1. Introduction
Oils or oily plants are used as spices, therapeutic agents, in herbal medicine and aromatherapy as well as flavouring components of perfume or toilet water in the cosmetics industry. Due to their disinfectant properties, some oils are used in the food industry and restaurant to disinfect potable water and preserve food, as well as in the cultivation of plants and tending pets [
1].
In recent years, due to the high survivability of microorganisms in the environment caused by resistance to antibiotics and preservative agents, the natural anti-microbiological preparations, such as extracts and essential oils, have become the centre of attention [
2].
Depending on the chemical composition, essential oils and extracts can be divided into terpene and non-terpene oils. Terpene oils contain mainly terpenes, most frequently mono-, sesqui-, and less frequently, di-terpenes, and the non-terpene oils contain phenylpropane derivatives. Compounds found in both of these oil groups are present, for example, in the form of hydrocarbons, alcohols, ketones, aldehydes, phenols, esters, and acids. Some oils may also contain compounds containing sulphur, nitrogen, and coumarins [
3].
The efficacy of antimicrobial action of oils and extracts depends to a great degree on the chemical composition, which is connected with the type of oily plants, the growth conditions, harvesting, and processing, as well as the manner of obtaining the extracts. Differences in the chemical composition and biological activity were observed in garlic oils containing sulphur compounds [
4,
5,
6,
7], nettle [
8,
9,
10], and chamomile [
11], which contain biogenic amines and angelica-containing coumarins [
12,
13,
14]. Furthermore, what is also significant is the qualitative-quantitative composition of various chemical compounds found in essential oils, since it is responsible for their antimicrobial efficacy.
Clove oil is an extremely important substance which inhibits the growth of the bacteria (e.g.,
Escherichia coli,
Bacillus subtilis), probably thanks to the presence of eugenol, eugenol acetate, and caryophyllene [
15,
16], and so is oregano oil, perhaps due to the presence of carvacrol [
17,
18]. On the other hand,
α- and
β-pinene present in juniper and marjoram oil and, additionally, limonene in marjoram oil may be responsible for the anti-viral, anti-fungal, and antibacterial properties [
19,
20,
21,
22].
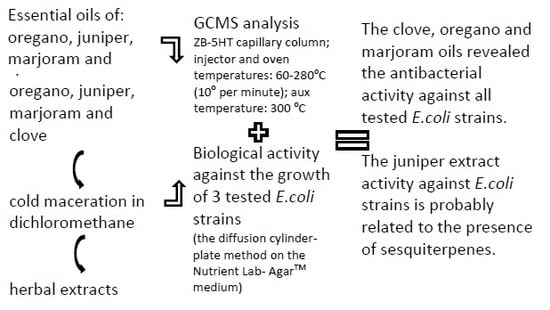
The aim of the paper is to compare the chemical composition of chosen commercial oils with extracts obtained from plant raw material (spices) in the process of cold maceration and hot extraction in the Soxhlet apparatus, as well as to determine their biological activity against the E. coli strains.
2. Results
Regardless of the applied procedure, the extraction efficiency of the tested plant raw material was similar (
Table 1).
The tested oils revealed a diversified qualitative-quantitative composition. In clove oil, a different number of chemical compounds was identified: the most in the commercial oil (20 components), and the least in cold and hot extracts, 14 and 13 components, respectively (
Table 2). Monoterpenes constituted the main components of these oils and in the commercial oil and after maceration they amounted to 78% and, after hot extraction, 82% (
Table 2). Eugenol was the most important part of this group of compounds, which made up 62% of the whole composition while, in the remaining extracts, it comprised approximately 55%. However, eugenol acetate was present in considerable amounts (23.5% and 27.5%) only in extracts (
Figure 1). The sesquiterpene-type compounds constituted around 20% of the commercial oil and maceration-obtained compositions, while 16% of the composition of the extract obtained as the result of extraction in the Soxhlet apparatus. In this group of compounds,
β-caryophyllene was observed in the highest amounts (16% and 13%). On the other hand, monoterpenes, which were mostly represented by eucalyptol, were present only in the commercial oil (
Table 2).
