The Effects of 4′-Esterified Resveratrol Derivatives on Calcium Dynamics in Breast Cancer Cells
Abstract
:1. Introduction
2. Results
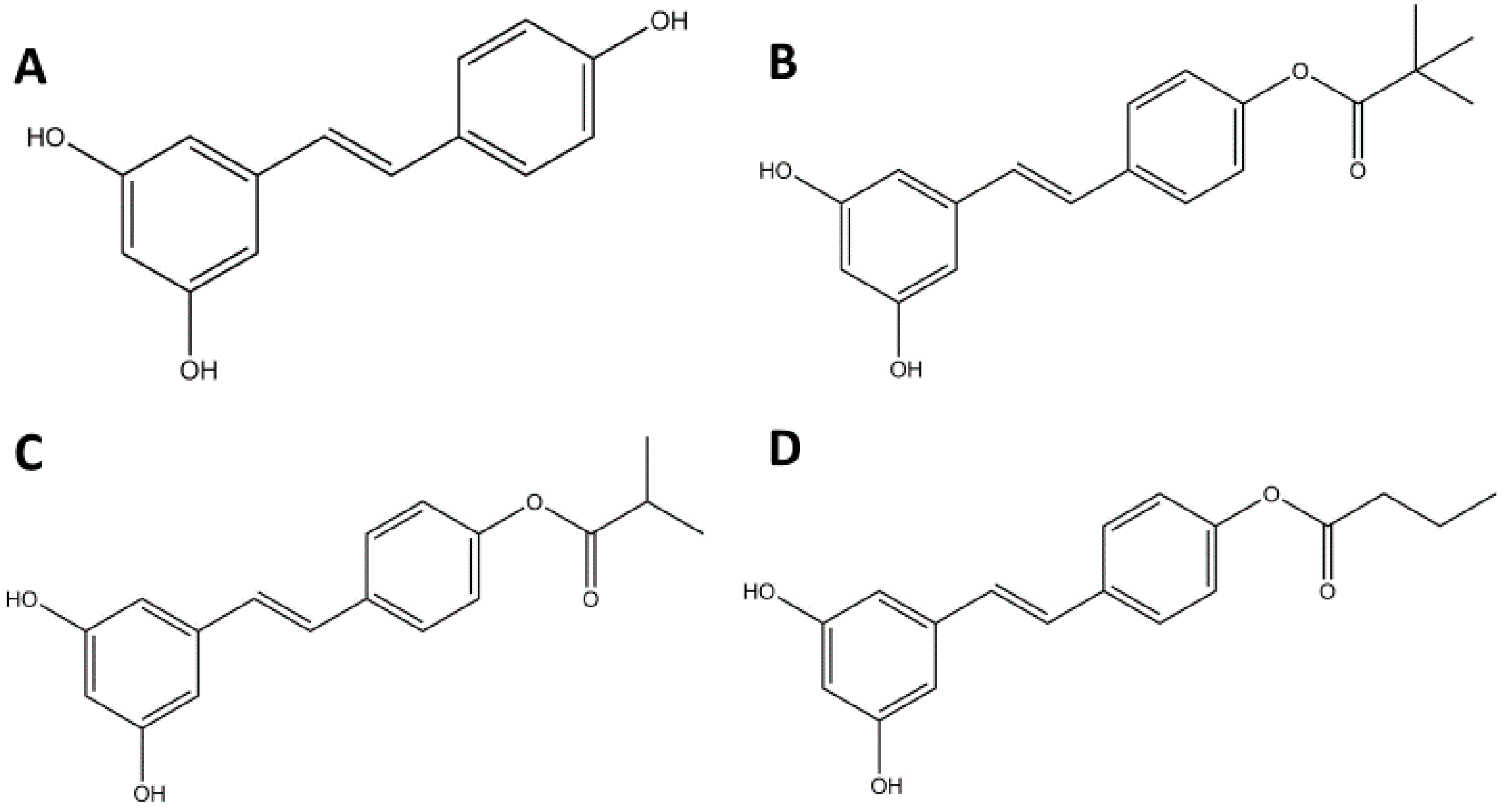
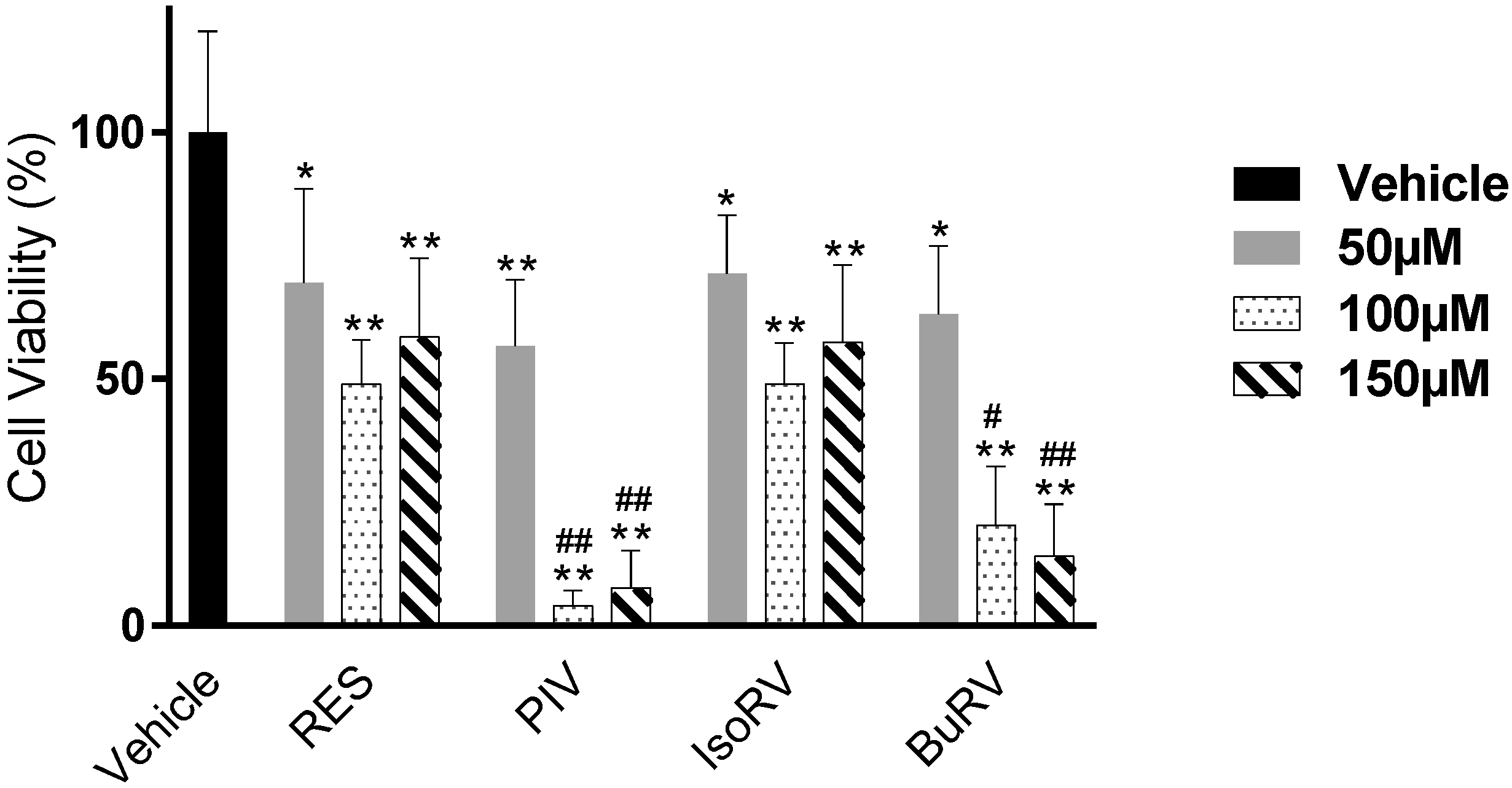
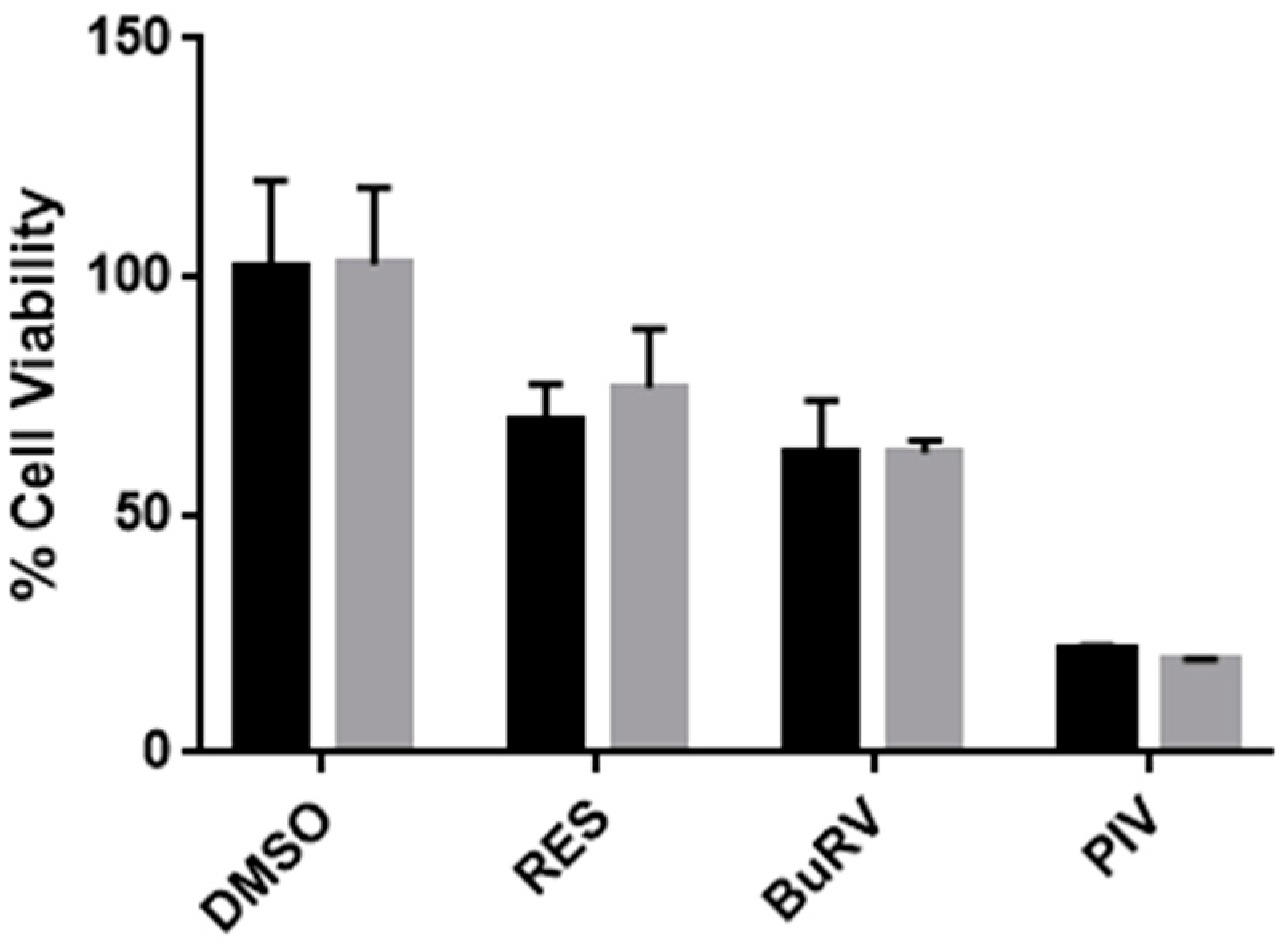
