New Biflavonoids with α-Glucosidase and Pancreatic Lipase Inhibitory Activities from Boesenbergia rotunda
Abstract
:1. Introduction
2. Results and Discussion
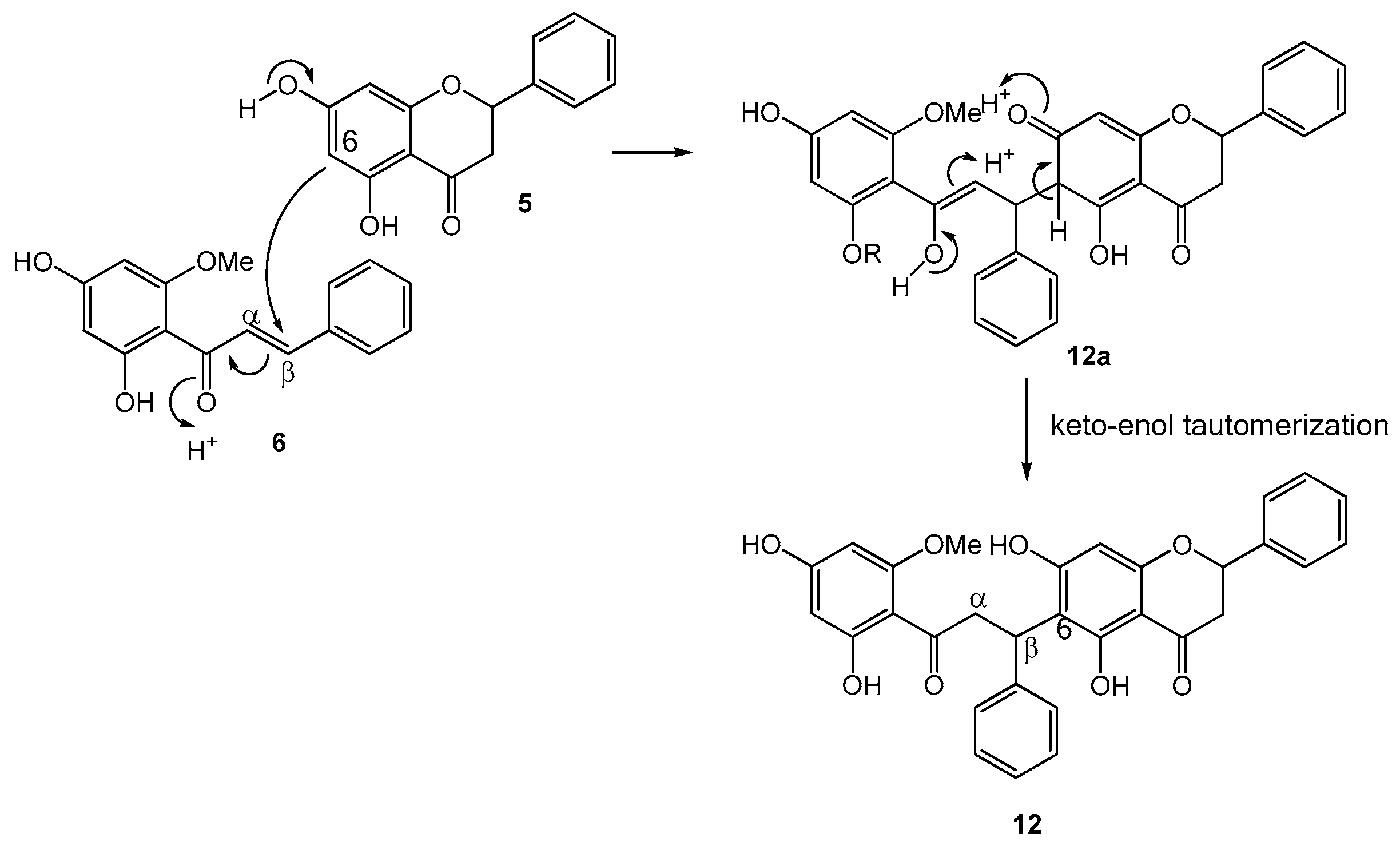
2.1. Structure Elucidation of Bioactive Components
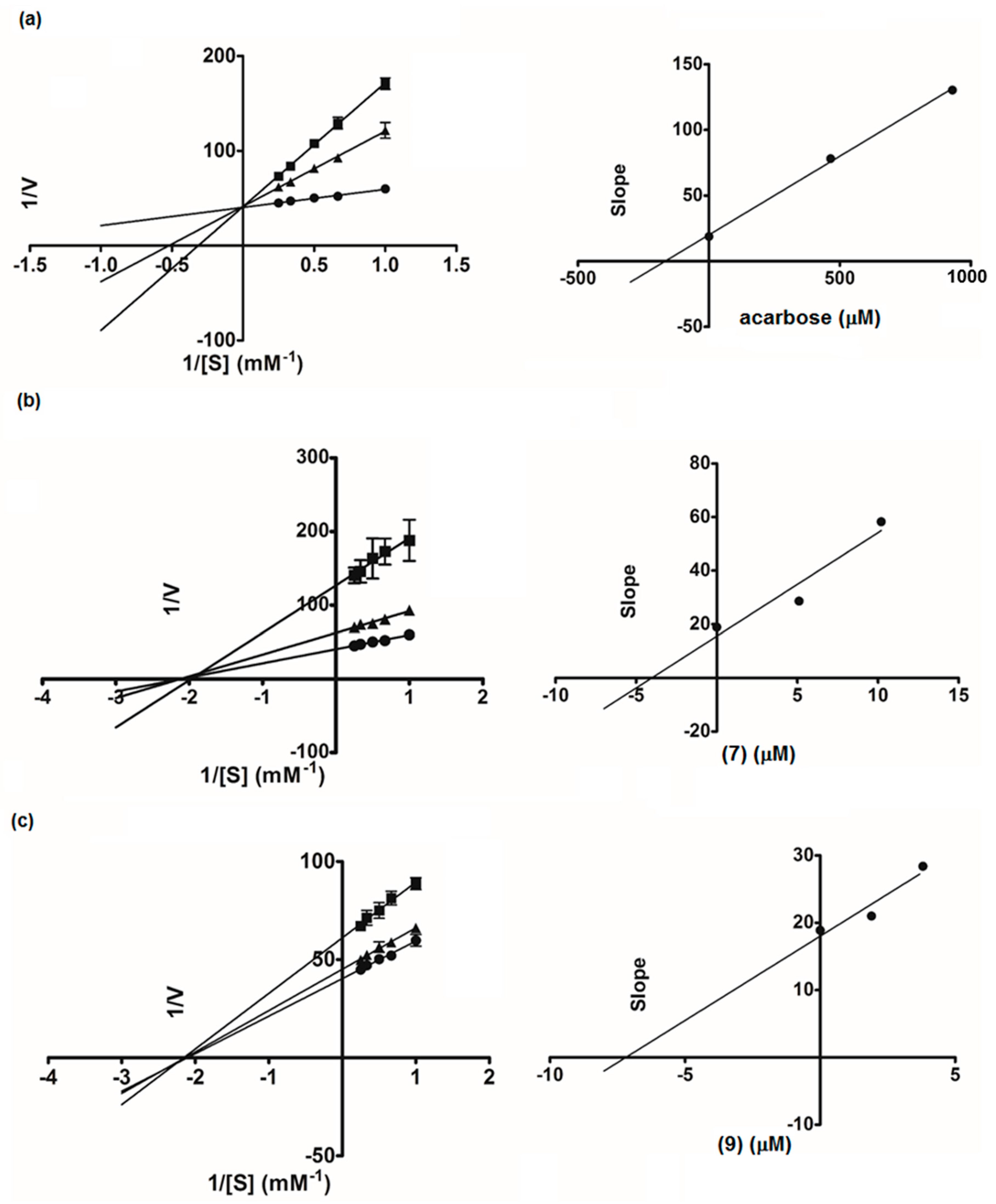
2.2. α-Glucosidase Inhibitory Activity
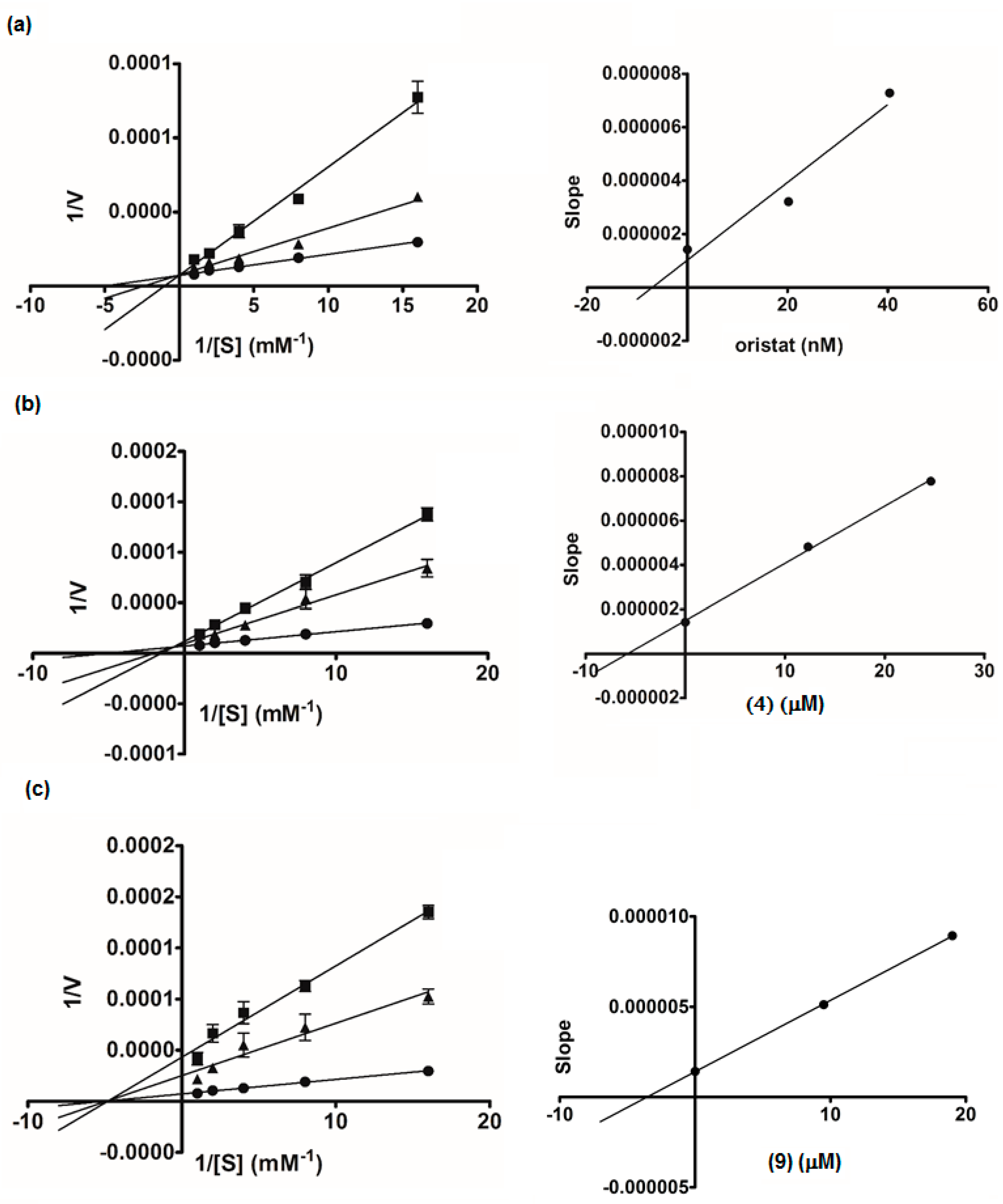
2.3. Lipase Inhibitory Activity
2.4. Therapeutic Implications
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction, Isolation, and Purification
3.4. Assays for α-Glucosidase Inhibitory Activity
3.5. Evaluation of Lipase Inhibitory Activity
3.6. Kinetic Study of α-Glucosidase and Lipase Inhibition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. n-3 Fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83, 1499S–1504S. [Google Scholar] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Bardini, G.; Rotella, C.M.; Giannini, S. Dyslipidemia and diabetes: reciprocal impact of impaired lipid metabolism and Beta-cell dysfunction on micro- and macrovascular complications. Rev. Diabetes Stud. 2012, 9, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.L.; Raskin, I.; Cefalu, W.T.; Ribnicky, D.M. Plant-derived therapeutics for the treatment of metabolic syndrome. Curr. Opin. Investig. Drugs 2010, 11, 1107–1115. [Google Scholar] [PubMed]
- Chuakul, W.; Boonpleng, A. Ethnomedical uses of Thai Zingiberaceous plant (1). Thai J. Phytopharm. 2003, 10, 33–39. [Google Scholar]
- Ongwisespaiboon, O.; Jiraungkoorskul, W. Fingerroot, Boesenbergia rotunda and its aphrodisiac activity. Pharmacog. Rev. 2017, 11, 27–30. [Google Scholar]
- Eng-Chong, T.; Yean-Kee, L.; Chin-Fei, C.; Choon-Han, H.; Sher-Ming, W.; Li-Ping, C.T.; Gen-Teck, F.; Khalid, N.; Abd Rahman, N.; Karsani, S.A.; et al. Boesenbergia rotunda: From ethnomedicine to drug discovery. Evid.-Based Complement. Altern. Med. 2012, 2012, e473637. [Google Scholar] [CrossRef] [PubMed]