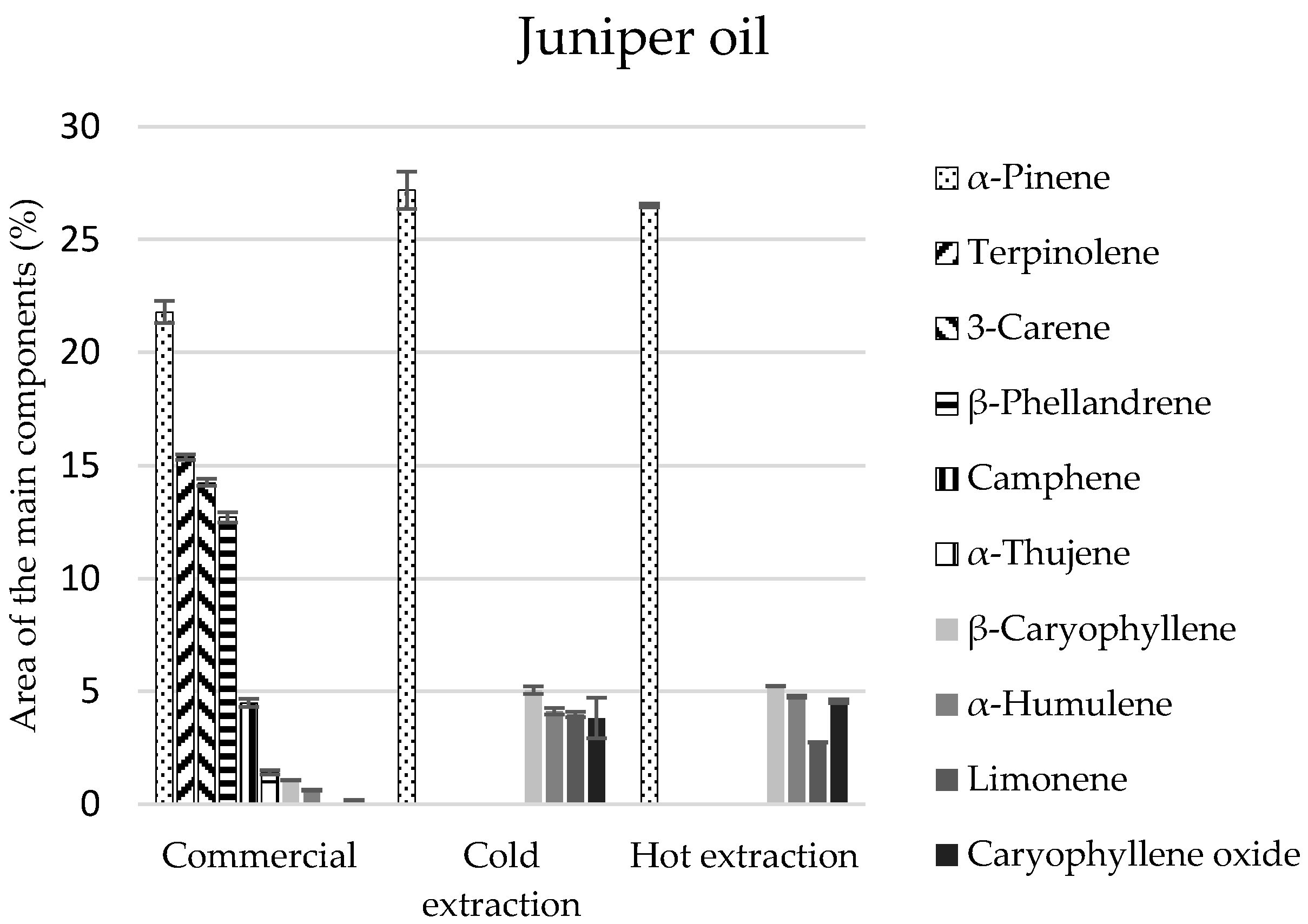
In the commercial juniper oil, there were 38 identified components, and in the extracted oils, 23 in each of the extracts (
Table 3). Monoterpenes constituted 88% of the commercial juniper oil composition and in the extracts their amount was two times lower and equalled 45%, on average. The second group of compounds in extracts, regardless of the manner of obtaining them, was sesquiterpenes in the amount of 21%, on average (
Table 3).
α-Pinene was the main component to be found in the greatest amounts in all the tested juniper oils (approximately 22%) (
Figure 2). However, these oils differed in terms of the quantitative-qualitative composition of the remaining components. Terpinolene, 3-carene,
β-phellandrene of the monoterpene group were identified only in the commercial oil, and the extracts contained
β-thujene and sesquiterpenes in considerable amounts (
β-caryophyllene, and
α-humulene).
