Antimalarial Activity of Acetylenic Thiophenes from Echinops hoehnelii Schweinf
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acute Oral Toxicity
2.2. Antimalarial Activity of 80% Methanol Root Extract of E. hoehnelii
2.3. Antimalarial Activity of Fractions
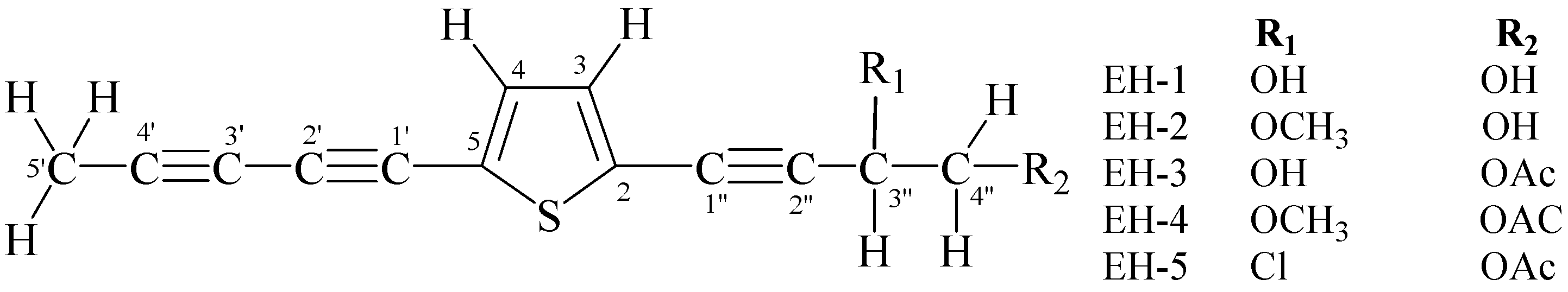
2.4. Isolation and Structural Elucidation of Compounds from the Dichloromethane Fraction
2.5. Antimalarial Activity of Isolated Compounds
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Experimental Animals and Parasite
3.4. Preparation of Extract and Fractions
3.5. Isolation of Compounds
3.6. Acute Oral Toxicity Test
3.7. Antimalarial Activity Test of 80% Methanol Root Extract of E. hoehnelii
3.8. Antimalarial Activity Test of the Fractions
3.9. Antimalarial Activity of Isolated Compounds
3.10. Data Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gay, F.; Zougbédé, S.; N’Dilimabaka, N.; Rebollo, A.; Mazier, D.; Moreno, A. Cerebral malaria: What is known and what is on research. Rev. Neurol. 2012, 168, 239–256. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Malaria Report. 2014. Available online: http://www.who.int/malaria/publications/world_malaria_report_2014/en/ (accessed on 21 January 2015).
- World Health Organization (WHO). World Malaria Report. 2015. Available online: www.who.int/entity/malaria/publications/world-malaria-report-2015/wmr2015-profiles.pdf (accessed on 8 June 2016).
- Grimberg, B.; Jaworska, M.M.; Hough, L.B.; Zimmerman, P.A.; Phillips, J.G. Addressing the malaria drug resistance challenge using flow cytometry to discover new antimalarials. Bioorg. Med. Chem. Lett. 2009, 19, 5452–5457. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, H.; Deharo, E. A call for using natural compounds in the development of new antimalarial treatments-an introduction. Malar. J. 2010, 10 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.N. Natural products as starting points for future anti-malarial therapies: Going back to our roots. Malar. J. 2011, 10 (Suppl. 1), S3. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, I.; Friis, I.; Edwards, S. Flora of Ethiopia and Eritrea; Part 2; Addis Ababa University: Addis Ababa, Ethiopia, 2004; Volume 4, pp. 15–23. [Google Scholar]
- Sánchez-Jiménez, I.; Lazkov, G.A.; Hidalgo, O.; Garnatje, T. Molecular systematics of Echinops L. (Asteraceae, Cynareae): A phylogeny based on ITS and trnL-trnF sequences with emphasis on sectional delimitation. Taxon 2010, 59, 698–708. [Google Scholar]
- Abegaz, B.M.; Tadesse, M.; Majinda, R. Distribution of sesquiterpene lactones and polyacetylenicthiophens in Echinops. Biochem. Syst. Ecol. 1991, 19, 323–328. [Google Scholar] [CrossRef]
