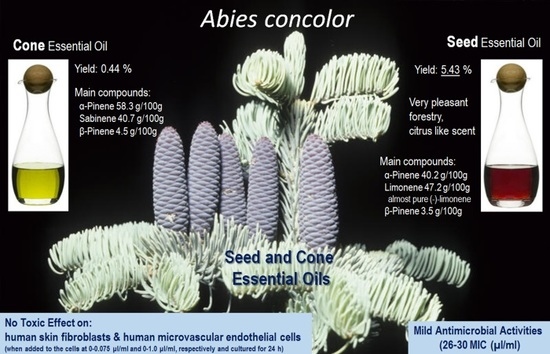
Abies Concolor Seeds and Cones as New Source of Essential Oils—Composition and Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry of Essential Oils
2.2. Biological Activities of Essential Oils
3. Experimental
3.1. Plant Material
3.2. Essential Oil Isolation
3.3. Chromatographic Analysis
3.4. Identification of Compounds
3.5. Isolation of Components
3.6. Essential Oil Constituents Quantification
3.7. Antimicrobial Activity
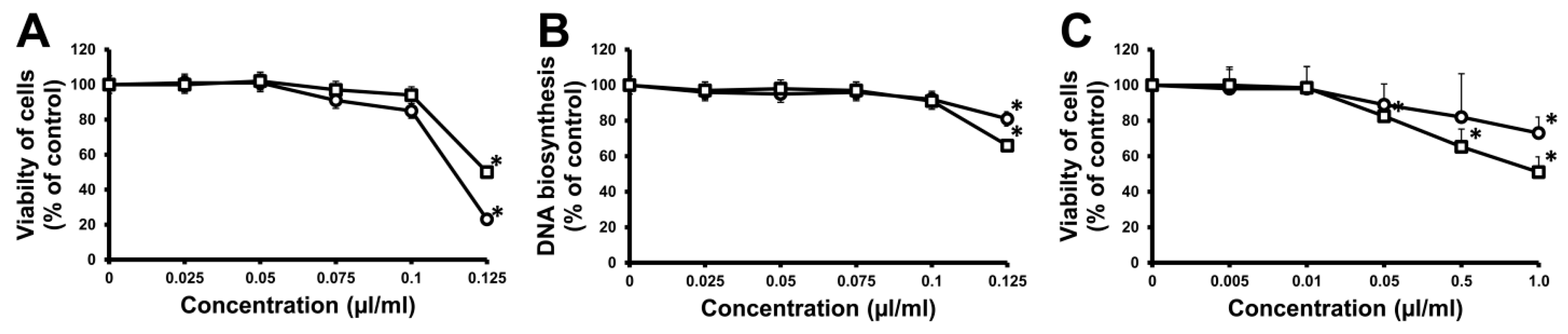
3.8. Cell Culture
3.9. Cell-Viability Assay
3.10. DNA Biosynthesis Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hamrick, J.L.; Libby, W.J. Variation and selection in western U.S. Montane species. I. White fir. Silvae Genet. 1972, 21, 29–35. [Google Scholar]
- Wright, J.W.; Lemmien, W.A.; Bright, J.N. Genetic variation in southern Rocky Mountain white fir. Silvae Genet. 1971, 20, 148–150. [Google Scholar]
- Harlow, W.M.; Harrar, E.S. Textbook of Dendrology; American Forestry Press: Arcadia, CA, USA, 1968. [Google Scholar]
- Boyce, O.M. Plant Uses by New Mexico’s Early Natives; Rydal Press: Santa Fe, NM, USA, 1974; pp. 13–15. [Google Scholar]
- Ulubelen, A.; Caldwell, M.E.; Cole, J.R. Phytochemical investigation of Abies concolor. J. Pharm. Sci. 1966, 55, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Cvrkal, H.; Janak, J. Identification of some terpenes in essential oils of conifers by gas chromatography. Collect. Czechoslov. Chem. Commun. 1959, 24, 1967–1974. [Google Scholar] [CrossRef]
- Lewis, M.; Nelson, J.K.; Grant, K.E. Analysis of the major volatile and semi-volatile components of essential needle oil from Abies concolor by gas chromatography/mass spectrometry. In Proceedings of the Joint 65th Northwest and 22nd Rocky Mountain Regional Meeting of the American Chemical Society, Pullman, WA, USA, 20–23 June 2010. [Google Scholar]
- Wagner, M.R.; Clancy, K.M.; Tinus, R.W. Maturational variation in needle essential oils from Pseudotsuga menziesii, Abies concolor and Picea engelmannii. Phytochemistry 1989, 28, 765–770. [Google Scholar] [CrossRef]
- Wagner, M.R.; Clancy, K.M.; Tinus, R.W. Seasonal patterns in the allelochemicals of Pseudotsuga menziesii, Picea engelmanii and Abies concolor. Biochem. Syst. Ecol. 1990, 18, 215–220. [Google Scholar] [CrossRef]
- Popina, O.A.; Vladykina, D.S.; Lamotkin, S.A. Variability of the main components in essential oil of Abies concolor growing in urban environment. Vestsi Akad. Navuk BSSR Ser. Biyal. Navuk 2015, 1, 107–110. [Google Scholar]