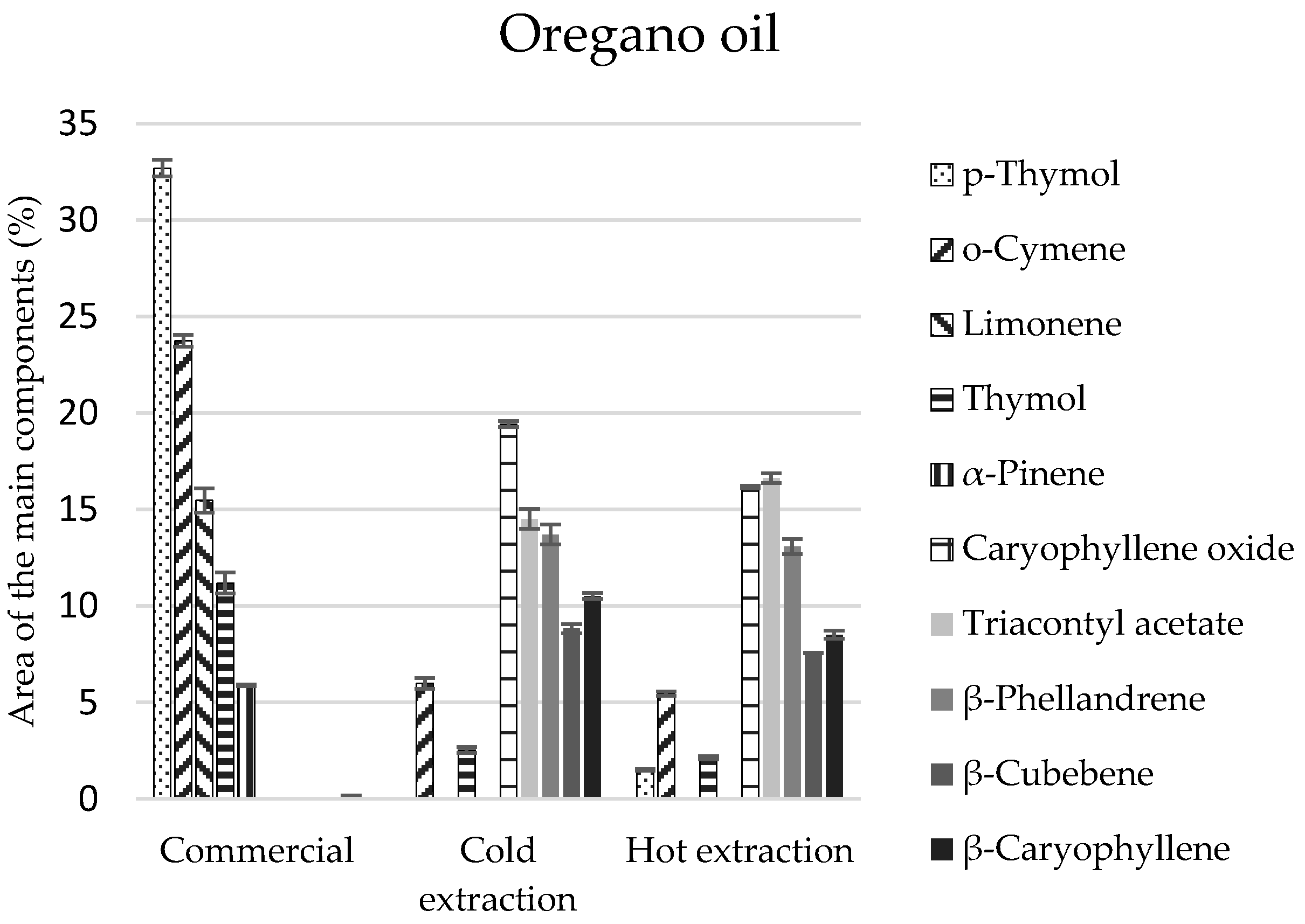
Eighteen compounds were identified in the commercial oregano oil, and more in extracts (21 and 23 components) (
Table 4). The commercial oregano oil contained monoterpenes in comparable amounts (51%) and their oxygen derivatives (49%) (
Table 4). Compositions of oils obtained by the extraction methods were differentiated and different from the composition of the commercial oregano oil—monoterpenes and their derivatives constituted 36% each, and sesquiterpenes and sesquiterpenoids constituted 40–50% (
Table 4).
p-Thymol and thymol of the monoterpenoid group constituted the main components of the commercial oil (total of 43.9%) (
Figure 3). Their presence in extracts obtained in the cold and hot manner was significantly lower and equalled 2.5% and 3.6%, respectively (
Table 4). However, the main elements of extracts included the following: caryophyllene, triacontyl acetate, and
β-phellandrene (
Figure 3), which were not identified in the commercial oil. On the other hand, carvacrol, a compound revealing biocidal properties, was identified only in the cold extract, in the amount of 1.8% (
Table 4), although it is enumerated as one of the main components of commercial oils. The lack of this compound can be caused by genetic changes in the plants, as well as the environmental conditions in oregano plantations.