- Chahyadi, A.; Hartati, R.; Wirasutisna, K.R.; Elfahmi. Boesenbergia pandurata Roxb., An Indonesian medicinal plant: Phytochemistry, biological activity, plant biotechnology. Procedia Chem. 2014, 13, 13–37. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.S.; Sa, B.K.; Kim, M.B.; Hwang, J.K. Boesenbergia pandurata attenuates diet-induced obesity by activating AMP-activated protein kinase and regulating lipid metabolism. Int. J. Mol. Sci. 2012, 13, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, U.L.B.; Ratnayake, R.M.S.; Medawala, M.M.W.S.; Fujimoto, Y. Dihydrochalcones with radical scavenging properties from the leaves of Syzygium jambos. Nat. Prod. Res. 2007, 21, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Pegnyemb, D.E.; Tih, R.G.; Sondengam, B.L.; Blond, A.; Bodo, B. Flavonoids of Ochna afzelii. Phytochemistry 2003, 64, 661–665. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-w.; Li, X.; Sun, W.-l.; Xing, Y.; Xiu, Z.-L.; Zhuang, C.-L.; Dong, Y.-S. Dietary flavonoids and acarbose synergistically inhibit α-glucosidase and lower postprandial blood glucose. J. Agric. Food Chem. 2017, 65, 8319–8330. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, H.; Nagao, M.; Harada, T.; Nakajima, Y.; Tanimura-Inagaki, K.; Okajima, F.; Tamura, H.; Inazawa, T.; Otonari, T.; Kawakami, M.; et al. Comparison of three α-glucosidase inhibitors for glycemic control and bodyweight reduction in Japanese patients with obese type 2 diabetes. J. Diabetes Investig. 2014, 5, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, M.S.; Jo, K.; Lee, K.E.; Hwang, J.K. Therapeutic potential of panduratin A, LKB1-dependent AMP-activated protein kinase stimulator, with activation of PPARα/δ for the treatment of obesity. Diabetes Obes. Metab. 