Atractylenolide II Inhibits Proliferation, Motility and Induces Apoptosis in Human Gastric Carcinoma Cell Lines HGC-27 and AGS
Abstract
:1. Introduction
2. Results
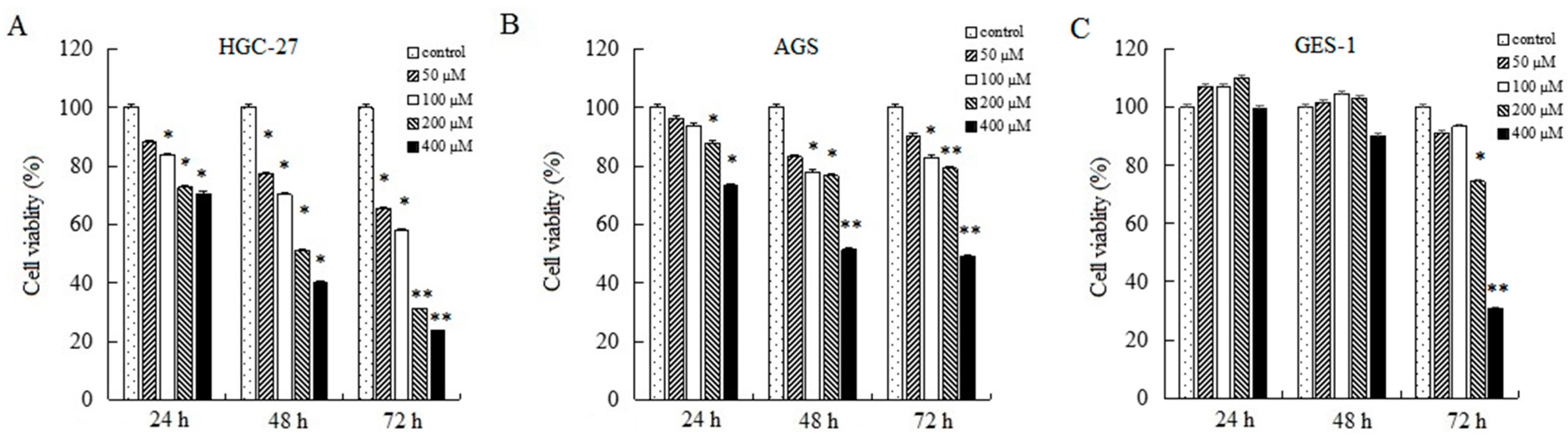
2.1. AT-II Inhibits Proliferation in HGC-27 and AGS Cells
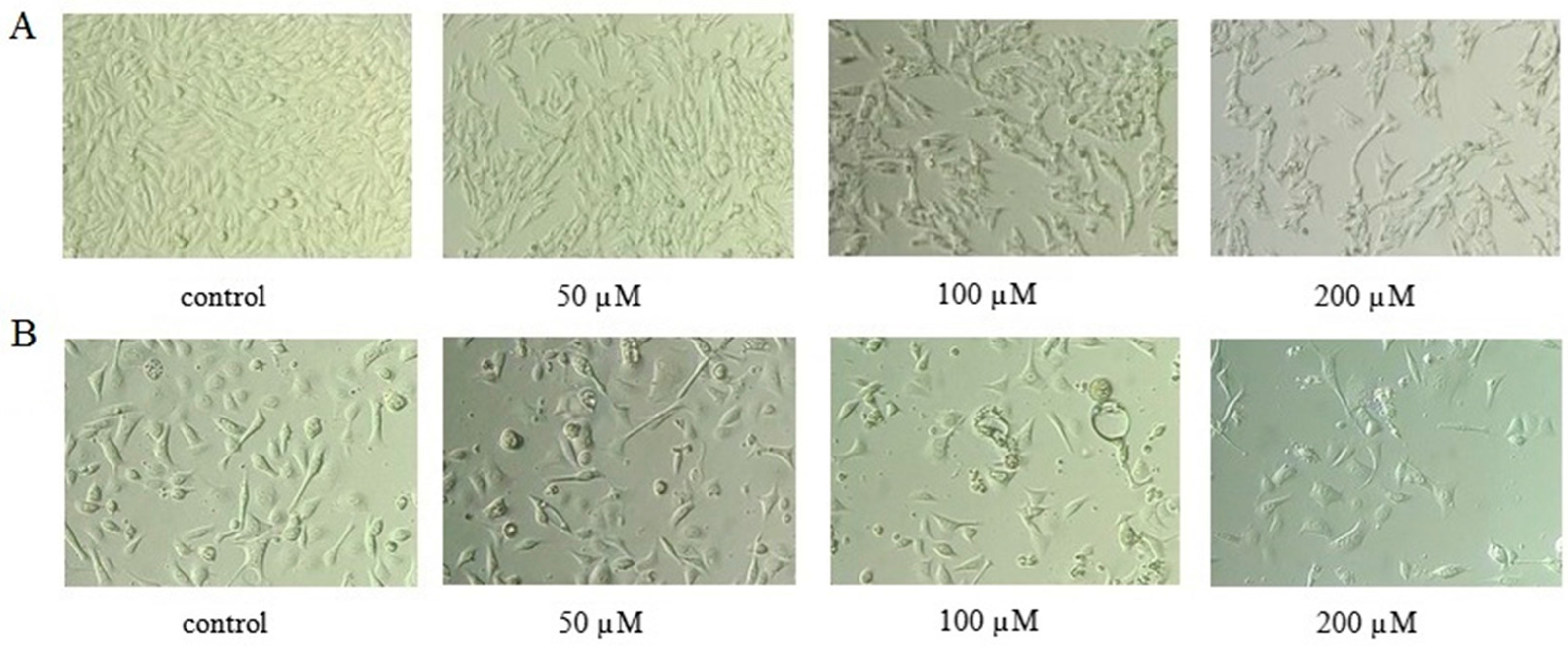
2.2. AT-II Affects Morphological Changes
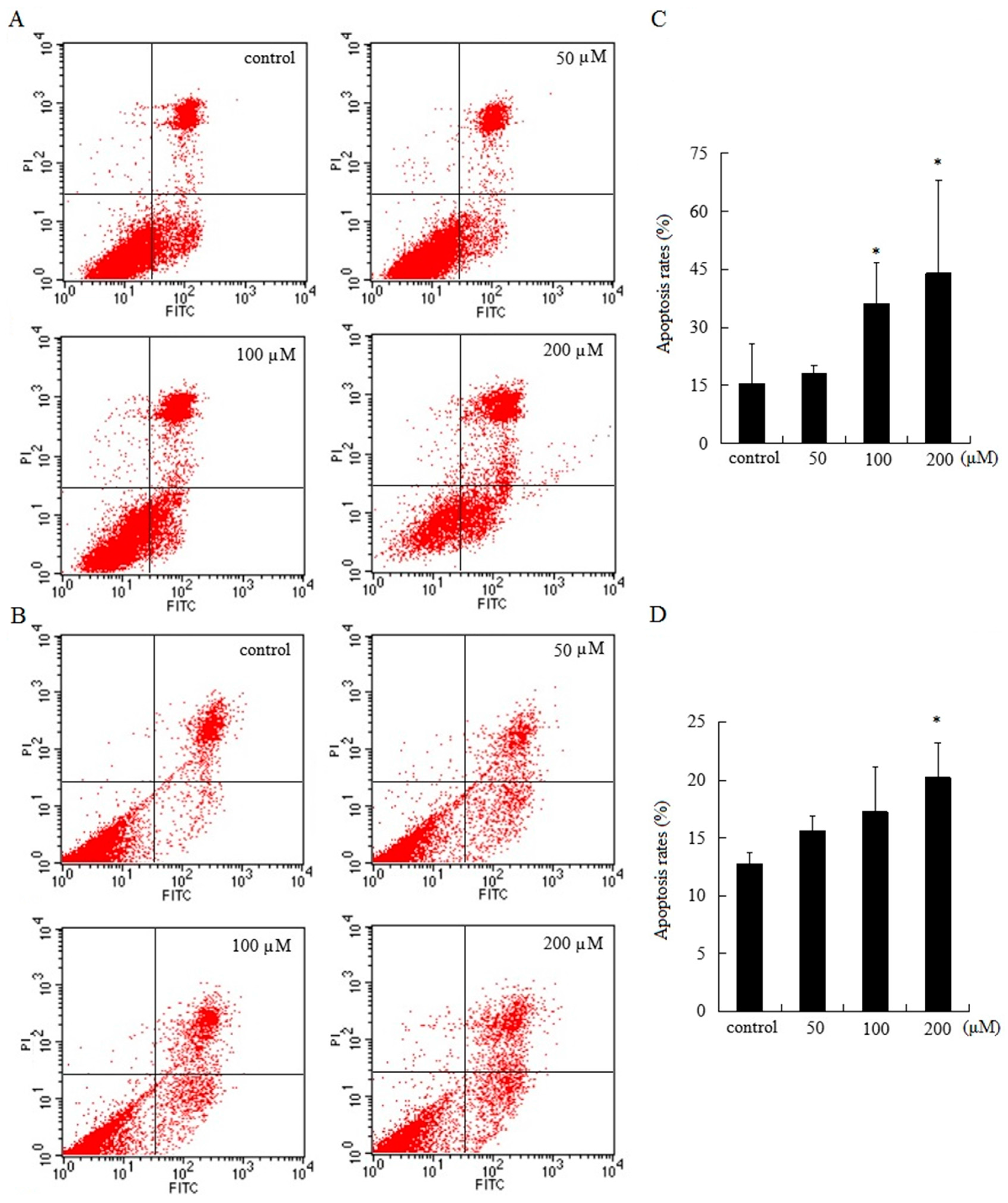
2.3. AT-II Induces Apoptosis in HGC-27 and AGS Cells
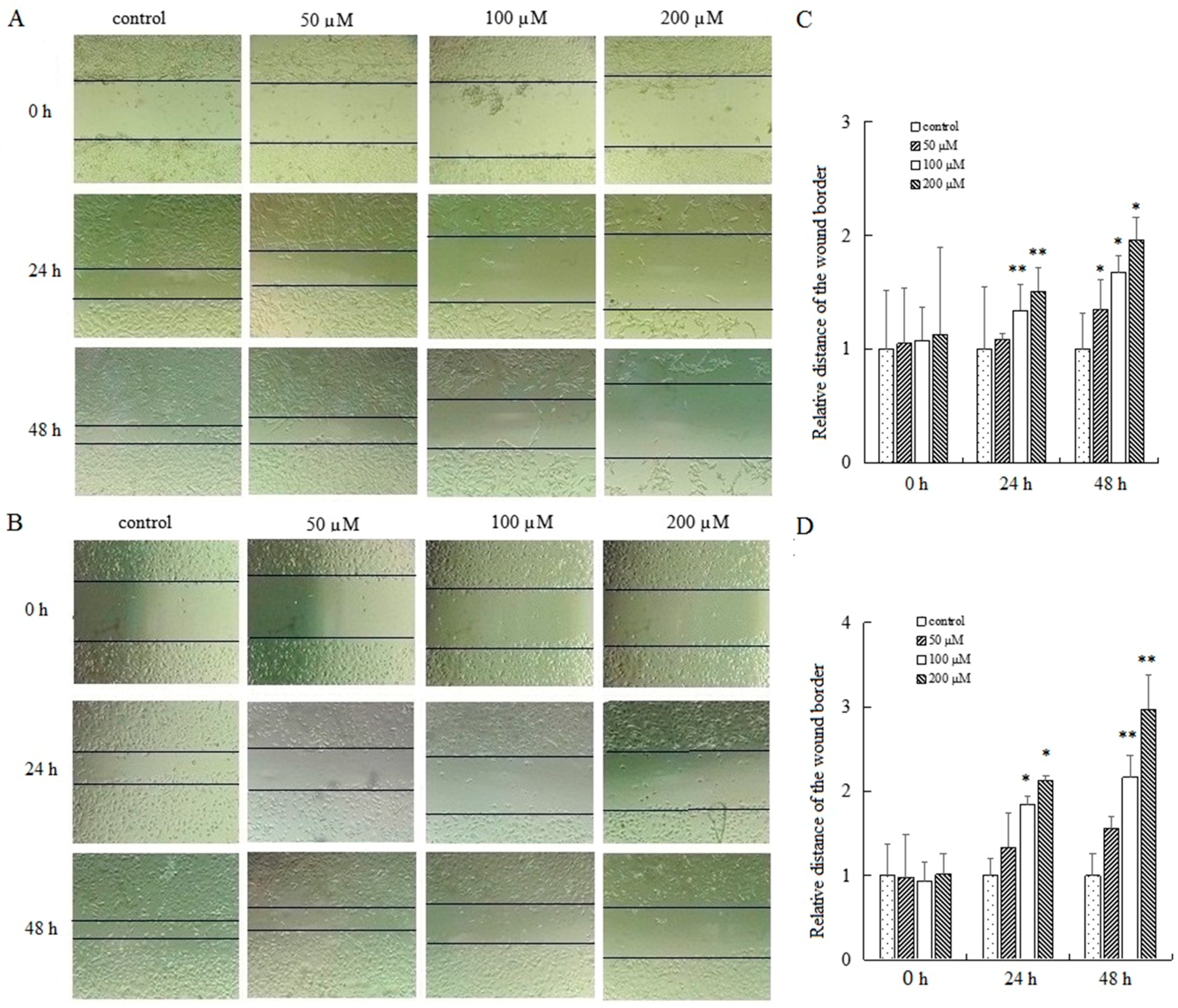
2.4. AT-II Suppresses the Capability of Cell Motility
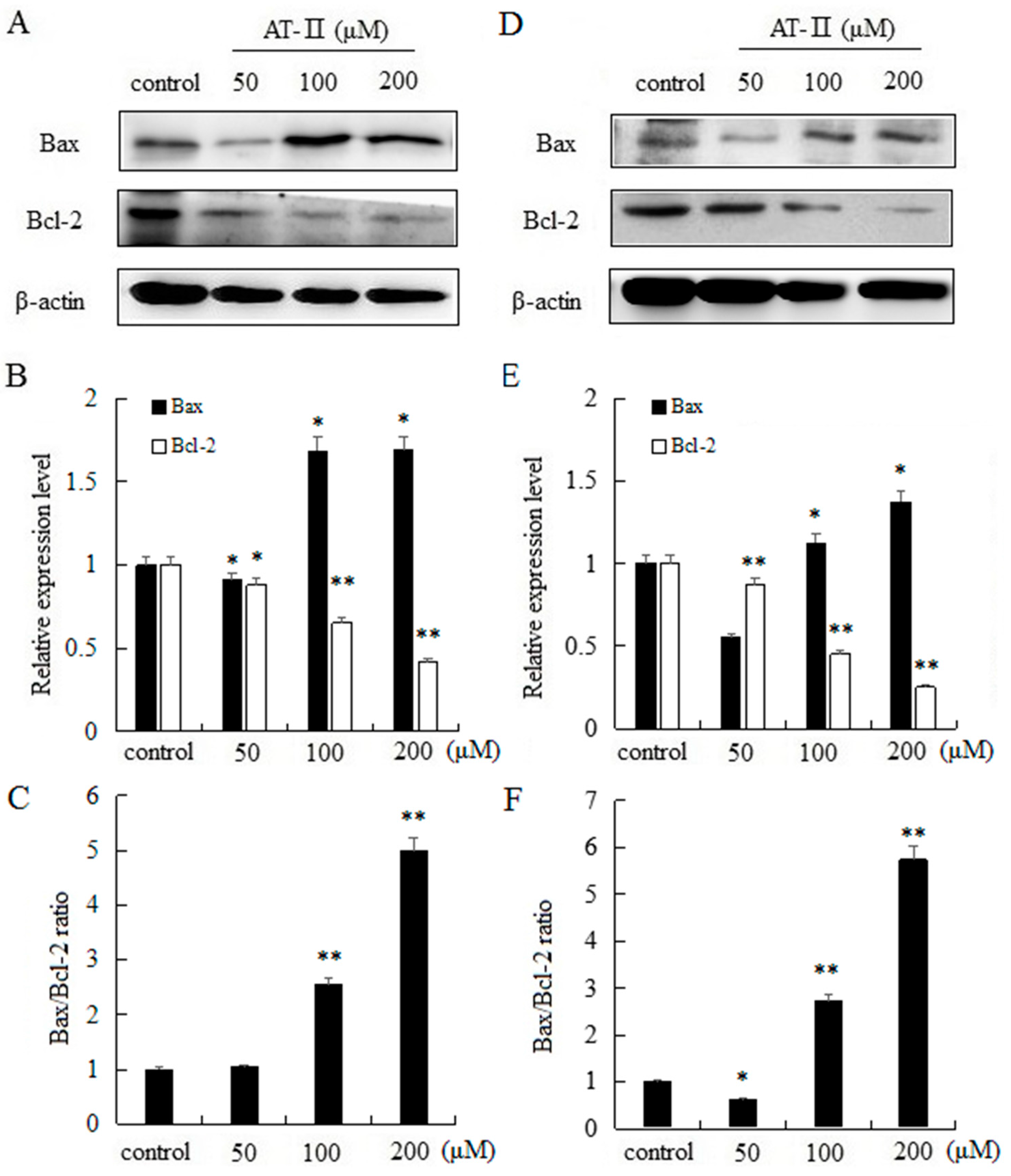
2.5. AT-II Regulates the Protein Expression Levels of Bax and Bcl-2
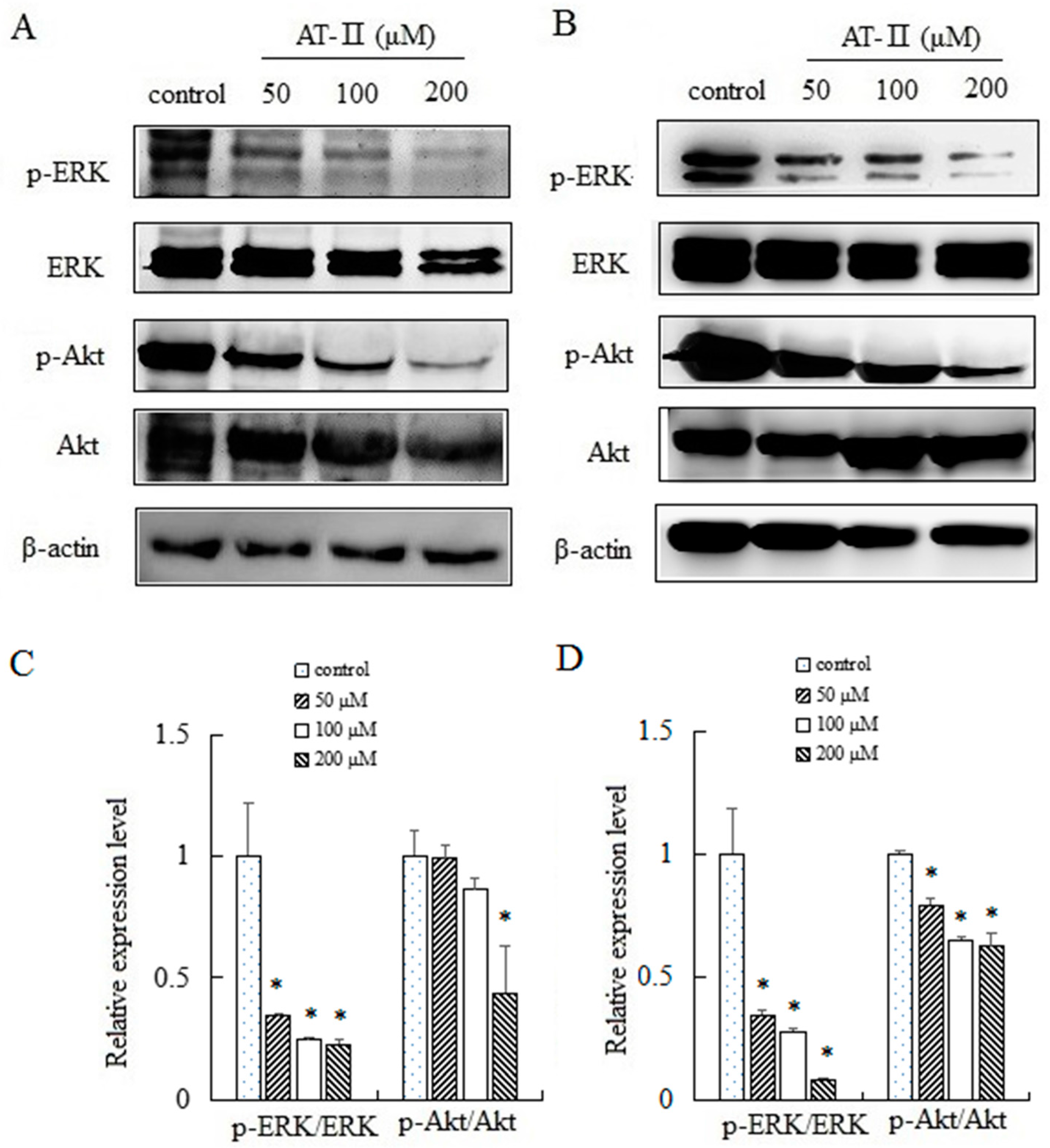
2.6. AT-II Inhibits the Phosphorylation of ERK and Akt in HGC-27 and AGS Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Cell Culture
4.3. Cell Growth Assay
4.4. Cell Morphological Assessment
4.5. Apoptosis Assay
4.6. Wound Healing Assay
4.7. Western Blot Analysis
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mather, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Labianca, R.; Beretta, G.D.; Gatta, G.; De, B.F.; Van, C.E. Gastric cancer. Oncol. Hematol. 