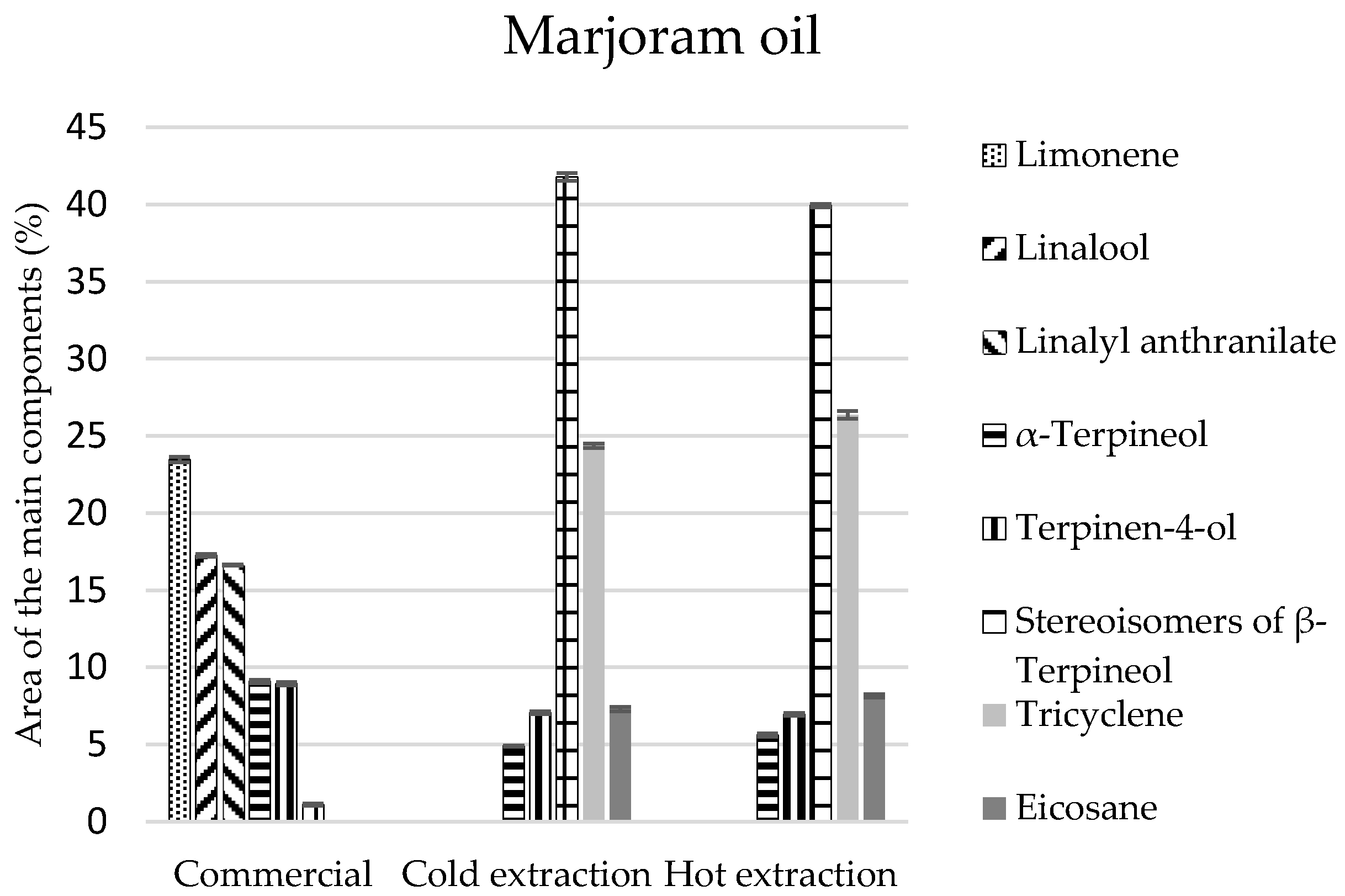
As for marjoram oils, the greatest number of compounds was identified in the commercial oil (21 components), while in the extract there were 15 components for cold and 12 components for hot extraction (
Table 5). The chemical analysis of these oils revealed that they are mainly composed of monoterpenes and monoterpenoids. In the commercial oil their composition was similar, and equalled 40%. In the oils obtained by extraction methods, the percentage of monoterpenes was 26–27%, and the amount of monoterpenoids was two times larger and equalled 55% (
Table 5). Limonene constituted the most abundant component of the commercial oil (at least 23%) (
Figure 4), and
β-terpineol stereoisomers were present in the extracts (approximately 40% of the composition), which were present in the commercial oil in the amount of 1% (
Figure 4).
The biological activity of commercial oils and extracts obtained in the process of cold and hot maceration against three strains of
E. coli was differentiated (
Table 6,
Figure 5,
Figure 6,
Figure 7 and
Figure 8).
The degree of growth inhibition of the tested bacterial strains depended both on the concentration and the chemical composition related to the methods of obtaining oils (
Table 6).