2011, 13, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Charoensin, S.; Punvittayagul, C.; Pompimon, W.; Meevatee, U.; Wongpoomchai, R. Toxicological and clastogenic evaluation of pinocembrin and pinostrobin isolated from Boesenbergia pandurata in Wistar rats. Thai J. Toxicol. 2010, 25, 29–40. [Google Scholar]
- Salama, S.M.; Bilgen, M.; Al Rashdi, A.S.; Abdulla, M.A. Efficacy of Boesenbergia rotunda treatment against thioacetamide-induced liver cirrhosis in a rat model. Evid.-Based Complement. Altern. Med. 2012, 2012, e137083. [Google Scholar] [CrossRef] [PubMed]
- Saraithong, P.; Saenphet, S.; Saenphet, K. Safety evaluation of ethanol extracts from Bosenbergia rotunda (L.) Mansf. in male rats. Trends Res. Sci.Technol. 2010, 2, 19–22. [Google Scholar]
- Ching, A.Y.L.; Wah, T.S.; Sukari, M.A.; Lian, G.E.C.; Rahmani, M.; Khalid, K. Characterization of flavonoid derivatives from Boesenbergia rotunda (L.). Malays. J. Anal. Sci. 2007, 11, 154–159. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 339–340. [Google Scholar]
- Tuntiwachwuttikul, P.; Pancharoen, O.; Reutrakul, V.; Byrne, L. (1′RS,2′SR,6′RS)-(2,6-Dihydroxy-4-Methoxyphenyl)-[3′-Methyl-2′-(3″-Methylbut-2″-Enyl)-6′-Phenylcyclohex-3′-Enyl]Methanone (Panduratin-a)—A Constituent of the Red Rhizomes of a Variety of Boesenbergia Pandurata. Aust. J. Chem. 1984, 37, 449–453. [Google Scholar] [CrossRef]
- Pandji, C.; Grimm, C.; Wray, V.; Witte, L.; Proksch, P. Insecticidal constituents from four species of the zingiberaceae. Phytochemistry 1993, 34, 415–419. [Google Scholar] [CrossRef]
- Itokawa, H.; Morita, M.; Mihashi, S. Phenolic compounds from the rhizomes of Alpinia speciosa. Phytochemistry 1981, 20, 2503–2506. [Google Scholar] [CrossRef]