- Hymete, A.; Rohloff, J.; Kjosen, H.; Iversen, T.H. Acetylenicthiophenes from the roots of Echinopsellenbeckii from Ethiopia. Nat. Prod. Res. 2005, 19, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Giday, M.; Asfaw, Z.; Woldu, Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J. Ethnopharmacol. 2010, 132, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Vathsala, P.G.; Dende, C.; Nagaraj, V.A.; Bhattacharya, D.; Das, G.; Rangarajan, P.N.; Padmanaban, G. Curcumin-Arteether combination therapy of Plasmodium berghei-infected mice prevents recrudescence through immune modulation. PLoS ONE 2012, 7, e29442. [Google Scholar] [CrossRef] [PubMed]
- Dahanukar, S.A.; Kulkarni, F.A.; Rege, N.N. Pharmacology of medicinal plants and natural products. Indian J. Pharmacol. 2000, 32, 81–118. [Google Scholar]
- Nafiu, M.O.; Abdulsalam, T.A.; Akanji, M.A. Phytochemical analysis and antimalarial activityaqueous extract of Lecaniodiscus cupanioides root. J. Trop. Med. Hyg. 2013, 2013, 1–4. [Google Scholar]
- Qiu, Y.Q.; Qi, S.H.; Zhang, S.; Tian, X.P.; Xiao, Z.H.; Li, M.L.; Li, Q.X. Thiophene derivatives from the aerial part of Plucheaindica. Heterocycles 2008, 75, 1757–1764. [Google Scholar]
- Shi, J.; Zhang, X.; Jiang, H. 2-(Penta-1,3-diynyl)-5-(3,4-dihydroxybut-1-ynyl) thiophene, a novel NQO1 inducing agent from Echinopsgrijsii Hance. Molecules 2010, 15, 5273–5281. [Google Scholar] [CrossRef] [PubMed]
- Fokialakis, N.; Cantrell, C.; Duke, S.O.; Skaltsounis, A.L.; Wedge, D.E. Antifungal activity of thiophenes from Echinopsritro. J. Agric. Food Chem. 2006, 54, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Institute for Laboratory Animal Research (ILAR). Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). OECD Guidelines for Testing of Chemicals: Guideline 425: Acute Oral Toxicity; Fixed Dose Method; Office of Economic and Community Development: Paris, France, 2008.
- Centre for Drug Evaluation and Research. Guidance for Industry Single Dose Acute Toxicity Testing for Chemicals; CDER: Rockville, MD, USA, 1996. [Google Scholar]
- Peters, W. The chemotherapy of rodent malaria, XXII. The value of drug resistant strains of P. berghei in screening for blood schizontocidal activity. Ann. Trop. Med. Parasitol. 1975, 69, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Kalra, B.S.; Chawla, S.; Gupta, P.; Valecha, N. Screening of antimalarial drugs: An overview. Indian J. Pharmacol. 2006, 38, 5–12. [Google Scholar] [CrossRef]