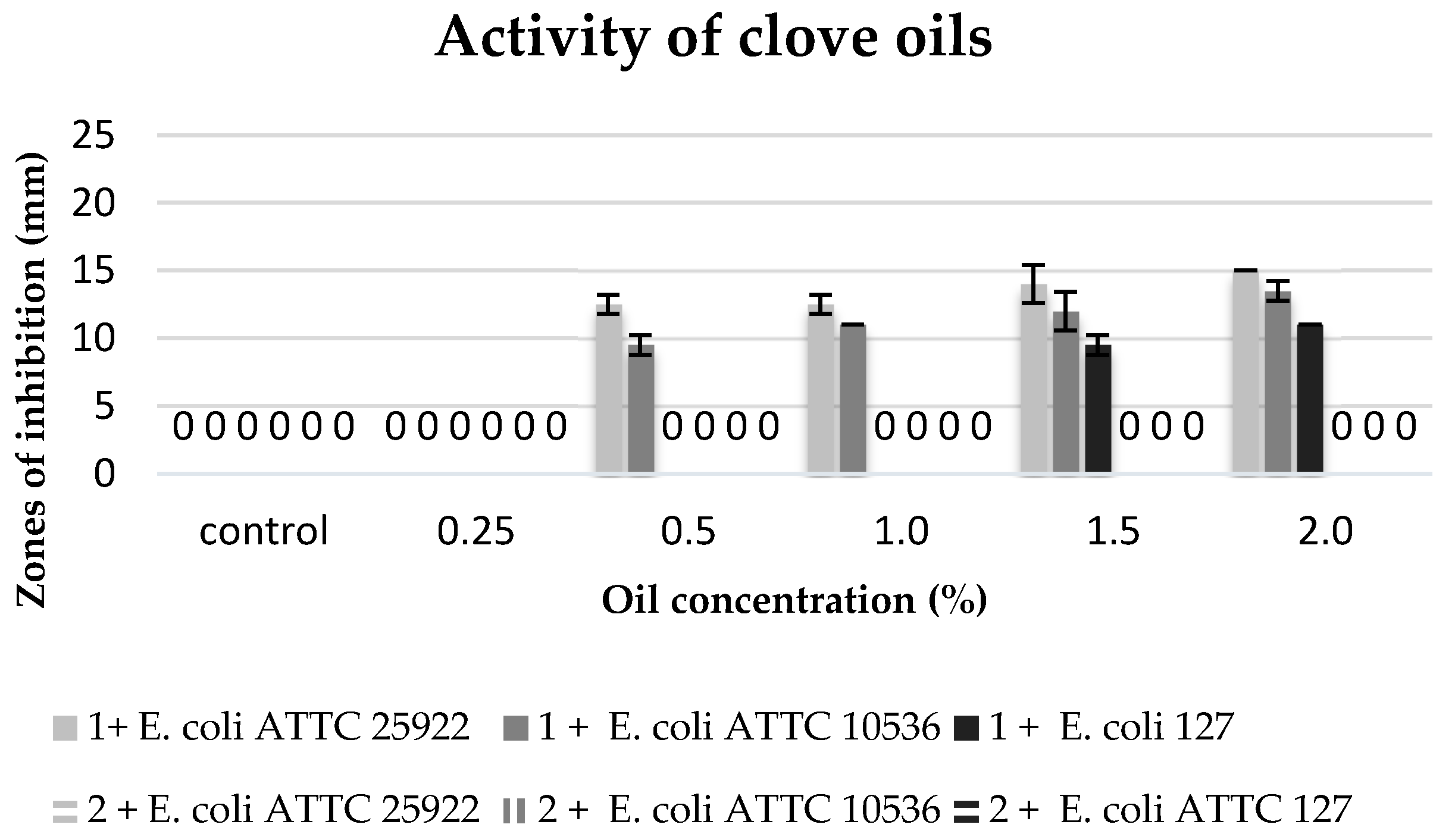
The commercial clove oil with the concentration of 0.5–2% inhibited the growth of the stains in the collection (
E. coli ATTC 25922 and
E. coli ATTC 10536). The
E. coli ATTC 25922 strain proved to be more sensitive, and the growth inhibition zones were 12.5 to 15 mm. On the other hand, the environmental isolate
E. coli 127 revealed sensitivity only to higher concentrations of this oil (1.5–2%). However, no biocidal effect was observed of clove extracts against the tested bacterial strains (
Figure 5).
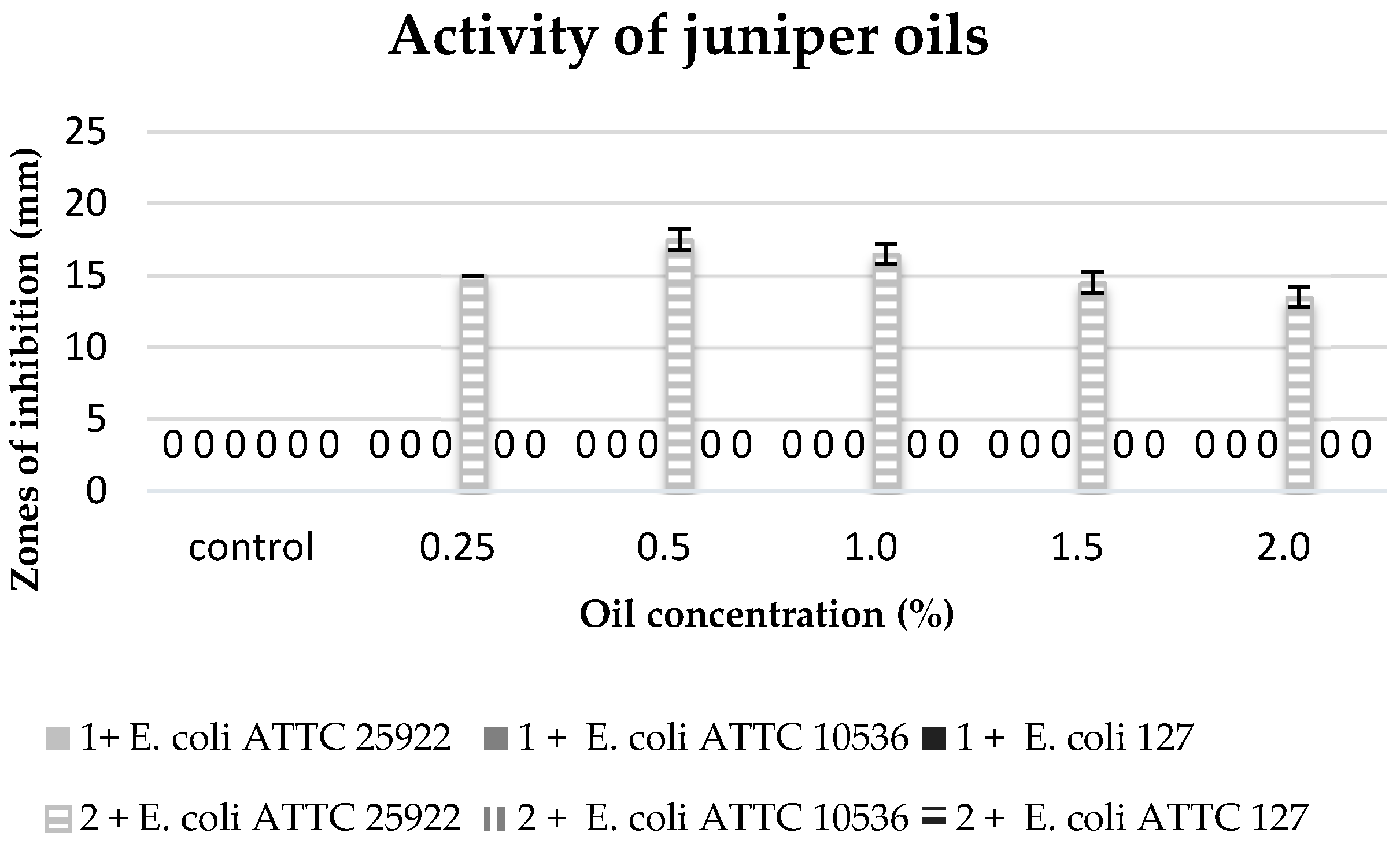
