Detection of Rare Somatic GNAS Mutation in McCune-Albright Syndrome Using a Novel Peptide Nucleic Acid Probe in a Single Tube
Abstract
:1. Introduction
2. Results
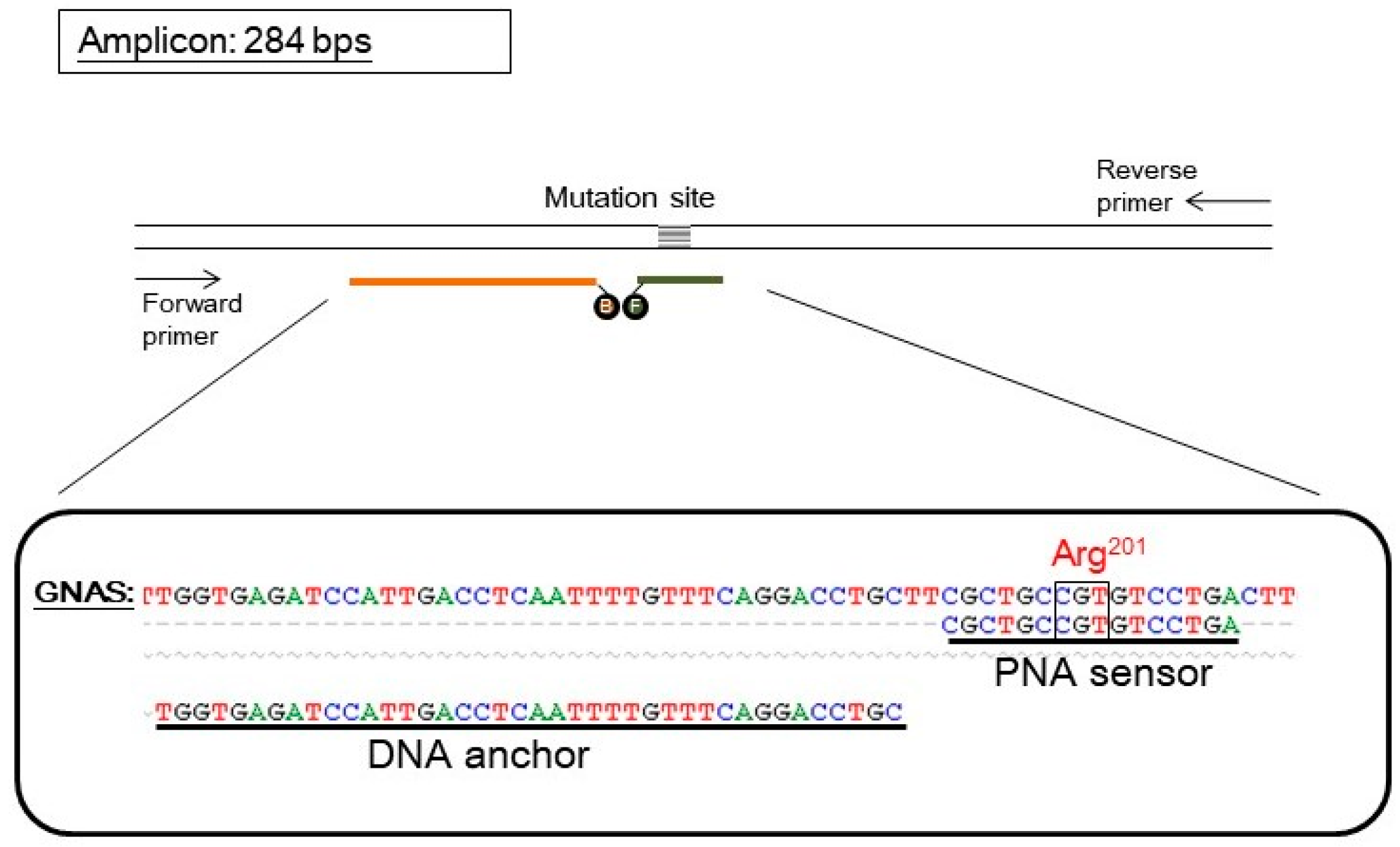
2.1. Assay Design
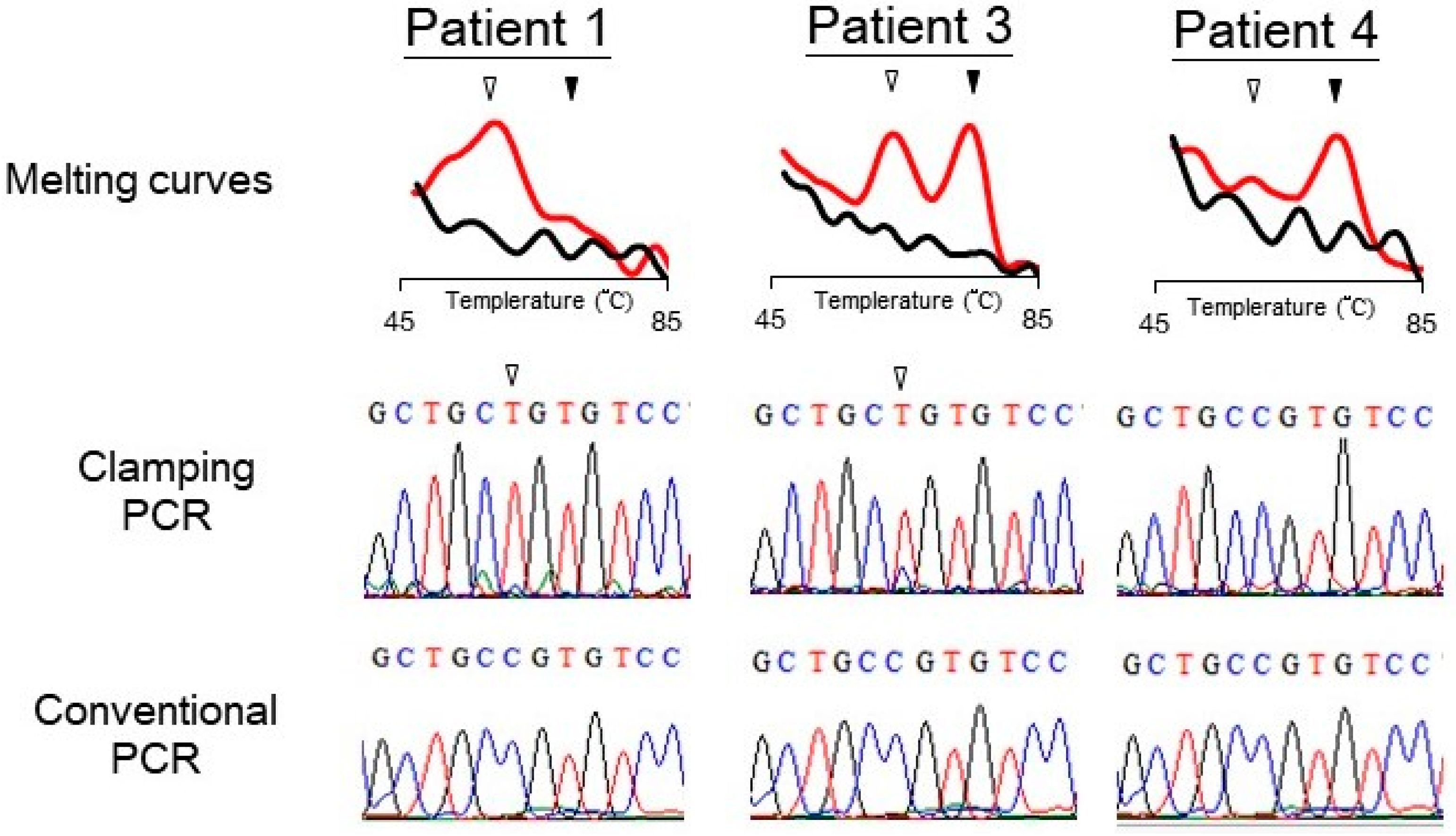
2.2. Genotypes Detected by the PNA Probe Assay
3. Discussion
4. Materials and Methods
4.1. Design of Probes and Primers
4.2. Patients
4.3. Preparation of Template DNA
4.4. Detection of GNAS Mutation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schwindinger, W.F.; Francomano, C.A.; Levine, M.A. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc. Natl. Acad. Sci. USA 1992, 89, 5152–5256. [Google Scholar] [CrossRef] [PubMed]
- Shenker, A.; Weinstein, L.S.; Sweet, D.E.; Spiegel, A.M. An activating Gs alpha mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 1994, 79, 750–755. [Google Scholar] [PubMed]
- Weinstein, L.S.; Shenker, A.; Gejman, P.V.; Merino, M.J.; Friedman, E.; Spiegel, A.M. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N. Engl. J. Med. 1991, 325, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.M.; Weinstein, L.S.; Shenker, A. Abnormalities in G protein-coupled signal transduction pathways in human disease. J. Clin. Investig. 1993, 92, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Hannon, T.S.; Noonan, K.; Steinmetz, R.; Eugster, E.A.; Levine, M.A.; Pescovitz, O.H. Is McCune-Albright syndrome overlooked in subjects with fibrous dysplasia of bone? J. Pediatr. 2003, 142, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, E.R.; Nam, H.J.; Chin, M.O.; Moon, Y.H.; Oh, M.R.; Yeo, U.C.; Song, S.M.; Kim, J.S.; Uhm, M.R.; et al. Activating mutation of GS alpha in McCune-Albright syndrome causes skin pigmentation by tyrosinase gene activation on affected melanocytes. Horm. Res. 1999, 52, 235–240. [Google Scholar] [PubMed]