- Tuchinda, P.; Reutrakul, V.; Claeson, P.; Pongprayoon, U.; Sematong, T.; Santisuk, T.; Taylor, W.C. Anti-inflammatory cyclohexenyl chalcone derivatives in Boesenbergia pandurata. Phytochemistry 2002, 59, 169–173. [Google Scholar] [CrossRef]
- Dharmaratne, H.R.W.; Nanayakkara, N.P.D.; Khan, I.A. Kavalactones from Piper methysticum, and their 13C NMR spectroscopic analyses. Phytochemistry 2002, 59, 429–433. [Google Scholar] [CrossRef]
- Mustafa, K.; Kjaergaard, H.G.; Perry, N.B.; Weavers, R.T. Hydrogen-bonded rotamers of 2′,4′,6′-trihydroxy-3′-formyldihydrochalcone, an intermediate in the synthesis of a dihydrochalcone from Leptospermum recurvum. Tetrahedron 2003, 59, 6113–6120. [Google Scholar] [CrossRef]
- Morimoto, M.; Kumeda, S.; Komai, K. Insect antifeedant flavonoids from Gnaphalium affine D. Don. J. Agric. Food Chem. 2000, 48, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Chen, S.X.; Xu, H.X.; Kadota, S.; Namba, T. A new antiplatelet diarylheptanoid from Alpinia blepharocalyx. J. Nat. Prod. 1998, 61, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Norbedo, C.; Ferraro, G.; Coussio, J.D. A new flavanone from Achyrocline flaccida. J. Nat. Prod. 1982, 45, 635–636. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.-H. Comparison of inhibitory activities and mechanisms of five mulberry plant bioactive components against α-glucosidase. J. Agric. Food Chem. 2013, 61, 8110–8119. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Vanderstraeten, J.; Winand, J.; Beguin, P.; Schneider, Y.J. Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chem. 2012, 135, 68–73. [Google Scholar] [CrossRef]
- Rahim, A.T.M.A.; Takahashi, Y.; Yamaki, K. Mode of pancreatic lipase inhibition activity in vitro by some flavonoids and non-flavonoid polyphenols. Food Res. Int. 2015, 75, 289–294. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Position | 9 | 12 | 13 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| Flavanone unit | ||||||
