Marrubium vulgare L. Leave Extract: Phytochemical Composition, Antioxidant and Wound Healing Properties
Abstract
:1. Introduction
2. Results
2.1. Extract Preparation
2.2. Phytochemical Analysis
2.2.1. Preliminary Screening
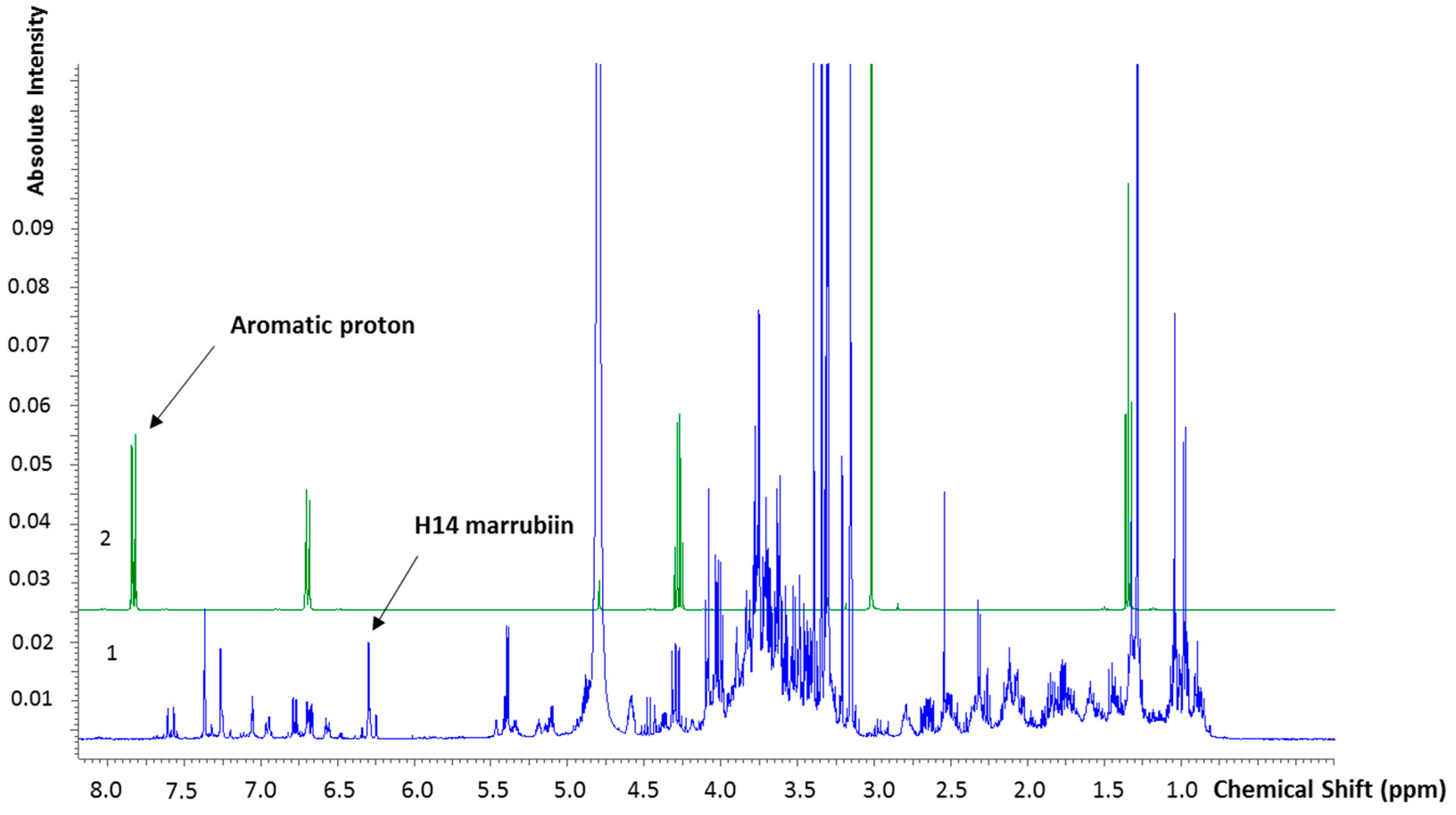
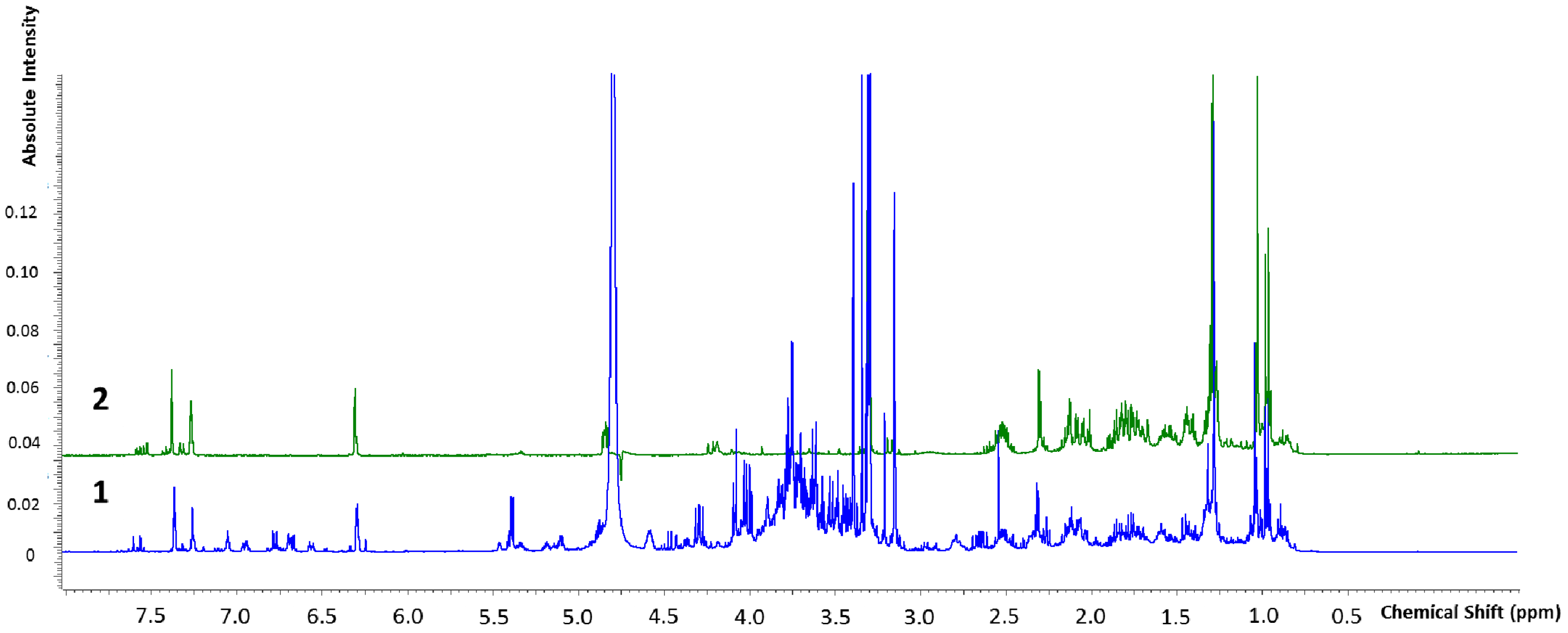
2.2.2. Marrubiin Content Determination
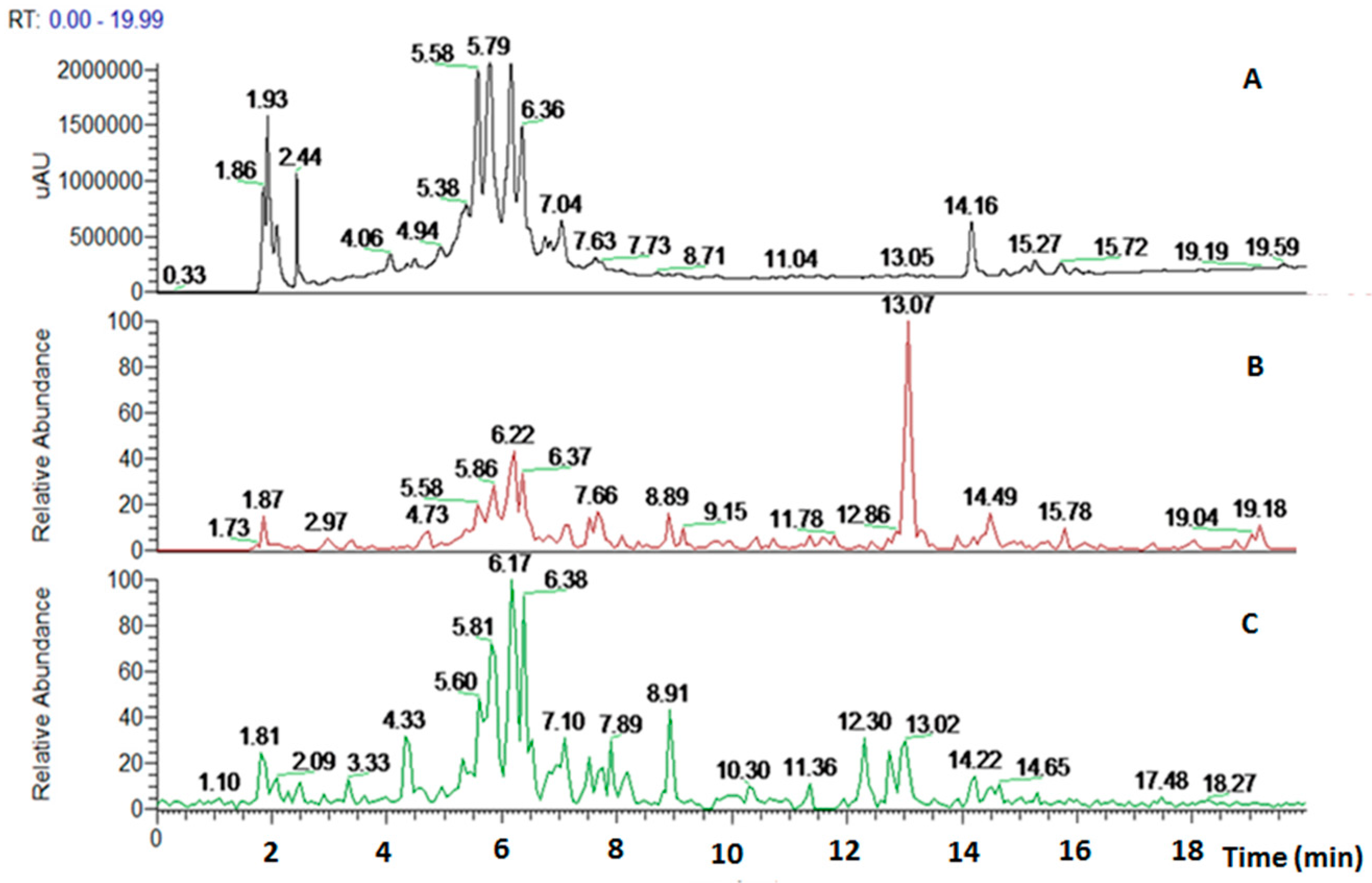
2.2.3. HPLC-UV/PAD MS Analysis
2.3. Biological Investigation
2.3.1. Antioxidant Activity
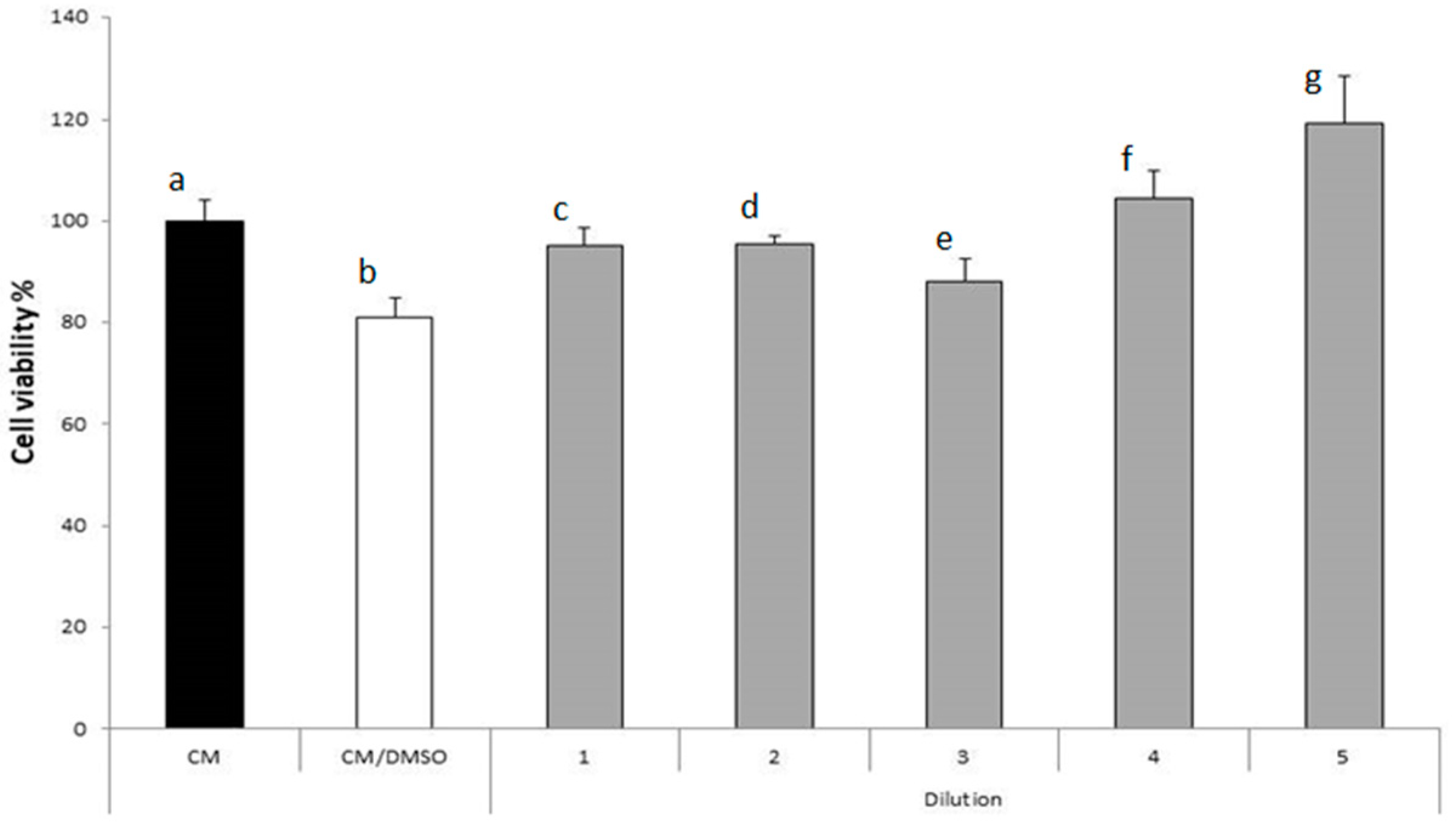
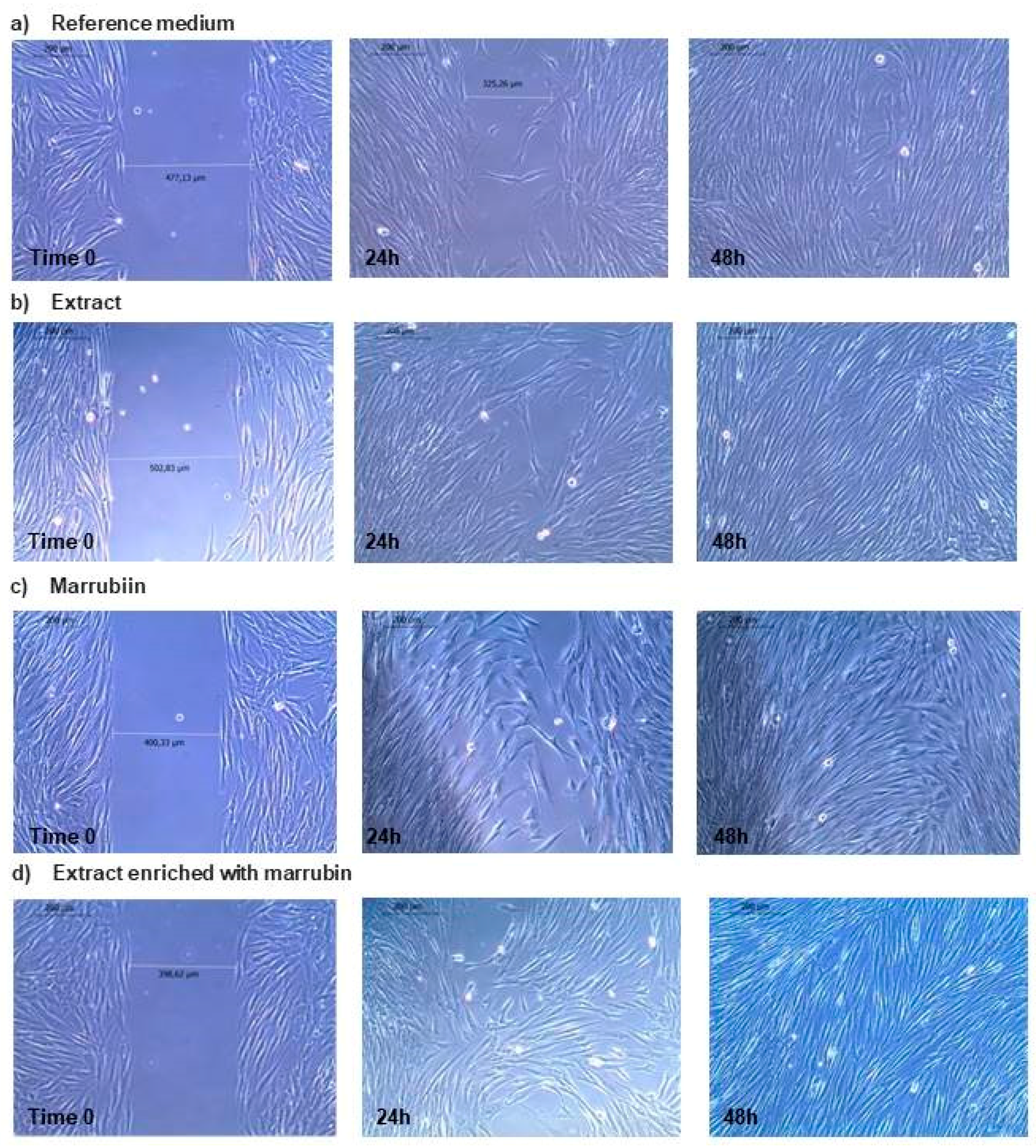
2.3.2. Activity on Fibroblast Proliferation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Instrumentation
4.3. Plant Growth
4.4. Microwave-Assisted Extraction
4.5. Phytochemical Screening
4.5.1. Determination of Total Phenolic Contents (TPC)
4.5.2. Determination of Total Flavonoid Contents (TFC)
4.5.3. NMR Characterization and Quantification
4.5.4. HPLC-PAD/UV-CD and HPLC-ESI-MS/MS Analysis
4.6. Antioxidant Activity
Free Radical Scavenging Activity
4.7. Wound Healing Activity
4.7.1. Cytoxicity Test towards Human Dermal Fibroblasts
4.7.2. Wound Healing Test towards Human Dermal Fibroblasts
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shsrms, S.; Thokchom, R. A review on endangered medicinal plants of India and their conservation. J. Crop Weed 2014, 10, 205–218. [Google Scholar]
- Das, S.; Roy, A.; Das, P.K. Ethnomedicinal uses of plants for wound healing in Khargone district, MP: A survey over Nimari communities. Res. J. Pharmacol. Pharmacodyn. 2014, 6, 21–29. [Google Scholar]
- Traditional, Complementary and Integrative Medicine. Available online: http://who.int/traditional-complementary-integrative-medicine/en/ (accessed on 10 October 2017).
- Hsu, S. Green tea and the skin. J. Am. Acad. Dermatol. 2005, 52, 1049. [Google Scholar] [CrossRef] [PubMed]
