Synthesis, Biological Evaluation and Molecular Docking Study of 2-Substituted-4,6-Diarylpyrimidines as α-Glucosidase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
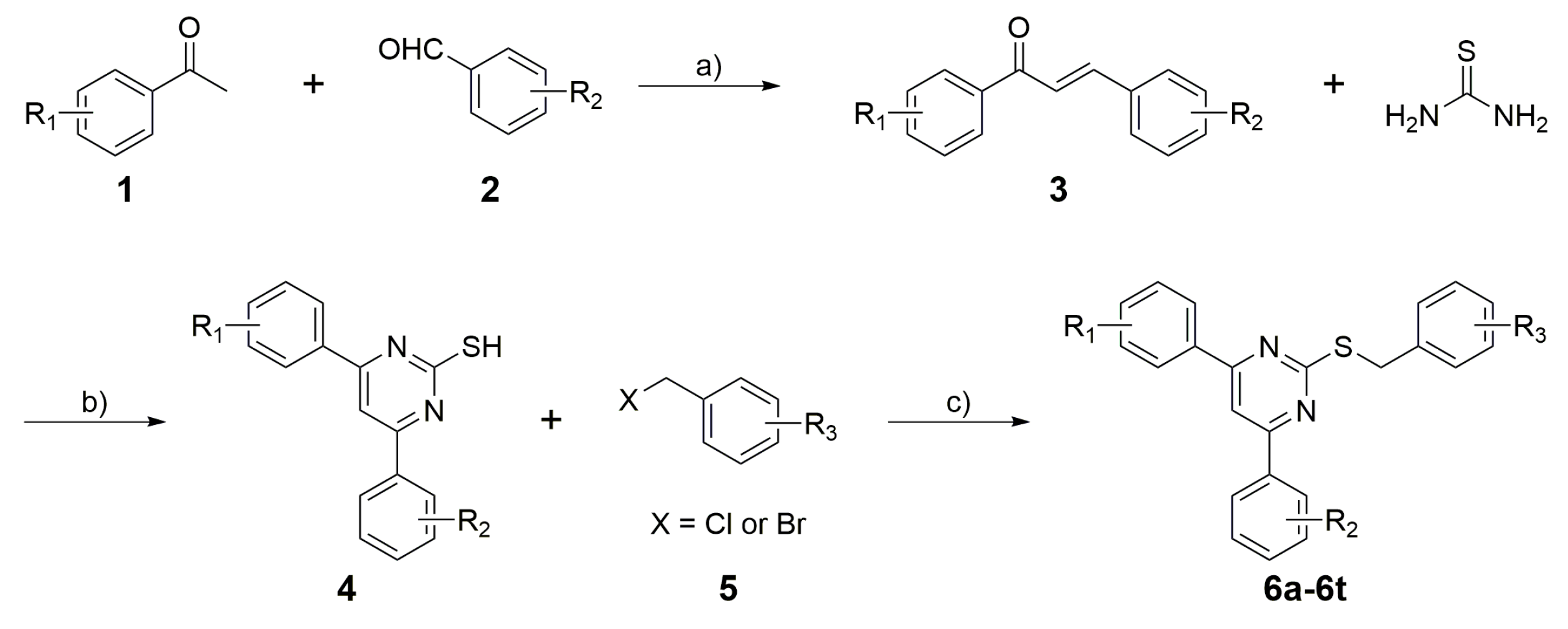
2.1. Chemistry
2.2. α-Glucosidase Inhibition Assay
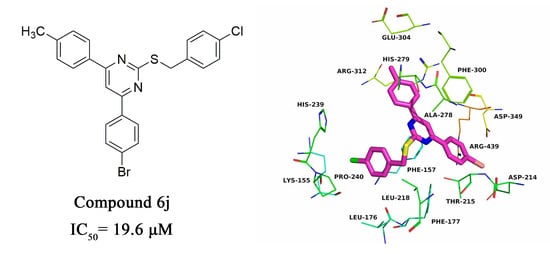
2.3. Molecular Docking
3. Materials and Methods
3.1. General
3.2. General Procedure for the Synthesis of 2-Substituted-4,6-Diarylpyrimidines (6a–6t)
3.3. In Vitro Assay of α-Glucosidase Inhibitory Activity
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Van de Laar, F.A. α-Glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef] [PubMed]
- Suresh, L.; Kumar, P.S.V.; Poornachandra, Y.; Kumar, C.G.; Chandramouli, G.V.P. Design, synthesis and evaluation of novel pyrazolo-pyrimido[4,5-d]pyrimidine derivatives as potent antibacterial and biofilm inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, J.-F.; Bai, Y.-B.; Wang, P.; Wang, T.; Gao, J.-M.; Zhang, Z.-T. Synthesis of pyrazolo[1,5-a]pyrimidine derivatives and their antifungal activities against phytopathogenic fungi in vitro. Mol. Divers. 2016, 20, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Kamel, R.; Fatahala, S.S. Synthesis and biological evaluation of some thio containing pyrrolo[2,3-d]Pyrimidine derivatives for their anti-inflammatory and anti-microbial activities. Eur. J. Med. Chem. 2010, 45, 2994–3004. [Google Scholar] [CrossRef] [PubMed]