| 2 | 5.51 dd (13, 2.7) | 79.9 | 5.52 dd (13, 2.8) | 79.9 | 5.52 dd (13, 2.4) | 79.9 |
| 3 | 2.74 dd (17, 2.7) | 43.7 | 2.75 dd (17, 2.8) | 43.7 | 2.75 dd (17, 2.4) | 43.7 |
| 3.15 dd (17, 13) | 3.15 dd (17, 13) | 3.15 dd (17, 13) | ||||
| 2.74 dd (17, 2.7) | 2.75 dd, (17, 2.8) | 2.75 dd, (17, 2.4) | ||||
| 4 | 197.1 | 197.1 | 197.1 | |||
| 5 | 163.0 | 162.9 | 163.0 | |||
| 6 | 112.4 | 112.5 | 112.5 | |||
| 7 | 162.0 | 164.5 | 162.0 | |||
| 8 | 6.04 s | 95.7 | 6.04 s | 95.7 | 6.04 s | 95.7 |
| 9 | 165.2 | 162.0 | 165.0 | |||
| 10 | 103.1 | 103.1 | 103.2 | |||
| 1 | 140.1 | 140.1 | 140.1 | |||
| 2′(6′) | 7.08–7.55 m | 127.3 | 7.06–7.56 m | 127.3 | 7.06–7.56 m | 127.3 |
| 3′(5′) | 7.08–7.55 m | 129.4 | 7.06–7.56 m | 128.8 | 7.06–7.56 m | 128.8 |
| 4′ | 7.08–7.55 m | 129.4 | 7.06–7.56 m | 126.3 | 7.06–7.56 m | 129.4 |
| Dihydrochalcone unit | ||||||
| 1′′ | 145.6 | 145.5 | 145.7 | |||
| 2′′(6′′) | 7.08–7.55 m | 128.7 | 7.06–7.56 m | 129.4 | 7.06–7.56 m | 129.4 |
| 3′′(5′′) | 7.08–7.55 m | 128.5 | 7.06–7.56 m | 128.5 | 7.06–7.56 m | 128.5 |
| 4′′ | 7.08–7.55 m | 126.2 | 7.06–7.56 m | 129.4 | 7.06–7.56 m | 126.2 |
| 1′′′ | 105.9 | 106.0 | 105.4 | |||
| 2′′′ | 165.2 | 168.2 | 165.2 | |||
| 3′′′ | 5.98 s | 94.3 | 5.92 d (2.2) | 96.8 | 5.92 s | 95.8 |
| 4′′′ | 166.6 | 165.4 | 165.4 | |||
| 5′′′ | 5.98 s | 94.3 | 6.03 br s | 91.8 | 5.92 s | 95.8 |
| 6′′′ | 165.2 | 165.1 | 165.2 | |||
| C=O | 205.6 | 205.2 | 205.2 | |||
| α | 4.05–4.09 m | 47.1 | 3.80–3.99 m | 47.3 | 3.98–4.09 m | 47.0 |
| 4.24–4.32 m | 4.07–4.19 m | 4.22–4.30 m | ||||
| β | 5.33 t (7.3) | 35.5 | 5.26 t (7.2) | 35.8 | 5.33 t (7.3) | 35.5 |
| HO-5 | 12.75 s | - | 12.76 s | - | 12.73 s | - |