2.1. RES Derivatives Decrease Cell Viability
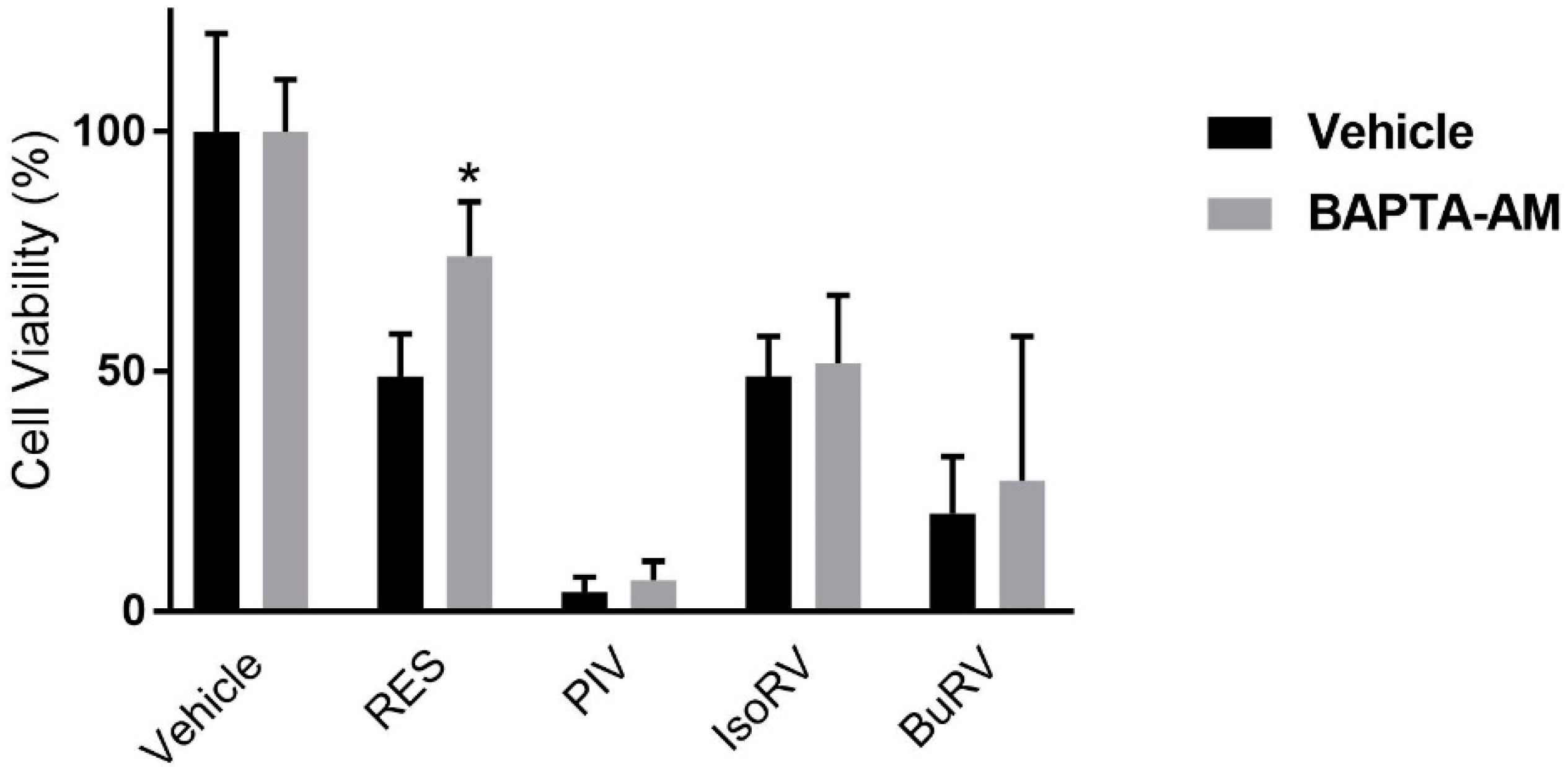
2.2. BAPTA-AM Mitigates