- Alam, O.; Khan, S.A.; Siddiqui, N.; Ahsan, W.; Verma, S.P.; Gilani, S.J. Antihypertensive activity of newer 1,4-dihydro-5-pyrimidine carboxamides: Synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2010, 45, 5113–5119. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.N.; Hussein, H.A.; El-Gazzar, A.-R. Synthesis of substituted thieno[2,3-d]pyrimidine-2,4-dithiones and their S-glycoside analogues as potential antiviral and antibacterial agents. Eur. J. Med. Chem. 2010, 45, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Hese, S.V.; Meshram, R.J.; Kamble, R.D.; Mogle, P.P.; Patil, K.K.; Kamble, S.S.; Gacche, R.N.; Dawane, B.S. Antidiabetic and allied biochemical roles of new chromeno-pyrano pyrimidine compounds: Synthesis, in vitro and in silico analysis. Med. Chem. Res. 2017, 26, 805–818. [Google Scholar] [CrossRef]
- Wang, S.-B.; Deng, X.-Q.; Zheng, Y.; Yuan, Y.-P.; Quan, Z.-S.; Guan, L.-P. Synthesis and evaluation of anticonvulsant and antidepressant activities of 5-alkoxytetrazolo[1,5-c]thieno[2,3-e]pyrimidine derivatives. Eur. J. Med. Chem. 2012, 56, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Ismail; Kuthati, B.; Thalari, G.; Bommarapu, V.; Mulakayala, C.; Chitta, S.K.; Mulakayala, N. Synthesis of novel spiro pyrazolo [4,3-d] pyrimidinones and spiro[benzo[4,5]thieno[2,3-d]pyrimidine-2,3′-indoline]-2′,4(3H)-diones and their evaluation for anticancer activity. Bioorg. Med. Chem. Lett. 2017, 27, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Chitranshi, N.; Agarwal, A.K. Significance and biological importance of pyrimidine in the microbial world. Int. J. Med. Chem. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Yar, M.; Bajda, M.; Shahzadi, L.; Shahzad, S.A.; Ahmed, M.; Ashraf, M.; Alam, U.; Khan, I.U.; Khan, A.F. Novel synthesis of dihydropyrimidines for α-glucosidase inhibition to treat type 2 diabetes: In vitro biological evaluation and in silico docking. Bioorg. Chem. 2014, 54, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Al-Majid, A.M.; Ghabbour, H.A.; Fun, H.-K.; Javed, K.; Imad, R.; Yousuf, S.; Choudhary, M.I.; Wadood, A. Synthesis, in vitro biological activities and in silico study of dihydropyrimidines derivatives. Bioorg. Med. Chem. 2015, 23, 6740–6748. [Google Scholar] [CrossRef] [PubMed]
- Shahidpour, S.; Panahi, F.; Yousefi, R.; Nourisefat, M.; Nabipoor, M.; Khalafi-Nezhad, A. Design and synthesis of new antidiabetic α-glucosidase and α-amylase inhibitors based on pyrimidine-fused heterocycles. Med. Chem. Res. 2015, 24, 3086–3096. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Al-Majid, A.M.; Ghabbour, H.A.; Yousuf, S.; Ashraf, M.; Shaikh, N.N.; Choudhary, M.I.; Khalil, R.; Ul-Haq, Z. Synthesis of pyrimidine-2,4,6-trione derivatives: Anti-oxidant, anti-cancer, α-glucosidase, β-glucuronidase inhibition and their molecular docking studies. Bioorg. Chem. 2016, 68, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Suresh, L.; Onkara, P.; Kumar, P.S.V.; Pydisetty, Y.; Chandramouli, G.V.P. Ionic liquid-promoted multicomponent synthesis of fused tetrazolo[1,5-a]pyrimidines as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 4007–4014. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, Z.; Wang, J.; Li, J.; Li, X. Synthesis, biological evaluation and molecular docking study of N-arylbenzo[d]oxazol-2-amines as potential α-glucosidase inhibitors. Bioorg. Med. Chem. 2016, 24, 5374–5379. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; He, D.; Li, X.; Li, J.; Peng, Z. One-pot and three-component synthesis, characterization and biological evaluation of some new 1,2,4-triazine-coumarins. Heterocycles 2016, 92, 1430–1439. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Z.; Wang, J.; Li, X.; Li, J. Synthesis, in vitro evaluation and molecular docking studies of novel triazine-triazole derivatives as potential α-glucosidase inhibitors. Eur. J. Med. Chem. 2017, 125, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Xie, Z.; Chen, M.; Li, L.; Peng, Y.; Chen, S.; Li, W.; Deng, B. Discovery of 3,3-di(indolyl)indolin-2-one as a novel scaffold for α-glucosidase inhibitors: In silico studies and SAR predictions. Bioorg. Chem. 2017, 72, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Niaz, H.; Kashtoh, H.; Khan, J.A.J.; Khan, A.; Atia tul, W.; Alam, M.T.; Khan, K.M.; Perveen, S.; Choudhary, M.I. Synthesis of diethyl 4-substituted-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylates as a new series of inhibitors against yeast α-glucosidase. Eur. J. Med. Chem. 2015, 95, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Malik, F.; Ullah, H.; Wadood, A.; Khan, F.; Javid, M.T.; Taha, M.; Rehman, W.; Rehman, A.U.; Khan, K.M. Isatin based Schiff bases as inhibitors of α-glucosidase: Synthesis, characterization, in vitro evaluation and molecular docking studies. Bioorg. Chem. 2015, 60, 42–48. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 6a–6t are available from the authors. |

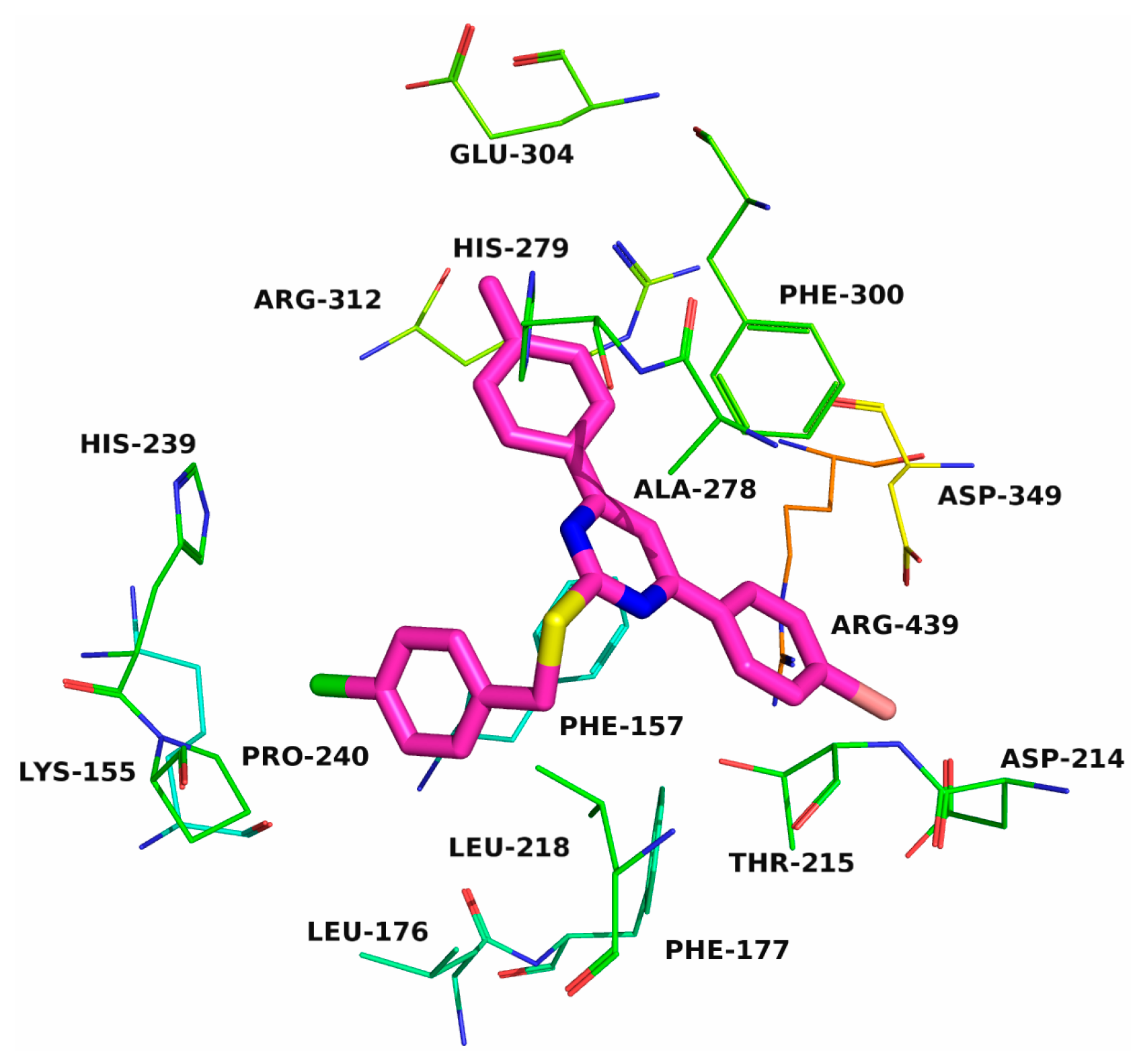
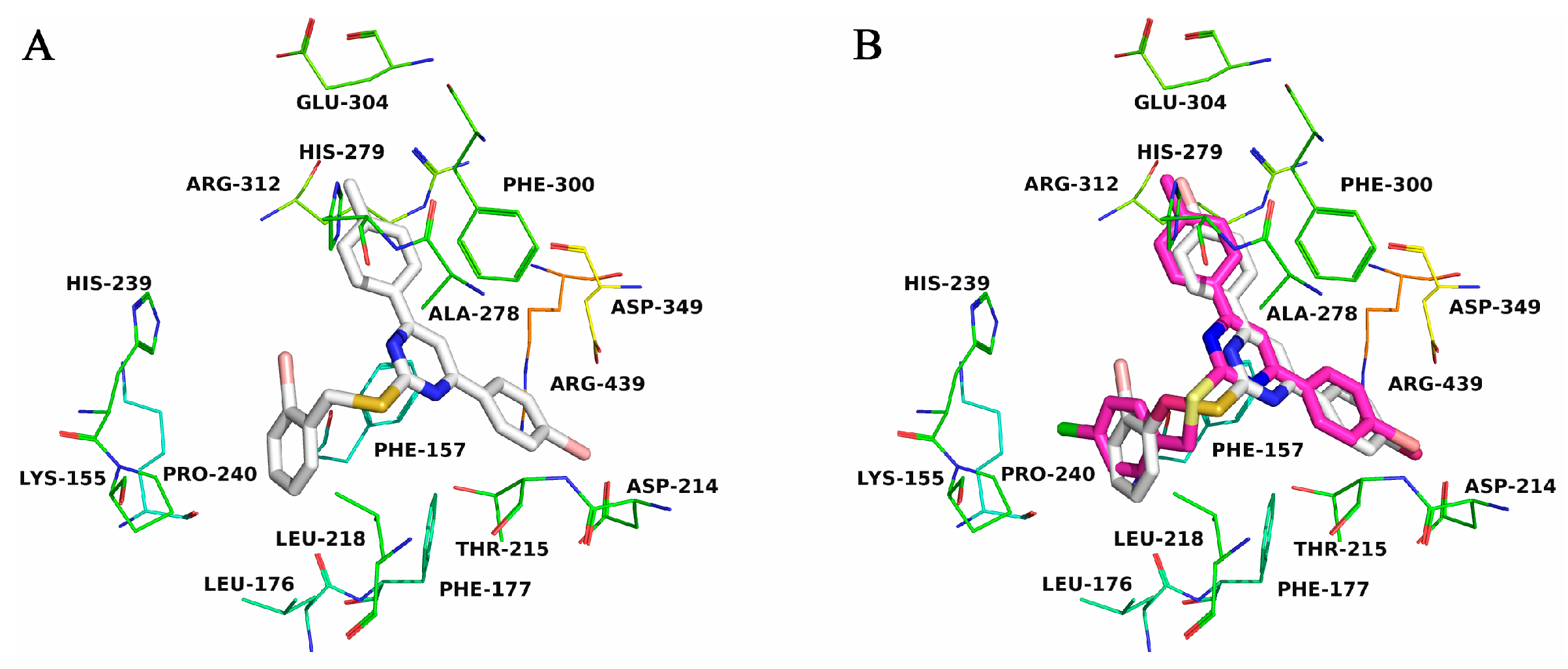

| Compound | R1 | R2 | R3 | IC50 (μM) |