- Ammar, I.; Bardaa, S.; Mzid, M.; Sahnoun, Z.; Rebaii, T.; Attia, H.; Ennouri, M. Antioxidant, antibacterial and in vivo dermal wound healing effects of Opuntia flower extracts. Int. J. Biol. Macromol. 2015, 81, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Nasti, R.; Bignardi, E.; Della Volpe, S.; Rossino, G.; Rossi, D.; Collina, S. PKC in Regenerative Therapy: New Insights for Old Targets. Pharmaceuticals (Basel) 2017, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Zaru, M.; Bacchetta, G.; Biggio, T.; Cappai, N.; Cabras, A.; Falchi, M.A.; Manconi, M.; Fadda, A.M. A new technological approach to improve the efficacy of a traditional herbal medicinal product in wound healing. Ind. Crops Prod. 2015, 63, 71–78. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: an overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Howley, P.; Cohen, I.K. In vitro inhibition of human neutrophil elastase by oleic acid albumin formulations from derivatized cotton wound dressings. Int. J. Pharm. 2004, 284, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.; Miller, A.L. Nutritional Support for Wound Healing. Altern. Med. Rev. 2003, 8, 359–377. [Google Scholar] [PubMed]
- Schwentker, A.; Vodovotz, Y.; Weller, R.; Billiar, T.R. Nitric oxide and wound repair: role of cytokines? Nitric Oxide 2002, 7, 1–10. [Google Scholar] [CrossRef]
- Reddy, M.; Gill, S.S.; Wu, W.; Kalkar, S.R.; Rochon, P.A. Does this patient have an infection of a chronic wound? JAMA 2012, 307, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Mughrabi, F.F.; Hashim, H.; Mahmood, A.A.; Suzy, S.M.; Salmah, I.; Zahra, A.A.; Kamal, K. Acceleration of wound healing activity by Polygonatum odoratum leaf extract in rats. J. Med. Plant Res. 2014, 8, 523–528. [Google Scholar]
- Fikru, A.; Makonnen, E.; Eguale, T.; Debella, A.; Mekonnen, G.A. Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. J. Ethnopharmacol. 2012, 143, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Amini-Nik, S. The role of phytochemicals in the inflammatory phase of wound healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Behera, S.S.; Pramanik, K. Ethno-herbal-medico in wound repair: An incisive review. Phytother. Res. 2017, 31, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Villanueva, J.; Martin