RES-Induced Decreases in Cell Viability
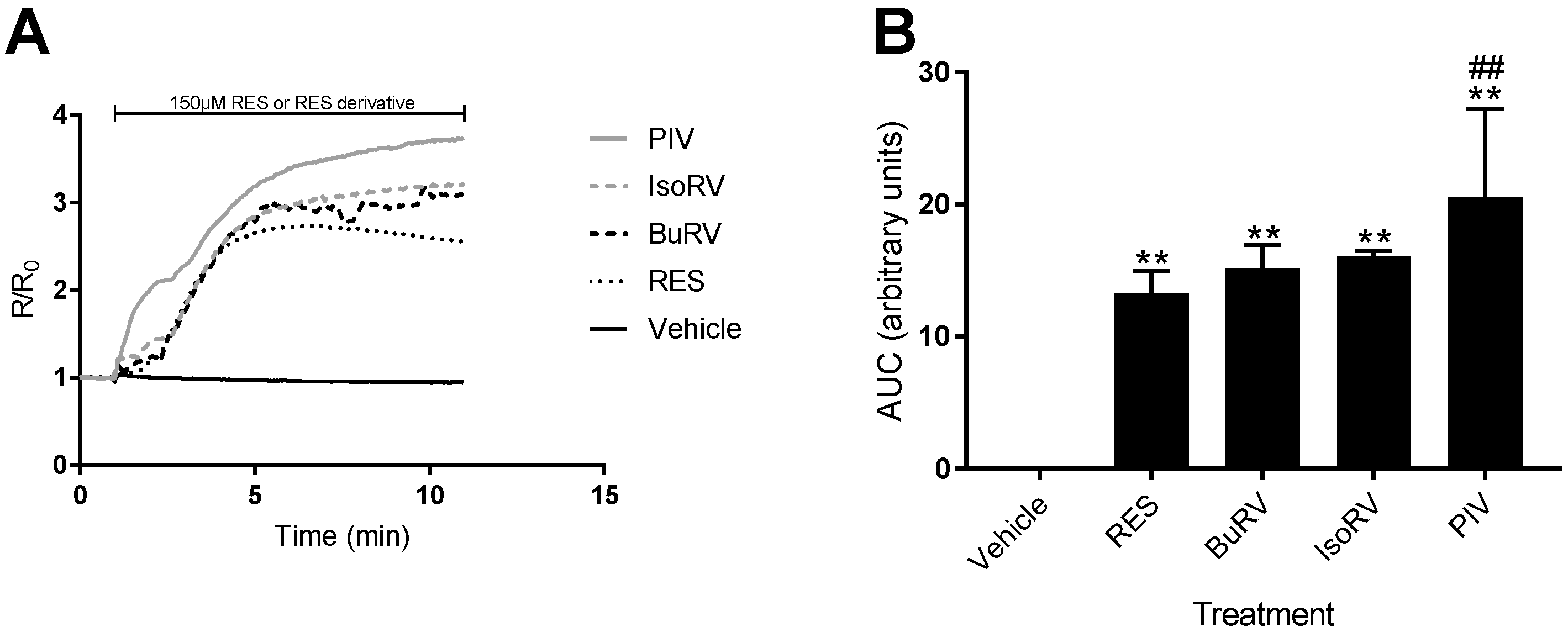
2.3. RES Derivatives Induce Increases in [Ca2+]i