- Wolff, R.L.; Lavialle, O.; Pédrono, F.; Pasquier, E.; Destaillats, F.; Marpeau, A.M.; Angers, P.; Aitzetmüller, K. Abietoid seed fatty acid composition—A review of the genera Abies, Cedrus, Hesperopeuce, Keteleeria, Pseudolarix, and Tsuga and preliminary inferences on the taxonomy of Pinaceae. Lipids 2002, 37, 17–26. [Google Scholar]
- Wolff, R.L.; Deluc, L.G.; Marpeau, A.M. Conifer Seeds: Oil Content and Fatty Acid Composition. JAOCS 1996, 73, 765–772. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Olejnik, K.; Bonikowski, R.; Banaszczak, P. Composition of essential oils from seeds of Abies koreana. Nat. Prod. Commun. 2013, 8, 227–230. [Google Scholar] [PubMed]
- Wajs, A.; Urbańska, J.; Zaleśkiewicz, E.; Bonikowski, R. Composition of essential oil from seeds and cones of Abies alba. Nat. Prod. Commun. 2010, 5, 1291–1294. [Google Scholar] [PubMed]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Maciag, A.; Szoka, Ł.; Karna, E. Chemical composition and biological activity of Abies alba and A. koreana seed and cone essential oils and characterization of their seed hydrolates. Chem. Biodivers. 2015, 12, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Wajs-Bonikowska, A.; Olejnik, K.; Bonikowski, R.; Banaszczak, P. Analysis of volatile components, fatty acids, and phytosterols of Abies koreana growing in Poland. Nat. Prod. Commun. 2013, 8, 1297–1300. [Google Scholar] [PubMed]
- Baser, K.H.C.; Buchbauer, G. (Eds.) Handbook of Essential Oils: Science, Technology and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Knobloch, K.; Weigand, H.; Weis, N.; Schwarm, H.M.; Vigenschow, H. Progress in Essential Oil Research; Brunke, E.J., Ed.; Walter de Gruyter: Berlin, Germany, 1986. [Google Scholar]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Microbios 1995, 82, 181. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. Establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowska-Owczarek, A. The effect of diclofenac on proliferation and production of growth factors by endothelial cells (HMEC-1) under hypoxia and inflammatory conditions. Acta Pharm. 2014, 64, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; Degraff, W.; Gazdar, A.; Minna, J.; Mitchell, J. Evaluation of a tetrazolium-based semiautomated colorimetrie assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Karna, E.; Szoka, Ł.; Pałka, J.A. The mechanism of hydralazine-induced collagen biosynthesis in cultured fibroblasts. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 303–309. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the essential oils are available from the authors. |