Esteban, J. An insight into a blockbuster phytomedicine; Marrubium vulgare L. herb more of a myth than a reality? Phytother. Res. 2016, 30, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Spencer, W.G. Celsus: De Medicina; IV Book; Harvard University Press: Cambridge, MA, USA, 1971; (Republication of the 1935 edition). [Google Scholar]
- El Bardai, S.; Lyoussi, B.; Wibo, M.; Morel, N. Comparative study of the antihypertensive activity of Marrubium vulgare and of the dihydropyridine calcium antagonist amlodipine in spontaneously hypertensive Rat. Clin. Exp. Hypertens. 2004, 26, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Meyre-Silva, C.; Yunes, R.A.; Schlemper, V.; Campos-Buzzi, F.; Cechinel-Filho, V. Analgesic potential of marrubiin derivatives, a bioactive diterpene present in Marrubium vulgare (Lamiaceae). IL Farmaco 2005, 60, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Stulzer, H.K.; Tagliari, M.P.; Zampirolo, J.A.; Cechinel-Filho, V.; Schlemper, V. Antioedematogenic effect of marrubiin obtained from Marrubium vulgare. J. Ethnopharmacol. 2006, 108, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Elberry, A.A.; Harraz, M.F.; Ghareib, S.A.; Gabr, S.A.; Nagy, A.A.; Abdel Sattar, I. Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. Int. J. Diabetes Mellit. 2011, 3, 37–44. [Google Scholar] [CrossRef]
- El Bardai, S.; Morel, N.; Wibo, M.; Fabre, N.; Labres, G.; Lyoussi, B.; Quetin-Leclerccq, J. The vasorelaxant Activity of Marrubenol and Marrubiin from Marrubium vulgare. Planta Med. 2003, 69, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Boudjelal, A.; Henchiri, C.; Siracusa, L.; Sari, M.; Ruberto, G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia 2012, 83, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Zarai, Z.; Békir, A.; Gharsallah, N.; Damak, M.; Gdoura, R. Chemical composition and antioxidant activity of Marrubium vulgare L. essential oil from Tunisia. Afr. J. Biotechnol. 2011, 10, 3908–3914. [Google Scholar]