2009, 71, 127–164. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Bentrem, D.J.; Besh, S.; D’Amico, T.A.; Das, P.; Denlinger, C.; Fakih, M.G.; Fuchs, C.S.; Gerdes, H.; Glasgow, R.E.; et al. Gastric cancer, version 2.2013: Featured updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2013, 11, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Mao, R.R.; Shen, K.; Zheng, Y.H.; Li, Y.Q.; Liu, J.W.; Ni, L. Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem traits. BBRC 2014, 450, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tan, Z.J.; Chen, J.; Dong, C. Cyclovirobuxine D Inhibits cell proliferation and induces mitochondria-mediated apoptosis in human gastric cancer cells. Molecules 2015, 20, 20659–20668. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. NaiPlants as biofactories: postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front. Plant. Sci. 2016, 7, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippiagraveolens, Lippiapalmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Bang, W.Y.; Nair, V.; Angulo-Escalante, M.A.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of flavonoids and lipophilic compounds from jatrophaplatyphylla on the suppression of Lipopolysaccharide (LPS)-induced inflammation in macrophage Cells. J. Agric. Food Chem. 2016, 64, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Shao, Z.-D.; Wang, Z.-C.; Fu, C.-X. Isolation and characterization of polymorphic microsatellite markers from the Chinese medicinal herb Atractylodes macrocephala (Asteraceae). Int. J. Mol. Sci. 2012, 13, 16046–16052. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Zhu, Y.J.; Zhang, T.; Zhao, Z.G.; Zhao, Y.; Cheng, P.; Li, H.; Gao, H.; Su, X.M. Anti-tumor effects of atractylenolide I isolated from Atractylodes macrocephala in human lung carcinoma cell lines. Molecules 2013, 18, 13357–13368. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and antinociceptive constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.S.; Tran, M.H.; Lee, J.S.; Ngo, Q.M.T.; Woo, M.H.; Min, B.S. Inflammatory inhibitory activity of sesquiterpenoids from Atractylodes macrocephala rhizomes. Chem. Pharm. Bull. (Tokyo) 2016, 64, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, F.; Li, Z.; Ye, N.; Huang, C.; Yuan, X. Atractylodes macrocephala polysaccharides induces mitochondrial-mediated apoptosis in glioma C6 cells. Int. J. Biol. Macromol. 