The commercial juniper oil did not reveal any antibacterial properties against any of the tested bacterial strains, and the extract obtained in the process of maceration only inhibited the growth of the strain of
E. coli 25922 at all used concentrations of this extract (
Figure 6).
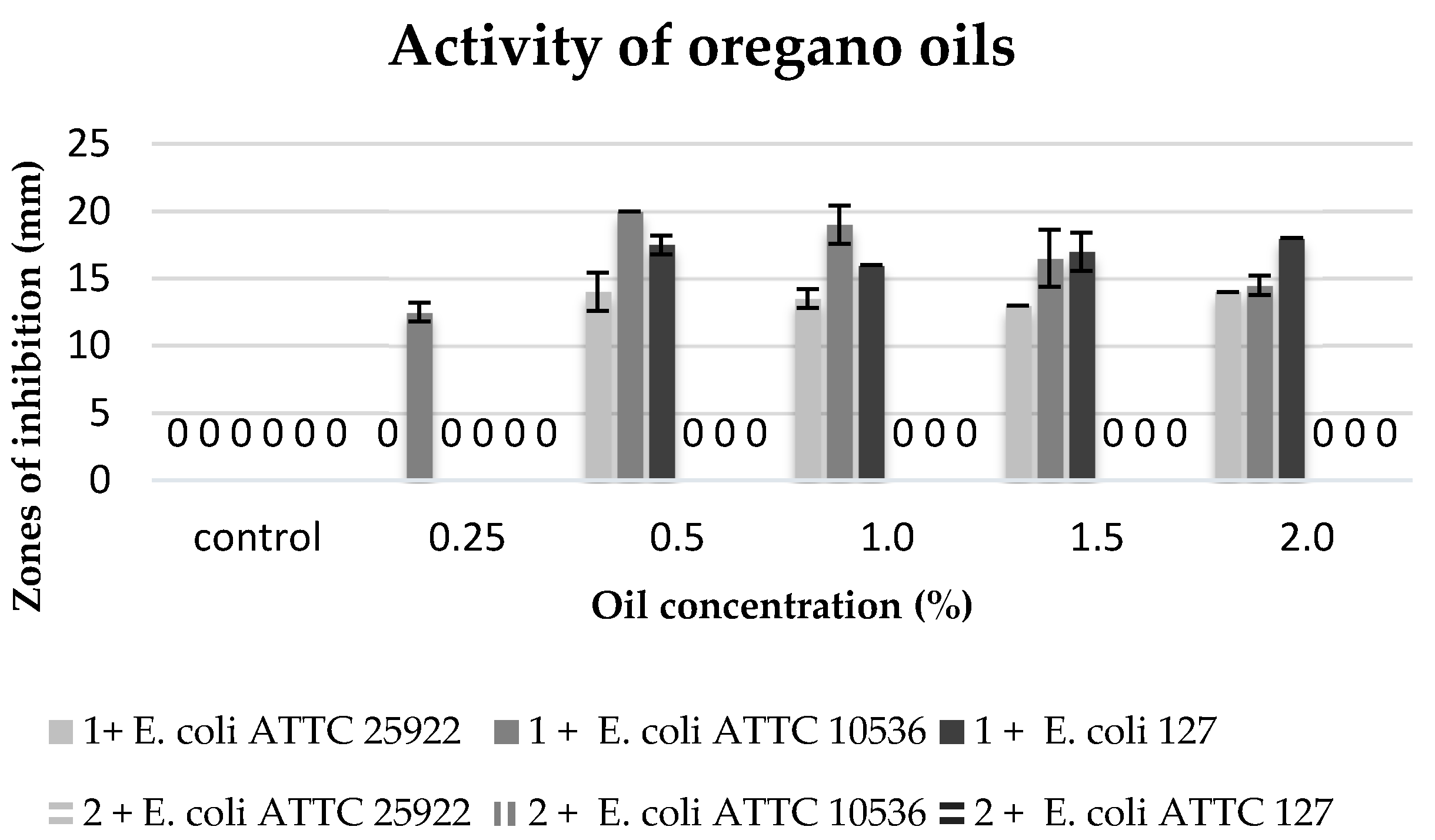
The commercial oregano oil inhibited the growth of all the tested strains of bacteria
E. coli in the concentrations ranging from 0.5% to 2%, yet, the maximum zone of inhibition bacteria growth was present at concentrations of 0.5% and 1%. The
E. coli ATTC 10536 strain proved to be the most sensitive to the oil activity. However, no biocidal activity was observed of clove macerate against the tested bacterial strains (
Figure 7).