- Masoodi, M.H.; Bahar, A.; Zagar, I.M.; Khan, S.A.; Khan, S.; Singh, P. Antibacterial activity of whole plant extract of Marrubium vulgare. Afr. J. Biotechnol. 2008, 7, 86–87. [Google Scholar]
- Pukalskas, A.; Venskutonis, P.R.; Salido, S.; de Waard, P.; van Beek, T.A. Isolation, identification and activity of natural antioxidants from horehound (Marrubium vulgare L.) cultivated in Lithuania. Food Chem. 2012, 130, 695–701. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Abu-Reidah, I.N.M.R.; Quirantes-Piné, R.; Madani, K.; Segura-Carretero, A. Phytochemical profiling, in vitro evaluation of total phenolic contents and antioxidant properties of Marrubium vulgare (horehound) leaves of plants growing in Algeria. Ind. Crops Prod. 2014, 61, 120–129. [Google Scholar] [CrossRef]
- Boulila, A.; Sanaa, A.; Ben Salem, I.; Rokbeni, N.; M’rabet, Y.; Hosni, K.; Fernandez, X. Antioxidant properties and phenolic variation in wild populations of Marrubium vulgare L. (Lamiaceae). Ind. Crops Prod. 2015, 76, 616–622. [Google Scholar] [CrossRef]
- Kurbatova, N.V.; Muzychkina, R.A.; Mukhitdinov, N.M.; Parshina, G.N. Comparative phytochemical investigation of the composition and content of biologically Active substances in Marrubium vulgare and M. Alternidens. Chem. Nat. Compd. 2003, 39, 501–502. [Google Scholar] [CrossRef]
- Akther, N.; Shawl, A.S.; Sultana, S.; Chandan, B.K.; Akhter, M. Hepatoprotective activity of Marrubium vulgare against paracetamol induced toxicity. J. Pharm. Res. 2013, 7, 565–570. [Google Scholar] [CrossRef]
- Gaggeri, R.; Rossi, D.; Mahmood, K.; Gozzini, D.; Mannucci, B.; Corana, F.; Daglia, M.; Avanzini, A.; Mantelli, M.; Martino, E.; et al. Towards elucidating Eremurus root remedy: Chemical profiling and preliminary biological investigations of Eremurus persicus and Eremurus spectabilis root ethanolic extracts. J. Med. Plant Res. 2015, 8, 1038–1048. [Google Scholar]