2014, 66, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Singhuber, J.; Baburin, I.; Kählig, H.; Urban, E.; Kopp, B.; Hering, S. GABA(A) receptor modulators from Chinese herbal medicines traditionally applied against insomnia and anxiety. Phytomedicine 2012, 19, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, C.; Sun, T.M.; Ran, X.K.; Kang, T.G.; Dou, D.Q. Two new compounds from Atractylodes macrocephala with neuroprotective activity. J. Asian Nat. Prod. Res. 2017, 19, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Yang, W.L.; Guo, L.Y.; Wu, X.L.; Zhang, T.T.; Liu, J.L.; Zhang, J.F. Atractylodes lactone compounds inhibit platelet activation. Platelets 2016, 28, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Q.; Chou, G.X.; Kwan, H.Y.; Tse, A.K.; Zhao, L.H.; Yuen, T.K.; Cao, H.H.; Yu, H.; Chao, X.J.; Su, T.; et al. Inhibition of STAT3 signaling contributes to the antimelanoma action of atractylenolide II. Exp. Dermatol. 2014, 23, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, H.; Chu, J.H.; Chou, G.X.; Chen, S.B.; Mo, H.B.; Fong, W.F.; Yu, Z.L. Atractylenolide II inducesG1 cell-cycle arrest and apoptosis in B16 melanomacells. J. Ethnopharmacol. 2011, 136, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, S.H.; Soudeh, K.F.; Mohammad, R.H.; Alireza, K.; Mohammad, R.M.; Hadis, A.; Reza, B.; Nesa, J.; Mehdi, M. Persian shallot, Allium hirtifolium Boiss, induced apoptosis in human hepatocellular carcinoma cells. Cytotechnology 2017, 69, 551–563. [Google Scholar]
- Fei, H.R.; Chen, G.; Wang, J.M.; Wang, F.Z. Perifosine induces cell cycle arrest and apoptosis in human hepatocellular carcinoma cell lines by blockade of Akt phosphorylation. Cytotechnology 2010, 62, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, Y.; Jiang, D. NT-3 attenuates the growth of human neuron cells through the ERK pathway. Cytotechnology 2016, 68, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Yu, F.M.; Zhang, G.M.; Yang, Z.S.; Huang, F.F.; Ding, G.F. A purified serine protease from Nereis virens and its impaction of apoptosis on human lung cancer cells. Molecules 2017, 22, 1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, X.; Liang, Y.N.; Wang, L.; Song, Z.X.; Liu, J.L.; Tang, Z.S. Cordycepin