. [Google Scholar] [CrossRef]
- Skillicorn, D.E.; Perkins, G.G.A.; Slark, A.; Dawkins, J.V. Molecular weight and solution viscosity characterization of PVC. J. Vinyl Addit. Technol. 1993, 15, 105–108. [Google Scholar] [CrossRef]
- Pepperl, G. Molecular weight distribution of commercial PVC. J. Vinyl Addit. Technol. 2000, 6, 88–92. [Google Scholar] [CrossRef]
- Campbell, D.; White, J.R. Polymer Characterization: Physical Techniques; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Jellinek, H.H.G. Aspects of Degradation and Stabilization of Polymers; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Kara, F.; Aksoy, E.A.; Yuksekdagd, Z.; Hasirci, N.; Aksoy, S. Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties. Carbohydr. Polym. 2014, 112, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-G.; Tang, L.-H.; Zhang, N.; Gao, Q.-H.; Zhang, C.-F.; Zhu, Z.-B. Dehydrochlorination of PVC materials at high temperature. Energy Fuels 2003, 17, 896–900. [Google Scholar] [CrossRef]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; pp. 48–49. [Google Scholar]
- González, A.; Pastor, J.; De Saja, J.A. Monitoring the UV degradation of PVC window frames by micro hardness analysis. J. Appl. Polym. Sci. 1989, 38, 1879–1882. [Google Scholar] [CrossRef]
- Rabek, J.F. Polymer Photodegradation: Mechanisms and Experimental Methods; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Mori, F.; Koyama, M.; Oki, Y. Studies on photodegradation of poly(vinyl chloride), part 3. Macromol. Mater. Eng. 1979, 75, 113–122. [Google Scholar] [CrossRef]
- Kwei, K.-P.S. Photo-oxidation of poly(vinyl chloride). J. Polym. Sci. A 1969, 7, 1075–1088. [Google Scholar] [CrossRef]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Mark, J.E. Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
Sample Availability: Samples of polyphosphates are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S. Polyphosphates as Inhibitors for Poly(vinyl Chloride) Photodegradation. Molecules 2017, 22, 1849. https://doi.org/10.3390/molecules22111849
Ahmed DS, El-Hiti GA, Yousif E, Hameed AS. Polyphosphates as Inhibitors for Poly(vinyl Chloride) Photodegradation. Molecules. 2017; 22(11):1849. https://doi.org/10.3390/molecules22111849
Chicago/Turabian StyleAhmed, Dina S., Gamal A. El-Hiti, Emad Yousif, and Ayad S. Hameed. 2017. "Polyphosphates as Inhibitors for Poly(vinyl Chloride) Photodegradation" Molecules 22, no. 11: 1849. https://doi.org/10.3390/molecules22111849