- Martino, E.; Ramaiola, I.; Urbano, M.; Bracco, F.; Collina, S. Microwave-assisted extraction of coumarin and related compounds from Melilotus officinalis (L.) Pallas as an alternative to Soxhlet and ultrasound assisted extraction. J. Chromatogr. A 2006, 1125, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Ahmed, K.M.; Gaggeri, R.; Volpe, S.D.; Maggi, L.; Mazzeo, G.; Longhi, G.; Abbate, S.; Corana, F.; Martino, E.; et al. (R)-(−)-Aloesaponol III 8-methyl ether from eremurus persicus: A novel compound against leishmaniosis. Molecules 2017, 22, 519. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Collina, S.; Rossi, D.; Bazzoni, D.; Gaggeri, R.; Bracco, F.; Azzolina, O. Influence of the extraction mode on the yield of hyperoside, vitexin and vitexin-2-O-rhamnoside from Crataegus monogyna Jacq. (Hawthorn). Phytochem. Anal. 2008, 19, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Gaggeri, R.; Rossi, D.; Christodoulou, M.S.; Passarella, D.; Leoni, F.; Azzolina, O.; Collina, S. Chiral flavanones from Amygdalus lycioides spach: Structural elucidation and identification of TNFalpha inhibitors by bioactivity-guided fractionation. Molecules 2012, 17, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.; Becker, K. Vanillin-HCl method for condensed tannins: Effect of organic solvents used for extraction of tannins. J. Chem. Ecol. 1993, 19, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Bladt, S. Plant Drug Analysis, II ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Mamadalieva, N.Z.; Bobakulov, K.M.; Vinciguerra, V.; Tiezzi, A.; Abdullaev, N.; Nahar, L.; Azimova, S.; Sarkerc, S.D. GC-MS and q-NMR based chemotaxonomic evaluation of two Leonurus species. Phytochem. Anal. 2016, 27, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Staneva, J.; Denkova, P.; Todorova, M.; Evstatieva, L. Quantitative analysis of sesquiterpene lactones in extract of Arnica montana L. by 1 H-NMR spectroscopy. J. Pharm. Biomed. Anal. 2011, 54, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Meyer, M.J.J.; Rodrìguez, B. Spectral assignment and Reference data. Magn. Res. Chem. 2003, 41, 147–151. [Google Scholar] [CrossRef]
- Popoola, O.K.; Elbagory, A.M.; Ameer, F.; Hussein, A.H. Review Marrubiin. Molecules 2013, 18, 9049–9060. [Google Scholar] [CrossRef] [PubMed]