| Seed Essential Oil | Cone Essential Oil | RI Exp. | Identification Method | ||
|---|---|---|---|---|---|
| No. | Compound | (g/100 g) | (g/100 g) | ||
| 1 | Santolinatriene | 0.03 | 914 | RI, MS | |
| 2 | Tricyclene | 0.02 | 0.02 | 918 | RI, MS |
| 3 | α-Thujene | 0.01 | 0.55 | 923 | RI, MS |
| 4 | α-Pinene | 40.22 | 58.31 | 933 | RI, MS |
| 5 | Camphene | 0.3 | 0.41 | 941 | RI, MS |
| 6 | Thuja-2,4-(10)-diene | 0.02 | 0.28 | 944 | RI, MS |
| 7 | Sabinene | 0.23 | 10.73 | 963 | RI, MS |
| 8 | β-Pinene | 3.54 | 4.45 | 967 | RI, MS |
| 9 | 1,8-Dehydrocineol | 0.02 | 979 | RI, MS | |
| 10 | Verbene | 0.16 | 980 | RI, MS | |
| 11 | β-Myrcene | 1.39 | 986 | ||
| 12 | δ-Car-2-ene | 0.01 | 0.02 | 992 | RI, MS |
| 13 | α-Phellandrene | 999 | |||
| 14 | δ-Car-3-ene | 0.13 | 1000 | RI, MS | |
| 15 | α-Terpinene | 0.08 | 0.2 | 1009 | RI, MS |
| 16 | p-Cymene | 0.83 | 1008 | RI, MS | |
| 17 | Limonene | 47.15 | 2.71 | 1025 | RI, MS |
| 18 | β-Phellandrene | 0.01 | 1026 | RI, MS | |
| 19 | γ-Terpinene | 0.01 | 1047 | RI, MS | |
| 20 | trans-Sabinene hydrate | 0.01 | 0.06 | 1052 | RI, MS |
| 21 | Fenchon | 0.02 | 1066 | RI, MS | |
| 22 | p-Cymenene | 0.02 | 1066 | RI, MS | |
| 23 | Terpinolene | 0.03 | 0.69 | 1081 | RI, MS |
| 24 | cis-Sabinene hydrate | 0.06 | 1083 | RI, MS | |
| 25 | Linallol | 0.03 | 1090 | RI, MS | |
| 26 | α-Thujone | 0.01 | 1090 | RI, MS | |
| 27 | α-Fenchol | 0.01 | 0.04 | 1099 | RI, MS |
| 28 | α-Campholenal | 0.01 | 0.24 | 1104 | RI, MS |
| 29 | cis-p-Menth-2-en-1-ol | 0.04 | 1106 | RI, MS | |
| 30 | cis-Limonene oxide | 0.02 | 1115 | RI, MS | |
| 31 | cis-p-Mentha-2,8-dien-1-ol | 0.01 | 1119 | RI, MS | |
| 32 | Camphor | 0.01 | 0.02 | 1124 | RI, MS |
| 33 | trans-Pinocarveol | 1.37 | 1125 | RI, MS | |
| 34 | cis-Verbenol | 0.02 | 1129 | 1H, RI, MS | |
| 35 | trans-Verbenol | 0.03 | 1.18 | 1129 | RI, MS |
| 36 | α-Phellandren-8-ol | 1133 | |||
| 37 | Pinocarvon | 0.03 | 1137 | RI, MS | |
| 38 | Cryptone | 0.27 | 1149 | RI, MS | |
| 39 | α-Thujenal | 0.07 | 1159 | RI, MS | |
| 40 | Terpinen-4-ol | 0.03 | 1.99 | 1162 | 1H, RI, MS |
| 41 | Myrtenal | 0.03 | 0.33 | 1169 | RI, MS |
| 42 | α-Terpineol | 0.03 | 0.28 | 1173 | RI, MS |
| 43 | trans-Dihydrocarvon | 0.01 | 1177 | RI, MS | |