- Yared, D.; Mekonnen, Y.; Debella, A. In vivo antimalarial activities of fractionated extracts of Asparagus africanus in mice infected Plasmodium berghei. Archives 2012, 3, 88–94. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Dose | % Parasitaemia ± SD | % Suppression | Mean Survival Time (Days) ± SD |
|---|---|---|---|
| Vehicle | 24.2 ± 2.3 | - | 6.4 ± 0.9 |
| 50 mg/kg | 23.1 ± 2.5 | 4.6 b3d1e3f3 | 7 ± 0.7 b3 |
| 100 mg/kg | 17.5 ± 5.4 | 27.8 a2b3c1e3f3 | 7.4 ± 0.5 b3 |
| 200 mg/kg | 7.6 ± 0.9 | 68.5 a3b2c3d3 | 7.8 ± 1.1 b3 |
| 400 mg/kg | 5.2 ± 1.2 | 78.7 a3c3d3 | 8 ± 0.7 a1b3 |
| CQ (25 mg/kg) | 0 ± 0.00 | 100 a3c3d3e2 | >28 a3c3d3e3f3 |
| Dose | Wt (g) D0 ± SD | Wt (g) D4 ± SD | % Change |
|---|---|---|---|
| Vehicle | 25.6 ± 0.3 | 24.9 ± 1.1 | −2.5 |
| 50 mg/kg | 25.0 ± 0.2 | 25.7 ± 2.1 | 2.9 |
| 100 mg/kg | 25.1 ± 0.3 | 25.7 ± 1.2 | 2.5 |
| 200 mg/kg | 25.6 ± 0.2 | 26.1 ± 0.6 | 2.3 |
| 400 mg/kg | 24.9 ± 0.7 | 25.6 ± 0.3 | 3.0 |
| CQ (25 mg/kg) | 23.9 ± 0.3 | 26.8 ± 0.6 | 12.0 3 |
| Fraction | Dose (mg/kg/Day) | % Parasitaemia ± SD | % Suppression | Mean Survival Time (Days) ± SD |
|---|---|---|---|---|
| Hexane | 100 | 28.2 ± 11.7 | 11.6 b3 | 7.2 ± 0.8 b3 |
| Hexane | 200 | 27.8 ± 1.5 | 12.9 b3 | 8.2 ± 0.8 a2b3 |
| Hexane | 400 | 22.6 ± 3.3 | 29.1 b3 | 8.2 ± 0.8 a2b3 |
| Vehicle | 0.5 mL | 31.9 ± 3.00 | 0 | 6.4 ± 0.5 |
| CQ | 25 | 0.00 ± 0.00 | 100 a3c3d3e3 | >28 ± 0.00 a3c3d3e3 |
| Dichloromethane | 100 | 32.3 ± 2.5 | 24.8 a3b3e3 | 8.0 ± 1.9 b3 |
| Dichloromethane | 200 | 28.6 ± 1.8 | 33.5 a3b3e3 | 8.4 ± 1.9 b3 |
| Dichloromethane | 400 | 24.5 ± 1.5 | 43.0 a3b3c3d1 | 9.0 ± 0.7 a1b3 |
| Vehicle | 0.5 mL | 43.00 ± 2.9 | 0 | 7.0 ± 1.6 |
| CQ | 25 | 0.00 ± 0.00 | 100 a3c3d3e3 | >28 ± 0.00 a3c3d3e3 |
| Ethyl acetate | 100 | 37.3 ± 7.3 | 9.8 b3 | 7.25 ± 1.3 b3 |
| Ethyl acetate | 200 | 37.0 ± 1.6 | 10.5 b3 | 7.0 ± 1.3 b3 |
| Ethyl acetate | 400 | 39.4 ± 2.1 | 4.80 b3 | 6.4 ± 0.9 b3 |
| Vehicle | 0.5 mL | 41.4 ± 4.0 | 0.00 | 6.54 ± 1.3 |
| CQ | 25 | 0.00 ± 0.00 | 100 a3c3d3e3 | >28 ± 0.00 a3c3d3e3 |
| Aqueous | 100 | 33.2 ± 4.0 | 19.8 b3 | 7.6 ± 1.3 b3 |
| Aqueous | 200 | 38.1 ± 15.1 | 7.8 b3 | 6.80 ± 1.3 b3 |
| Aqueous | 400 | 40.0 ± 1.4 | 3.3 b3 | 6.80 ± 0.1 b3 |
| Vehicle | 0.5 mL | 41.4 ± 4.0 | 0.00 | 6.54 ± 1.3 |
| CQ | 25 | 0.00 ± 0.00 | 100 a3c3d3e3 | >28 ± 0.00 a3c3d3e3 |
| Fraction | Dose (mg/kg/Day) | Wt (g) D0 ± SD | Wt (g) D4 ± SD | % Change |
|---|---|---|---|---|
| Hexane | 100 | 25.6 ± 0.2 | 26.0 ± 0.4 | 1.5 |
| Hexane | 200 | 25.5 ± 1.3 | 26.2 ± 0.5 | 2.4 |
| Hexane | 400 | 25.4 ± 0.3 | 26.3 ± 1.1 | 3.7 |
| Vehicle | 0.5 mL | 26.2 ± 0.2 | 24.5 ± 0.5 | −6.3 2 |