induces apoptosis and inhibits proliferation of human lung cancer cell line H1975 via Inhibiting the Phosphorylation of EGFR. Molecules 2016, 21, 1267. [Google Scholar] [CrossRef] [PubMed]
- Long, F.Y.; Wang, T.; Jia, P.; Wang, H.F.; Qing, Y.; Xiong, T.T.; He, M.J.; Wang, X.L. Anti-tumor effects of Atractylenolide-I on human ovarian cancer cells. Med. Sci. Monit. 2017, 23, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, S.P.; Cai, D.W.; Kong, G.Q. Induction of mitochondrial-dependent apoptosis in T24 cells by a selenium (Se)-containing polysaccharide from Ginkgo Biloba L. leaves. Int. J. Biol. Macromol. 2017, 101, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.J.; Chen, J.; Wang, S.G. A polysaccharide from Pinellia ternata inhibits cell proliferation and metastasis in human cholangiocarcinoma cells by targeting of Cdc42 and 67 kDa Laminin Receptor (LR). Int. J. Biol. Macromol. 2016, 93, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bi, T.T.; Shen, G.H.; Li, Z.M.; Wu, G.L.; Wang, Z.; Qian, L.Q.; Gao, Q.G. Lupeol induces apoptosis and inhibits invasion in gallbladder carcinoma GBC-SD cells by suppression of EGFR/MMP-9 signaling pathway. Cytotechnology 2016, 68, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chou, G.X.; Wang, H.; Chu, J.H.; Fong, W.F.; Yu, Z.L. Effects of sesquiterpenes isolated from largehead atractylodes rhizome on growth, migration, and differentiation of B16 melanoma cells. Integr. Cancer Ther. 2011, 10, 92–100. [Google Scholar]
- Yu, R.; Yu, B.X.; Chen, J.F.; Lv, X.Y.; Yan, Z.J.; Cheng, Y.; Ma, Q. Anti-tumor effects of Atractylenolide I on bladder cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Jiang, Y.N.; Fu, T.T.; Hao, Y.; Zhu, X.F.; Lu, Y. Naringin inhibits lipopolysaccharide-induced damage in human umbilical vein endothelial cells via attenuation of inflammation, apoptosis and MAPK pathways. Cytotechnology 2016, 68, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, G.; Zhang, P.F.; Zhang, J.; Huang, Y.X.; Lu, Y.M.; Da, W.; Sun, Q.; Zhu, J.S. Sanguinarine inhibits growth and invasion of gastric cancer cells via regulation of the DUSP4/ERK pathway. J. Cell. Mol. Med. 2017, 21, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Qian, C.J. Sporamin induce apoptosis in human tongue carcinoma cells by down-regulating Akt/GSK-3 signaling. Fundam. Clin. Pharmacol. 2010, 25, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.Z.; Chen, L.; Wei, X.B.; Xiang, Y.X.; Liu, X.Q.; Zhang, X.M. The Akt/GSK-3β pathway mediates flurbiprofen-induced neuroprotection against focal cerebral ischemia/reperfusion injury in rats. Biochem. Biophys. Res. Commun. 2011, 409, 808–813. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Yu, H. Atractylenolide II Inhibits Proliferation, Motility and Induces Apoptosis in Human Gastric Carcinoma Cell Lines HGC-27 and AGS. Molecules 2017, 22, 1886. https://doi.org/10.3390/molecules22111886
Tian S, Yu H. Atractylenolide II Inhibits Proliferation, Motility and Induces Apoptosis in Human Gastric Carcinoma Cell Lines HGC-27 and AGS. Molecules. 2017; 22(11):1886. https://doi.org/10.3390/molecules22111886
Chicago/Turabian StyleTian, Shuang, and Hongdan Yu. 2017. "Atractylenolide II Inhibits Proliferation, Motility and Induces Apoptosis in Human Gastric Carcinoma Cell Lines HGC-27 and AGS" Molecules 22, no. 11: 1886. https://doi.org/10.3390/molecules22111886