| 44 | Myrtenol | 2.17 | 1182 | 1H, RI, MS | |
| 45 | Verbenone | 0.01 | 1188 | ||
| 46 | cis-Carvotanacetone | 0.01 | 1190 | RI, MS | |
| 47 | trans-Carveol | 0.01 | 0.02 | 1199 | RI, MS |
| 48 | Citronellol | 0.01 | 1211 | RI, MS | |
| 49 | cis-Carveol | 0.01 | 1212 | RI, MS | |
| 50 | Thymol methyl ether | 0.02 | 0.01 | 1215 | RI, MS |
| 51 | Carvon | 0.01 | 1221 | RI, MS | |
| 52 | Piperitone | 0.01 | 1223 | RI, MS | |
| 53 | Bornyl acetate | 0.33 | 0.31 | 1267 | 1H, RI, MS |
| 54 | trans-Shisol | 0.01 | 1279 | RI, MS | |
| 55 | Perilla aldehyde | 0.01 | 1283 | RI, MS | |
| 56 | p-Mentha-1,4-dien-7-ol | 0.01 | 1305 | RI, MS | |
| 57 | α-Cubebene | 0.15 | 0.01 | 1346 | 1H, RI, MS |
| 58 | α-Copaene | 0.18 | 1373 | 1H, RI, MS | |
| 59 | α-Bourbonene | 0.39 | 1381 | RI, MS | |
| 60 | β-Bourbonene | 0.01 | 1385 | RI, MS | |
| 61 | (E)-β-Caryophyllene | 0.04 | 0.01 | 1414 | RI, MS |
| 62 | α-Bergamotene | 0.02 | 1424 | RI, MS | |
| 63 | trans-β-Farnesene | 0.02 | 1438 | RI, MS | |
| 64 | Cadina-3,5-diene | 0.01 | 1447 | RI, MS | |
| 65 | Cadina-4,11-diene | 0.01 | 1456 | RI, MS | |
| 66 | γ-Muurolen | 0.17 | 1467 | RI, MS | |
| 67 | Germacrene D | 0.05 | 0.03 | 1473 | RI, MS |
| 68 | β-Selinene | 0.02 | 1479 | RI, MS | |
| 69 | Bicyclosesquiphellandrene | 0.04 | 1485 | RI, MS | |
| 70 | 4-epi-Cubebol | 0.02 | 1488 | 1H, RI, MS | |
| 71 | α-Muurolene | 0.2 | 1490 | RI, MS | |
| 72 | γ-Cadinene | 0.97 | 0.01 | 1504 | ¹H, RI, MS |
| 73 | cis/trans-Calamenene | 0.01 | 1509 | RI, MS | |
| 74 | Cadina-1,4-diene | 0.01 | 1528 | RI, MS | |
| 75 | δ-Cadinene | 0.02 | 1528 | 1H, RI, MS | |
| 76 | (E)-Nerolidol | 0.01 | 1547 | RI, MS | |
| 77 | Gleenol | 0.01 | 1571 | RI, MS | |
| 78 | 1,10-di-epi-Cubenol | 0.01 | 1603 | RI, MS | |
| 79 | Selin-6-en-4-ol | ||||
| 80 | 1-epi-Cubenol | 0.02 | 1613 | RI, MS | |
| 81 | τ-Cadinol | 0.13 | 0.02 | 1625 | 1H, RI, MS |
| 82 | τ-Muurolol | RI, MS | |||
| 83 | Cubenol | 1629 | 1H, RI, MS | ||
| 84 | α-Cadinol | 0.01 | 0.02 | 1638 | 1H, RI, MS |
| 85 | cis-Calemenen-10-ol | 0.01 | 1640 | RI, MS | |
| 86 | trans-Calemenen-10-ol | 0.01 | 1647 | 1H, RI, MS | |
| 87 | Cembrene | 0.01 | 1918 | RI, MS | |
| 88 | 18-Norabieta-8,11,13-triene | 0.42 | 1965 | RI, MS | |
| 89 | Manoyl oxide | 0.08 | 1987 | RI, MS | |
| 90 | Pimara-8(14),15-diene | 0.21 | 1998 | RI, MS | |
| 91 | Methyl abietate | 1.10 | 1996 | RI, MS | |
| 92 | Kaur-16-ene | 0.04 | 2029 | RI, MS | |
| 93 | Abieta-8(14),9(11),13-triene | 0.06 | 0.35 | 2038 | RI, MS |