| MeO-4′′′ | 3.77 s | 55.7 | - | - | - | |
| MeO-6′′′ | - | - | 3.85 s | 56.2 | - | |
| Compound | α-Glucosidase | Lipase | ||
|---|---|---|---|---|
| % Inhibition (at 20 µg/mL) | IC50 (µM) | % Inhibition (at 20 µg/mL) | IC50 (µM) | |
| 1 | 10.0 ± 2.9 | - | 7.9 ± 0.8 | - |
| 2 | 9.6 ± 4.0 | - | 30.6 ± 2.0 | - |
| 3 | 94.7 ± 0.4 | 12.7 ± 1.3 | 72.3 ± 0.1 | 17.1 ± 3.7 |
| 4 | 97.8 ± 0.8 | 7.5 ± 0.6 | 75.2 ± 1.1 | 15.1 ± 3.3 |
| 5 | 19.8 ± 0.1 | - | 3.7 ± 1.3 | - |
| 6 | 19.7 ± 0.9 | - | 7.7 ± 1.9 | - |
| 7 | 98.5 ± 0.4 | 4.6 ± 0.4 | 49.3 ± 2.7 | - |
| 8 | 9.5 ± 2.5 | - | 33.7 ± 1.0 | - |
| 9 | 100.0 ± 0.0 | 2.4 ± 0.4 | 69.1 ± 1.6 | 25.8 ± 2.6 |
| 10 | 88.1 ± 0.9 | 32.0 ± 2.2 | 48.7 ± 3.5 | - |
| 11 | 7.0 ± 2.0 | - | 8.0 ± 1.9 | - |
| 12 | 100.0 ± 0.2 | 3.4 ± 0.9 | 67.8 ± 1.3 | 30.1 ± 2.3 |
| 13 | 100.0 ± 0.1 | 1.3 ± 0.2 | 80.5 ± 0.6 | 10.6 ± 1.2 |
| 14 | 97.1 ± 0.5 | - | 70.9 ± 2.0 | - |
| 15 | 20.9 ± 2.1 | - | 44.7 ± 1.7 | - |
| Acarbose | - | 1155.5 ± 23.0 | ||
| Orlistat | - | 31.4 ± 0.6 nM | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatsumpun, N.; Sritularak, B.; Likhitwitayawuid, K. New Biflavonoids with α-Glucosidase and Pancreatic Lipase Inhibitory Activities from Boesenbergia rotunda. Molecules 2017, 22, 1862. https://doi.org/10.3390/molecules22111862
Chatsumpun N, Sritularak B, Likhitwitayawuid K. New Biflavonoids with α-Glucosidase and Pancreatic Lipase Inhibitory Activities from Boesenbergia rotunda. Molecules. 2017; 22(11):1862. https://doi.org/10.3390/molecules22111862
Chicago/Turabian StyleChatsumpun, Nutputsorn, Boonchoo Sritularak, and Kittisak Likhitwitayawuid. 2017. "New Biflavonoids with α-Glucosidase and Pancreatic Lipase Inhibitory Activities from Boesenbergia rotunda" Molecules 22, no. 11: 1862. https://doi.org/10.3390/molecules22111862