|---|---|---|---|---|
| 6a | 4-Br | 4-Br | 2-Cl | 20.4 ± 0.23 |
| 6b | 4-Br | 4-Br | 4-Cl | 23.7 ± 0.27 |
| 6c | 4-Br | 4-Br | 3-F | 37.6 ± 0.34 |
| 6d | 4-Br | 4-Br | 2-F | 29.0 ± 0.28 |
| 6e | 4-Br | 4-Br | 4-F | 32.0 ± 0.26 |
| 6f | 4-Me | 3-Br | 2-Cl | 38.8 ± 0.37 |
| 6g | 4-Me | 3-Br | 3-F | 38.9 ± 0.35 |
| 6h | 4-Me | 3-Br | 4-Cl | >50 |
| 6i | 4-Me | 3-Br | 2-F | >50 |
| 6j | 4-Me | 4-Br | 4-Cl | 19.6 ± 0.21 |
| 6k | 4-Me | 4-Br | 2-Br | 21.2 ± 0.25 |
| 6l | 4-Me | 4-Br | 4-F | >50 |
| 6m | 4-Me | 4-Br | 2-F | >50 |
| 6n | 4-Me | 4-Br | 3-F | >50 |
| 6o | 4-Me | 4-Br | H | >50 |
| 6p | 4-Me | 4-Br | 2,4-Cl2 | >50 |
| 6q | 4-Me | 3-Br | 4-F | 32.1 ± 0.34 |
| 6r | 4-Me | 3-Br | 4-Br | >50 |
| 6s | 3-Br | 4-Br | 2-Cl | 26.2 ± 0.27 |
| 6t | 3-Br | 4-Br | 3-F | 25.3 ± 0.28 |
| Acarbose | 817.38 ± 6.27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Xie, Z.; Qiu, J.; Wang, G. Synthesis, Biological Evaluation and Molecular Docking Study of 2-Substituted-4,6-Diarylpyrimidines as α-Glucosidase Inhibitors. Molecules 2017, 22, 1865. https://doi.org/10.3390/molecules22111865
Gong Z, Xie Z, Qiu J, Wang G. Synthesis, Biological Evaluation and Molecular Docking Study of 2-Substituted-4,6-Diarylpyrimidines as α-Glucosidase Inhibitors. Molecules. 2017; 22(11):1865. https://doi.org/10.3390/molecules22111865
Chicago/Turabian StyleGong, Zipeng, Zhenzhen Xie, Jie Qiu, and Guangcheng Wang. 2017. "Synthesis, Biological Evaluation and Molecular Docking Study of 2-Substituted-4,6-Diarylpyrimidines as α-Glucosidase Inhibitors" Molecules 22, no. 11: 1865. https://doi.org/10.3390/molecules22111865