- Candeliere, G.A.; Roughley, P.J.; Glorieux, F.H. Polymerase chain reaction-based technique for the selective enrichment and analysis of mosaic arg201 mutations in G alpha s from patients with fibrous dysplasia of bone. Bone 1997, 21, 201–206. [Google Scholar] [CrossRef]
- Riminucci, M.; Liu, B.; Corsi, A.; Shenker, A.; Spiegel, A.M.; Robey, P.G.; Bianco, P. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: Site-specific patterns and recurrent histological hallmarks. J. Pathol. 1999, 187, 249–258. [Google Scholar] [CrossRef]
- Kyger, E.M.; Krevolin, M.D.; Powell, M.J. Detection of the hereditary hemochromatosis gene mutation by real-time fluorescence polymerase chain reaction and peptide nucleic acid clamping. Anal. Biochem. 1998, 260, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Riminucci, M.; Majolagbe, A.; Kuznetsov, S.A.; Collins, M.T.; Mankani, M.H.; Corsi, A.; Bone, H.G.; Wientroub, S.; Spiegel, A.M.; et al. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J. Bone Miner. Res. 2000, 15, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kalfa, N.; Philibert, P.; Audran, F.; Ecochard, A.; Hannon, T.; Lumbroso, S.; Sultan, C. Searching for somatic mutations in McCune-Albright syndrome: A comparative study of the peptidic nucleic acid versus the nested PCR method based on 148 DNA samples. Eur. J. Endocrinol. 2006, 155, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Lietman, S.A.; Ding, C.; Levine, M.A. A highly sensitive polymerase chain reaction method detects activating mutations of the GNAS gene in peripheral blood cells in McCune-Albright syndrome or isolated fibrous dysplasia. J. Bone Jt. Surg. Am. 2005, 87, 2489–2494. [Google Scholar]
- Karadag, A.; Riminucci, M.; Bianco, P.; Cherman, N.; Kuznetsov, S.A.; Nguyen, N.; Collins, M.T.; Robey, P.G.; Fisher, L.W. A novel technique based on a PNA hybridization probe and FRET principle for quantification of mutant genotype in fibrous dysplasia/McCune-Albright syndrome. Nucleic Acids Res. 2004, 32, e63. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.C.; Luo, J.D.; Chen, T.L. Single-tube reaction using peptide nucleic acid as both PCR clamp and sensor probe for the detection of rare mutations. Nat. Protoc. 2006, 1, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.D.; Chan, E.C.; Shih, C.L.; Chen, T.L.; Liang, Y.; Hwang, T.L.; Chiou, C.C. Detection of rare mutant K-ras DNA in a single-tube reaction using peptide nucleic acid as both PCR clamp and sensor probe. Nucleic Acids Res. 2006, 34, e12. [Google Scholar] [CrossRef] [PubMed]
- Lemli, L. Fibrous dysplasia of bone. Report of female monozygotic twins with and without the McCune-Albright syndrome. J. Pediatr. 1977, 91, 947–949. [Google Scholar] [CrossRef]
- Fukazawa, T.; Ohtawara, H.; Motozumi, H.; Senoo, I.; Hayashibara, H.; Hanaki, K.; Ohzeki, T. McCune-Albright syndrome in one of monozygotic twins. J. Jpn. Pediatr. Soc. 1990, 94, 1913. [Google Scholar]
- Endo, M.; Yamada, Y.; Matsuura, N.; Niikawa, N. Monozygotic twins discordant for the major signs of McCune-Albright syndrome. Am. J. Med. Genet. 1991, 41, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.M.; Shaw, C.A.; Stankiewicz, P.; Lupski, J.R. Somatic mosaicism: Implications for disease and transmission genetics. Trends Genet. 2015, 31, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, C.E.; Collins, M.T. McCune-Albright syndrome. Orphanet J. Rare Dis. 2008, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Matthaei, H.; Maitra, A.; Dal Molin, M.; Wood, L.D.; Eshleman, J.R.; Goggins, M.; Canto, M.I.; Schulick, R.D.; Edil, B.H.; et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl. Med. 2011, 3, 92ra66. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Fabre, M.; Couchy, G.; Pilati, C.; Jeannot, E.; Tran Van Nhieu, J.; Saint-Paul, M.C.; De Muret, A.; Redon, M.J.; Buffet, C.; et al. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J. Hepatol. 