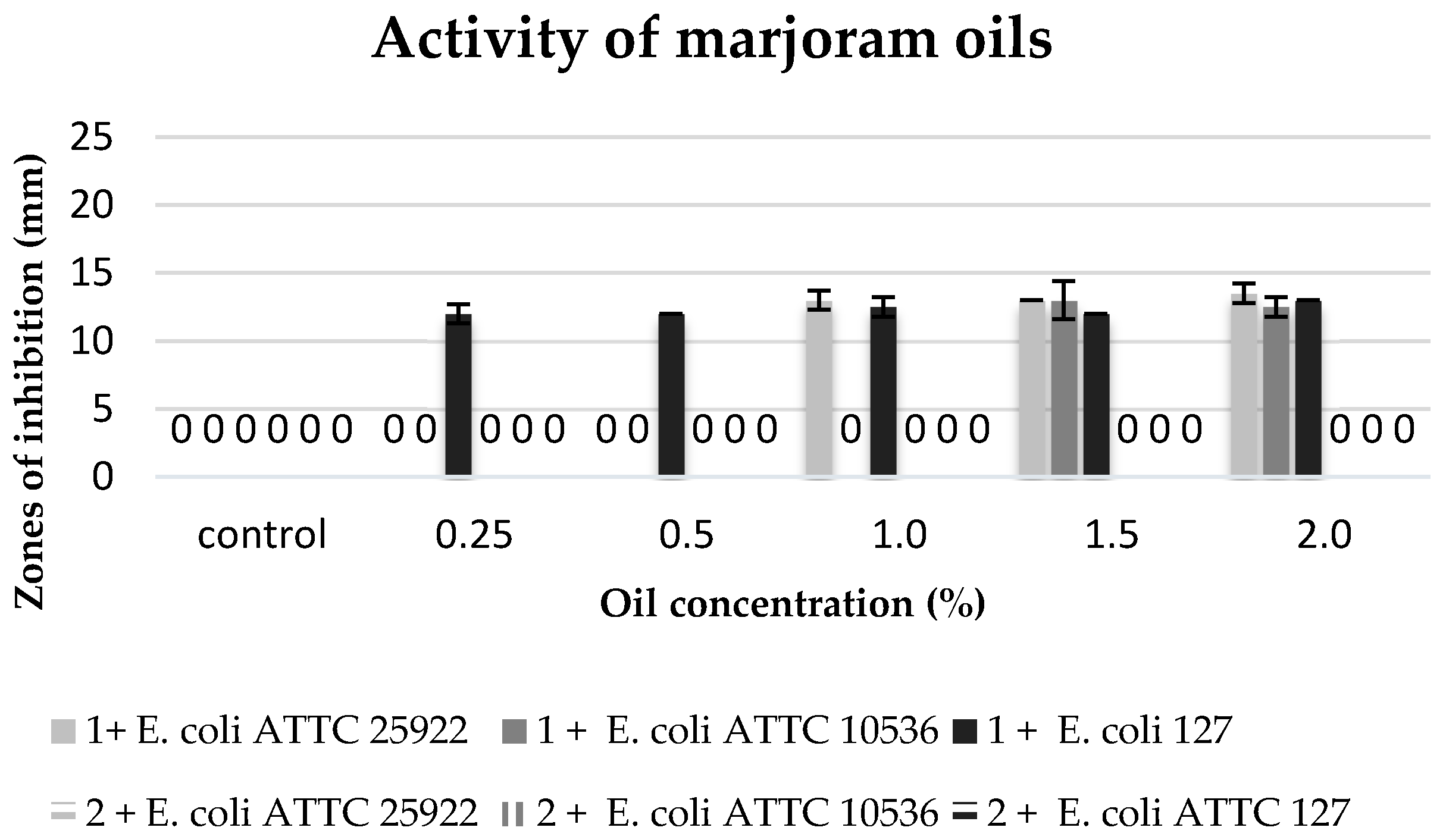
The commercial marjoram oil, within the range of concentration 1.5–2%, inhibited the growth of the tested
E. coli strains. It revealed a similar inhibitory activity against the strains of
E. coli ATTC 25922 and
E. coli 127.
E. coli ATTC 10536 showed a higher sensitivity to this oil (
Figure 8).