- Malz, F.; Jancke, H. Validation of quantitative NMR. J. Pharm. Biomed. Anal. 2005, 38, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Sahpaz, S.; Hennebelle, T.; Bailleul, F. Marruboside, a New Phenylethanoid glycoside from Marrubium vulgare L. Nat. Prod. Lett. 2002, 16, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sahpaz, S.; Garbacki, N.; Tits, M.; Bailleu, F. Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J. Ethnopharmacol. 2002, 79, 389–392. [Google Scholar] [CrossRef]
- Porter, E.A.; Kite, G.C.; Veitch, N.C.; Geoghegan, I.A.; Larsson, S.; Simmonds, M.S.J. Phenylethanoid glycosides in tepals of Magnolia salicifolia and their occurrence in flowers of Magnoliaceae. Phytochemistry 2015, 117, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Kırmızıbekmez, H.; Montoro, P.; Piacente, S.; Pizza, C.; Dönmez, A.; Çalış, I. Identification by HPLC-PAD-MS and quantification by HPLC-PAD of phenylethanoid glycosides of five Phlomis species. Phytochem. Anal. 2005, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Grassia, A.; Bruno, M.; Rosselli, S.; Piozzi, F.; Formisano, C.; Arnold, N.A.; Senatore, F. Labdane Diterpenoids from Marrubium globosum ssp. Libanoticum. J. Nat. Prod. 2006, 69, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Heilmann, J.; Skaltsa, H. Labdane diterpenes from Marrubium velutinum and Marrubium cylleneum. Phytochemistry 2005, 66, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Meyre-Silva, C.; Cechinel-Filho, V. A Review of the Chemical and Pharmacological Aspects of the Genus Marrubium. Curr. Pharm. Des. 2010, 16, 3503–3518. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, M.; Ali, Z.; Liang, S.; Yin, H.; Wang, W.; Khan, I.A. Labdane diterpenoids from Marrubium vulgare. Phytochem. Lett. 2015, 13, 275–279. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Savi, A.O.S.; Schlemper, V.; Reynaud, E.; Cechinel-Filho, V. An Improved Extraction of marrubiim from Marrubium vulgare. Chromatographia 1998, 47, 449–450. [Google Scholar] [CrossRef]