2012, 56, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Kalfa, N.; Lumbroso, S.; Boulle, N.; Guiter, J.; Soustelle, L.; Costa, P.; Chapuis, H.; Baldet, P.; Sultan, C. Activating mutations of Gsalpha in kidney cancer. J. Urol. 2006, 176, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Fecteau, R.E.; Lutterbaugh, J.; Markowitz, S.D.; Willis, J.; Guda, K. GNAS mutations identify a set of right-sided, RAS mutant, villous colon cancers. PLoS ONE 2014, 9, e87966. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |


| Patient | Sex | Age (Year) | PP 1 | Café-au-lait Spots | BFD 2 | GNAS Mutations | |
|---|---|---|---|---|---|---|---|
| 1 | twin A | Female | 0.39 | + | + | + | + |
| 2 | twin B | Female | 7.83 | + | − | + | + |
| 3 | Female | 5.28 | + | − | + | + | |
| 4 | Female | 11.95 | − | + | + | − | |
| 5 | Male | 12.05 | − | − | + | − | |
| 6 | Male | 6.25 | − | − | + | − |
| Name | Sequence (5′-3′ for DNA or N Terminal to C Terminal for PNA) | Length |
|---|---|---|
| Primers | ||
| Forward | AACTACTCCAGACCTTTGCTTTAGAT | 26 |
| Reverse | CAGCTGGTTATTCCAGAGGGAC | 22 |
| Probes | ||
| PNA sensor | (Fluorescein)-OO-CGCTGCCGTGTCCTGA | 16 |
| DNA anchor | TGGTGAGATCCATTGACCTCAATTTTGTTTCAGGACCTGC-(Bodipy630/650) | 40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, F.-S.; Chen, T.-L.; Chiou, C.-C. Detection of Rare Somatic GNAS Mutation in McCune-Albright Syndrome Using a Novel Peptide Nucleic Acid Probe in a Single Tube. Molecules 2017, 22, 1874. https://doi.org/10.3390/molecules22111874
Lo F-S, Chen T-L, Chiou C-C. Detection of Rare Somatic GNAS Mutation in McCune-Albright Syndrome Using a Novel Peptide Nucleic Acid Probe in a Single Tube. Molecules. 2017; 22(11):1874. https://doi.org/10.3390/molecules22111874
Chicago/Turabian StyleLo, Fu-Sung, Tai-Long Chen, and Chiuan-Chian Chiou. 2017. "Detection of Rare Somatic GNAS Mutation in McCune-Albright Syndrome Using a Novel Peptide Nucleic Acid Probe in a Single Tube" Molecules 22, no. 11: 1874. https://doi.org/10.3390/molecules22111874