3. Discussion
Essential oils show a differentiated chemical composition, which depends on various factors, such as the source of the raw material, climate conditions of plantations, the methods of obtaining the oils, etc. [
23,
24,
25,
26]. The tested commercial essential oils revealed considerable differences in terms of the composition and diversity of terpenes, terpenoids, and sesquiterpenes as compared with the extracts obtained from plant material. The clove, juniper, and marjoram oils contained a lower number of components as compared with their commercial counterparts, which may be caused by the loss of high-volatility compounds in the process of drying of the plant material used as the raw material for extraction [
27]. On the other hand, the choice of extraction method mainly affected the quantitative composition of the oils, e.g., a higher share of sesquiterpenes was observed as compared with monoterpenes, which also may result from the nature of the raw material used for extraction purposes.
The commercial clove, oregano, and marjoram oils in the concentrations ranging from 1.5% to 2% showed antibacterial properties against all the tested strains of E. coli. However, these strains were not sensitive to essential oils obtained from the plant material in the process of maceration.
This suggests that the tested strains of
E. coli show a high sensitivity mainly against monoterpenes, such as
α-pinene,
β-pinene,
α,
β,
γ-terpinene, limonene, and some terpenoids (thymol, carvacrol). Hajlaoui et al. [
28] linked the antimicrobial properties of marjoram essential oil with the high proportion of oxygenated monoterpenes, such as terpinen-4-ol,
α-terpinol,
α-pinene, and
p-cymene. Additionally, the oxygenated compound, especially oxygenated monoterpenes and phenylpropanoids, might be responsible for the antimicrobial activity of marjoram oil against
Clostridium perfringens [
29].
Other authors also connect the antibacterial properties of essential oils with the presence of monoterpenes. For instance, Król et al. [
30] and Dorman and Deans [
31] link the activity of clove oil with the presence of eugenol,
β-pinene, and
β-terpinene. Additionally, de Oliveira et al. [
32] showed in their study that eugenol is responsible for the phytotoxic activity of clove essential oil. On the other hand, Fun and Baerheim [
33] claim that the activity of marjoram oil is induced by limonene (23.5%),
α,
β-pinene and
γ-terpinene, and that a high concentration of monoterpene tricyclene leads to the reduction of the antibacterial activity of this oil. The latter statement is confirmed by the results of our own study, since the marjoram obtained in the extraction process with a high concentration of tricyclene (24%) did not inhibit the growth of any tested strain of
E. coli.
Furthermore, the high anti-microbiological activity of the oregano oil is connected with the high concentration of terpenoids, carvacrol in particular [
17,
18,
34]. However, the tested commercial oregano oil did not contain carvacrol, but only thymol (11%),
p-thymol (33%), and limonene (15.5%). Most probably, it was these compounds present in high concentrations that were responsible for the inhibition of
E. coli. The oil obtained in the process of maceration, despite the fact it contained carvacrol (1.8%) and thymol (2.5%), probably did not reveal any biological activity due to the low concentrations of these compounds.
A different reaction of bacteria
E. coli was observed against the commercial juniper oil. These strains did not show sensitivity to the components of this oil; however, they were sensitive to the action of the obtained juniper extracts. The bactericidal activity of juniper extracts against the strains of
E. coli is most probably connected with the presence of sesquiterpenes which were not isolated from the commercial essential oil. The commercial juniper oil contained mainly terpenes (88%) and terpenoids (8%), and the extracts (depending on the applied procedure) contained lower amounts of terpenes (42–48%) and terpenoids (approximately 1%), yet high amounts of sesquiterpenes (25–30%). The anti-microbiotic properties of the juniper oil obtained in the maceration process seem to be caused by the synergistic activity of mono- and sesquiterpenes, which was also observed in the case of the activity of coriander oil against yeasts
Candida albicans [
35]. The antioxidant activity of juniper berry oil from Bulgaria against
Saccharomyces cerevisiae depended on monoterpenes, mostly from α-pinene as main component [
36]. The biological activity of the tested essential oils not only depends on the presence of active compounds, but also on the sensitivity of the tested strains of
E. coli.
5. Conclusions
Commercial essential oils and oils obtained in the process of extraction from plant material differed in terms of the content and the quantitative ratio of terpenes, terpenoids, and sesquiterpenes, as well as their antibacterial activity.
The commercial clove, oregano, and marjoram oils in concentrations of 1.5–2% revealed the antibacterial activity against all tested E. coli strains.
Essential oils obtained from plant material in the process of maceration did not reveal bactericidal activity, except for the juniper oil.
The bactericidal activity of the juniper extract against E. coli strains is probably related to presence of sesquiterpenes which were not isolated from the commercial essential oil.