- Arpini, S.; Fuzzati, N.; Giori, A.; Martino, E.; Mombelli, G.; Pagni, L.; Ramaschi, G. HPLC-DAD-MS Fingerprint of Andrographis paniculata (Burn. f.) Nees (Acanthaceae). Nat. Prod. Commun. 2008, 3, 1981–1985. [Google Scholar]
- Radev, R. Pharmacological Effects of Phenylethanoid Glycosides. J. Clin. Med. 2010, 3, 20–23. [Google Scholar]
- Stanković, M.S. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac J. Sci. 2011, 33, 63–72. [Google Scholar]
- Suntar, I.; Akkol, E.K.; Nahar, L.; Sarker, S.D. Wound healing and antioxidant properties: Do they coexist in plants. Free Radic. Antioxid. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Mau, J.L.; Chao, G.R.; Wu, K.T. Antioxidant Properties of Methanolic Extracts from Several Ear Mushrooms. J. Agric. Food Chem. 2001, 49, 5461–5467. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.S.; Tang, M.C.; Wu, J.M. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Marrubium vulgare L. leave extract is available from the authors. |
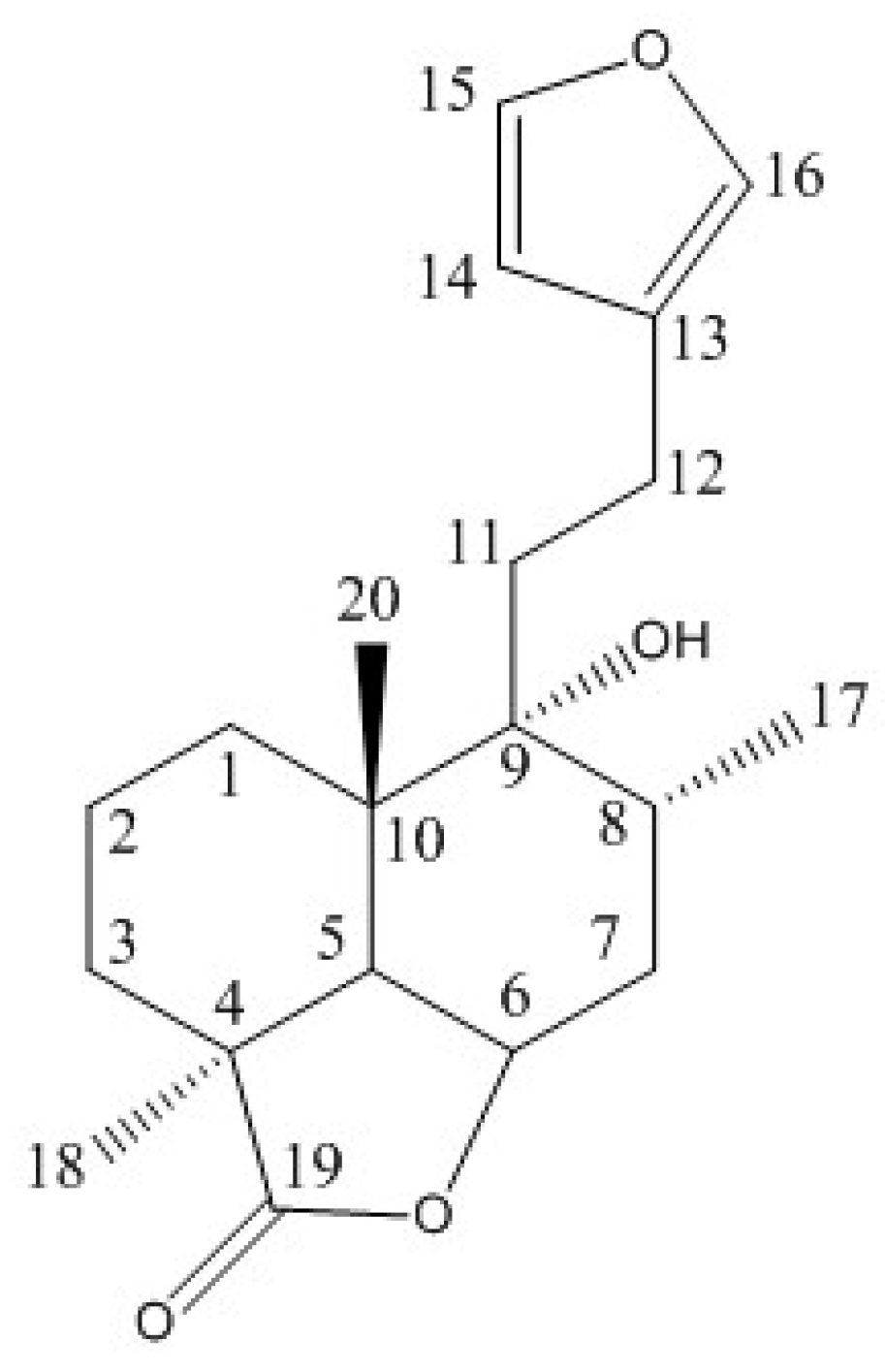

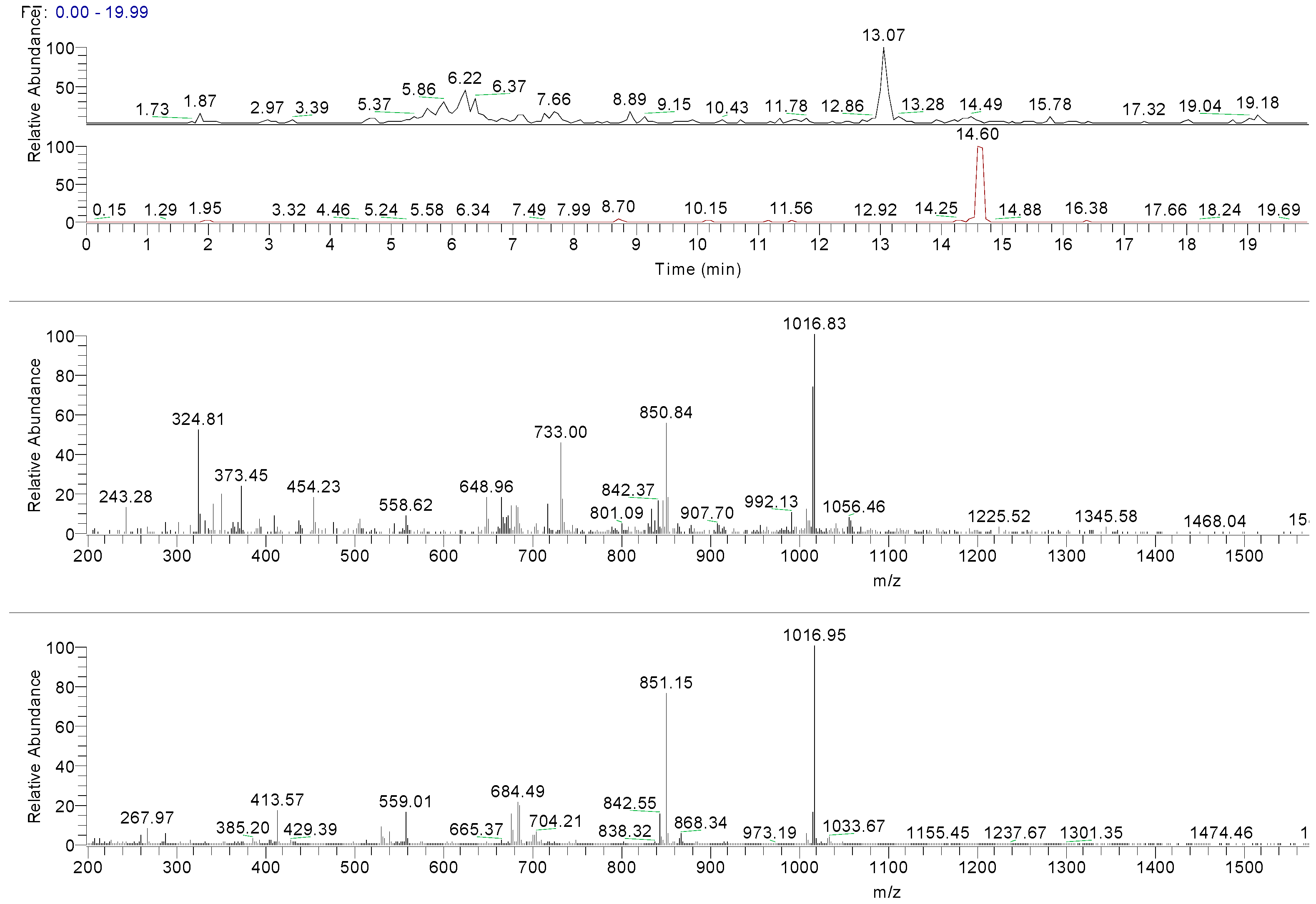
| 1H | δ (ppm) | 13C | δ (ppm) |
|---|---|---|---|
| 1α | 1.82 | 1 | 28.32 |
| 1β | 1.31 | 2 | 17.78 |
| 2α | 1.72 | 3 | 28.09 |
| 2β | 1.55 | 4 | 44.02 |
| 3α | 1.44 | 5 | 44.79 |
| 3β | 2.05 | 6 | 77.53 |
| 5 | 2.31 | 7 | 31.03 |
| 6 | 4.84 ddd | 8 | 31.72 |
| 7α | 1.77 | 9 | 75.52 |
| 7β | 2.13 | 10 | 39.63 |
| 8 | 2.13 | 11 | 35.50 |
| 11A | 1.77 | 12 | 20.65 |
| 11B | 1.83 | 13 | 125.56 |
| 12A | 2.52 | 14 | 110.42 |
| 12B | 2.52 | 15 | 142.69 |
| 14 | 6.31 dd | 16 | 138.29 |
| 15 | 7.38 t | 17 | 15.58 |
| 16 | 7.27 ddt | 18 | 21.93 |
| 17 | 0.97 s | 19 | 185.98 |
| 18 | 1.29 s | 20 | 21.93 |
| 20 | 1.03 s |
| Retention Time (min) | Negative Ions | Fragment Ions | Positive Ions | MW | Fragment Ions | Structure | Compound Name |
|---|---|---|---|---|---|---|---|
| 5.58 | 887.27 [M − H]− | 725.16 [M − H-162]− 593.18 [M − H-162-132]− | 906.02 [M + NH4]+ | 888 | 889.12 [M + NH4-NH3]+ 624.71 [M + NH4-NH3-132-132]+ 478.60 [M + NH4-NH3-132-132-146]+ 470.68 [M + NH4-NH3-132-132-154]+ 324.90 [M + NH4-NH3-132-132-146-154]+ |  | Marruboside |