| CQ | 25 | 25.4 ± 0.5 | 26.5 ± 0.3 | 4.2 2 |
| Dichloromethane | 100 | 28.3 ± 3.0 | 29.0 ± 1.5 | 2.6 |
| Dichloromethane | 200 | 26.8 ± 3.4 | 27.7 ± 3.7 | 3.5 |
| Dichloromethane | 400 | 25.9 ± 3.3 | 27.1 ± 3.7 | 4.7 |
| Vehicle | 0.5 mL | 27.9 ± 3.00 | 26.3 ± 4.4 | −5.8 |
| CQ | 25 | 25.4 ± 0.5 | 26.5 ± 0.3 | 4.2 2 |
| Ethyl acetate | 100 | 26.2 ± 0.7 | 26.4 ± 1.6 | 0.6 |
| Ethyl acetate | 200 | 25.0 ± 2.5 | 26.1 ± 2.5 | 4.3 |
| Ethyl acetate | 400 | 25.6 ± 2.6 | 26.5 ± 3.1 | 3.4 |
| Vehicle | 0.5 mL | 26.8 ± 3.5 | 26.1 ± 5.9 | −2.5 |
| CQ | 25 | 28.8 ± 5.1 | 29.8 ± 4.5 | 3.6 |
| Aqueous | 100 | 25.8 ± 2.1 | 26.7 ± 1.8 | 3.3 |
| Aqueous | 200 | 25.1 ± 2.5 | 25.7 ± 2.4 | 2.8 |
| Aqueous | 400 | 27.2 ± 2.3 | 27.9 ± 2.5 | 2.5 |
| Vehicle | 0.5 mL | 26.8 ± 3.5 | 26.1 ± 5.9 | −2.5 |
| CQ | 25 | 28.8 ± 5.1 | 29.8 ± 4.5 | 3.6 |
| Test | Dose | %Parasitaemia ± SD | % Suppression | Mean Survival Time (Days) ± SD |
|---|---|---|---|---|
| EH-1 | 50 mg/kg | 23.5 ± 2.1 | 43.2 a3b3 | 9.4 ± 1.5 a1b3 |
| EH-1 | 100 mg/kg | 15.3 ± 8.7 | 50.2 a3b3 | 9.7 ± 1.5 a1b3 |
| Vehicle | 0.5 mL | 30.8 ± 6.5 | 0 | 7.2 ± 1.2 |
| CQ | 25 mg/kg | 0.00 ± 0.00 | 100 a3c3d3 | >28 a3c3d3 |
| EH-5 | 50 mg/kg | 28.0 ± 3.2 | 18.8 b3 | 7.2 ± 1.0 b3 |
| EH-5 | 100 mg/kg | 23.2 ± 5.3 | 32.7 a2b3 | 7.8 ± 1.3 b3 |
| Vehicle | 0.5 mL | 34.4 ± 3.9 | 0 | 7.2 ± 0.8 |
| CQ | 25 mg/kg | 0.00 ± 0.00 | 100 a3c3d3 | >28 a3c3d3 |
| Test | Dose | Wt(g) D0 ± SD | Wt (g) D4 ± SD | % Change |
|---|---|---|---|---|
| EH-1 | 50 mg/kg | 26.3 ± 2.4 | 26.6 ± 1.6 | 1.1 |
| EH-1 | 100 mg/kg | 22.4 ± 1.9 | 22.6 ± 1.6 | 0.8 |
| Vehicle | 0.5 mL | 23.5 ± 1.6 | 23.1 ± 1.8 | −1.5 |
| CQ | 25 mg/kg | 27.6 ± 2.5 | 28.8 ± 2.7 | 4.4 1 |
| EH-5 | 50 mg/kg | 22.0 ± 1.0 | 22.2 ± 2.1 | 0.9 |
| EH-5 | 100 mg/kg | 24.2 ± 2.6 | 25.4 ± 3.0 | 4.9 |
| Vehicle | 0.5 mL | 24.0 ± 3.3 | 23.6 ± 2.9 | −1.7 |
| CQ | 25 mg/kg | 21.8 ± 1.6 | 24.1 ± 2.6 | 10.6 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitew, H.; Mammo, W.; Hymete, A.; Yeshak, M.Y. Antimalarial Activity of Acetylenic Thiophenes from Echinops hoehnelii Schweinf. Molecules 2017, 22, 1965. https://doi.org/10.3390/molecules22111965
Bitew H, Mammo W, Hymete A, Yeshak MY. Antimalarial Activity of Acetylenic Thiophenes from Echinops hoehnelii Schweinf. Molecules. 2017; 22(11):1965. https://doi.org/10.3390/molecules22111965
Chicago/Turabian StyleBitew, Helen, Wendimagegn Mammo, Ariaya Hymete, and Mariamawit Yonathan Yeshak. 2017. "Antimalarial Activity of Acetylenic Thiophenes from Echinops hoehnelii Schweinf" Molecules 22, no. 11: 1965. https://doi.org/10.3390/molecules22111965