| 94 | Abieta-8-(14),13(15)-dien | 0.30 | 2075 | RI, MS | |
| 95 | Cembrol | 0.02 | 2139 | RI, MS | |
| 96 | Pimara-7,15-dien-3-on | 0.03 | 2225 | RI, MS | |
| 97 | Dehydroabietal | 0.23 | 2233 | RI, MS | |
| 98 | Abietal | 0.06 | 2286 | RI, MS | |
| Monoterpene hydrocarbons | 92.98 | 78.88 | |||
| Oxygenated monoterpenes | 0.71 | 9.23 | |||
| Sesquiterpene hydrocarbons | 1.89 | 0.49 | |||
| Oxygenated sesquiterpenes | 0.21 | 0.04 | |||
| Diterpene hydrocarbons | 0.06 | 1.32 | |||
| Oxygenated diterpenes | - | 1.50 | |||
| Others | 0.01 | 0.03 | |||
| Total identified [g/100 g] | 95.86 | 91.87 | |||
| Yield of essential oil [%] | 5.43 | 0.44 | |||
| ± (standard deviation) | ±(0.09) | ±(0.03) | |||
| Abies concolor | ||
|---|---|---|
| Enantiomer | Seed | Cone |
| (S)-(−)-Limonene (%) | 97.1 | 84.1 |
| (R)-(+)-Limonene (%) | 2.9 | 15.9 |
| ee | 94.2 | 68.2 |
| (1S,5S)-(−)-α-Pinene (%) | 87.0 | 95.8 |
| (1R,5R)-(+)-α-Pinene (%) | 13.0 | 4.2 |
| ee | 74.0 | 91.6 |
| (1S,5S)-(−)-β-Pinene (%) | 10.1 | 41.2 |
| (1R,5R)-(+)-β-Pinene (%) | 89.9 | 58.8 |
| ee | 79.8 | 17.6 |
| (S)-(−)-Camphene (%) | - | 94.2 |
| (R)-(+)-Camphene (%) | - | 5.8 |
| ee | - | 88.4 |
| Essential Oil | Minimal Inhibitory Concentration MIC (μL/mL) | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 43300 | Enterococcus faecalis ATCC 51299 | Enterococcus faecium ATCC 35667 | Escherichia coli ATCC 25922 | Klebsiella pneumoniae ATCC 700603 | |
| Seed | 30 ± 1.4 | 26 ± 2.1 | 30 ± 3.5 | 26 ± 0.9 | 28 ± 1.8 |
| Cone | 30 ± 2.6 | 26 ± 0.7 | 26 ± 2.8 | 30 ± 2.2 | 28 ± 1.3 |
| Thymol | 2.5 | 0.13 | 0.39 | 0.63 | 0.76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajs-Bonikowska, A.; Szoka, Ł.; Karna, E.; Wiktorowska-Owczarek, A.; Sienkiewicz, M. Abies Concolor Seeds and Cones as New Source of Essential Oils—Composition and Biological Activity. Molecules 2017, 22, 1880. https://doi.org/10.3390/molecules22111880
Wajs-Bonikowska A, Szoka Ł, Karna E, Wiktorowska-Owczarek A, Sienkiewicz M. Abies Concolor Seeds and Cones as New Source of Essential Oils—Composition and Biological Activity. Molecules. 2017; 22(11):1880. https://doi.org/10.3390/molecules22111880
Chicago/Turabian StyleWajs-Bonikowska, Anna, Łukasz Szoka, Ewa Karna, Anna Wiktorowska-Owczarek, and Monika Sienkiewicz. 2017. "Abies Concolor Seeds and Cones as New Source of Essential Oils—Composition and Biological Activity" Molecules 22, no. 11: 1880. https://doi.org/10.3390/molecules22111880