| 5.79 | 755 [M − H]− | 593.18 [M − H-162]− 461.16 [M − H-162-132]− 447.14 [M − H-162-146]− | 773 [M + NH4]+ | 756 | 756.50 [M + NH4-NH3]+ 624.83 [M + NH4-NH3-132]+ 478.58 [M + NH4-NH3-132-146]+ 470.68 [M + NH4-NH3-132-154]+ 324.82 [M + NH4-NH3-132-154-146]+ |  | Forsythoside B |
| 6.17 | 755 [M − H]− | 593.18 [M − H-162]− 461.16 [M − H-162-132]− | 773 [M + NH4]+ | 756 | 756.50 [M + NH4-NH3]+ 624.83 [M + NH4-NH3-132]+ 478.58 [M + NH4-NH3-132-146]+ 470.68 [M + NH4-NH3-132-154]+ 324.82 [M + NH4-NH3-132-154-146]+ |  | Samioside |
| 6.36 | 623.18 [M − H]− | 461.16 [M − H-162-132]− 315.0 [M − H-162-132-146]− | 641.89 [M + NH4]+ | 624 | 624.71 [M + NH4-NH3]+ 478.51 [M + NH4-NH3-146]+ 470.54 [M + NH4-NH3-154]+ 324.82 [M + NH4-NH3-154-146]+ |  | Verbascoside |
| 13.07 | ND | ND | 336.66 [M + H]+ | 336 | 319.14 [M + H-H2O]+ 301.1 [M + H-2H2O]+ 289.1 [M + H-H2O-H2C=O]+ |  | Deacetylvitexilactone |
| 14.49 | ND | ND | 665.24 [2M + H]+ 1016.8 [6M + H + K]+ | 332 | ND |  | Marrubiin |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amri, B.; Martino, E.; Vitulo, F.; Corana, F.; Kaâb, L.B.-B.; Rui, M.; Rossi, D.; Mori, M.; Rossi, S.; Collina, S. Marrubium vulgare L. Leave Extract: Phytochemical Composition, Antioxidant and Wound Healing Properties. Molecules 2017, 22, 1851. https://doi.org/10.3390/molecules22111851
Amri B, Martino E, Vitulo F, Corana F, Kaâb LB-B, Rui M, Rossi D, Mori M, Rossi S, Collina S. Marrubium vulgare L. Leave Extract: Phytochemical Composition, Antioxidant and Wound Healing Properties. Molecules. 2017; 22(11):1851. https://doi.org/10.3390/molecules22111851
Chicago/Turabian StyleAmri, Bédis, Emanuela Martino, Francesca Vitulo, Federica Corana, Leila Bettaieb-Ben Kaâb, Marta Rui, Daniela Rossi, Michela Mori, Silvia Rossi, and Simona Collina. 2017. "Marrubium vulgare L. Leave Extract: Phytochemical Composition, Antioxidant and Wound Healing Properties" Molecules 22, no. 11: 1851. https://doi.org/10.3390/molecules22111851