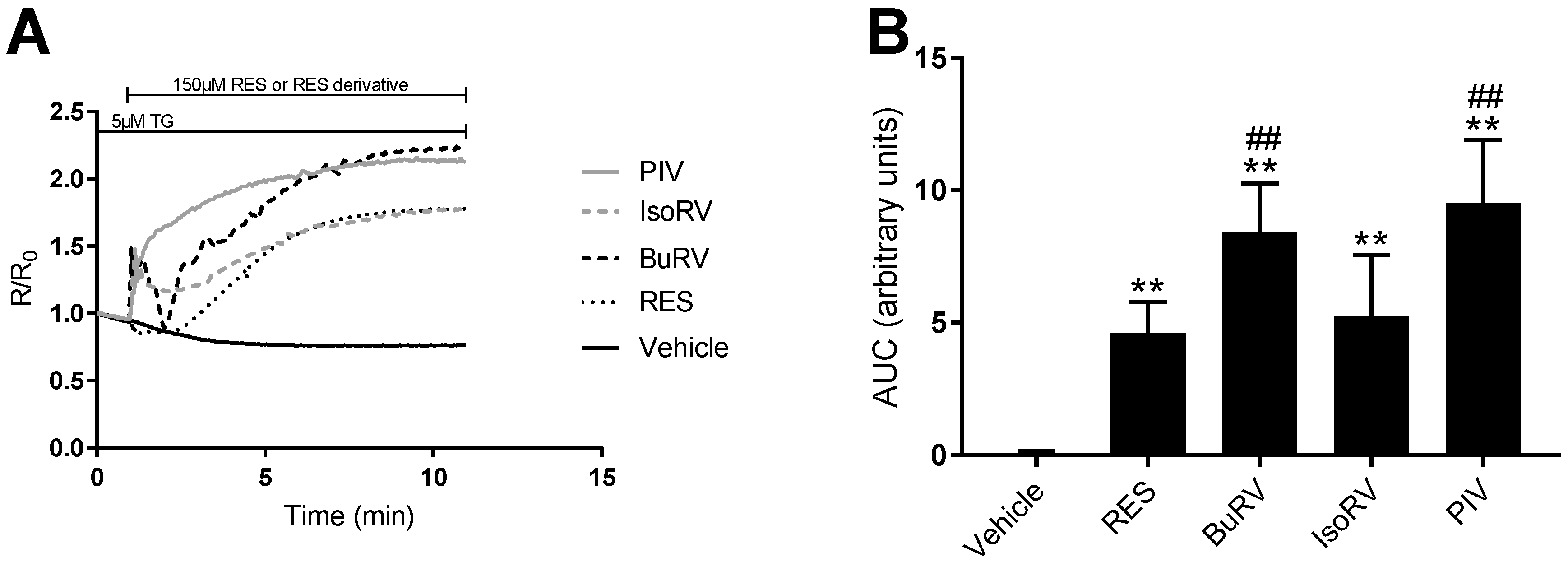
2.4. RES Derivatives Inhibit Plasma Membrane Calcium ATPase (PMCA)
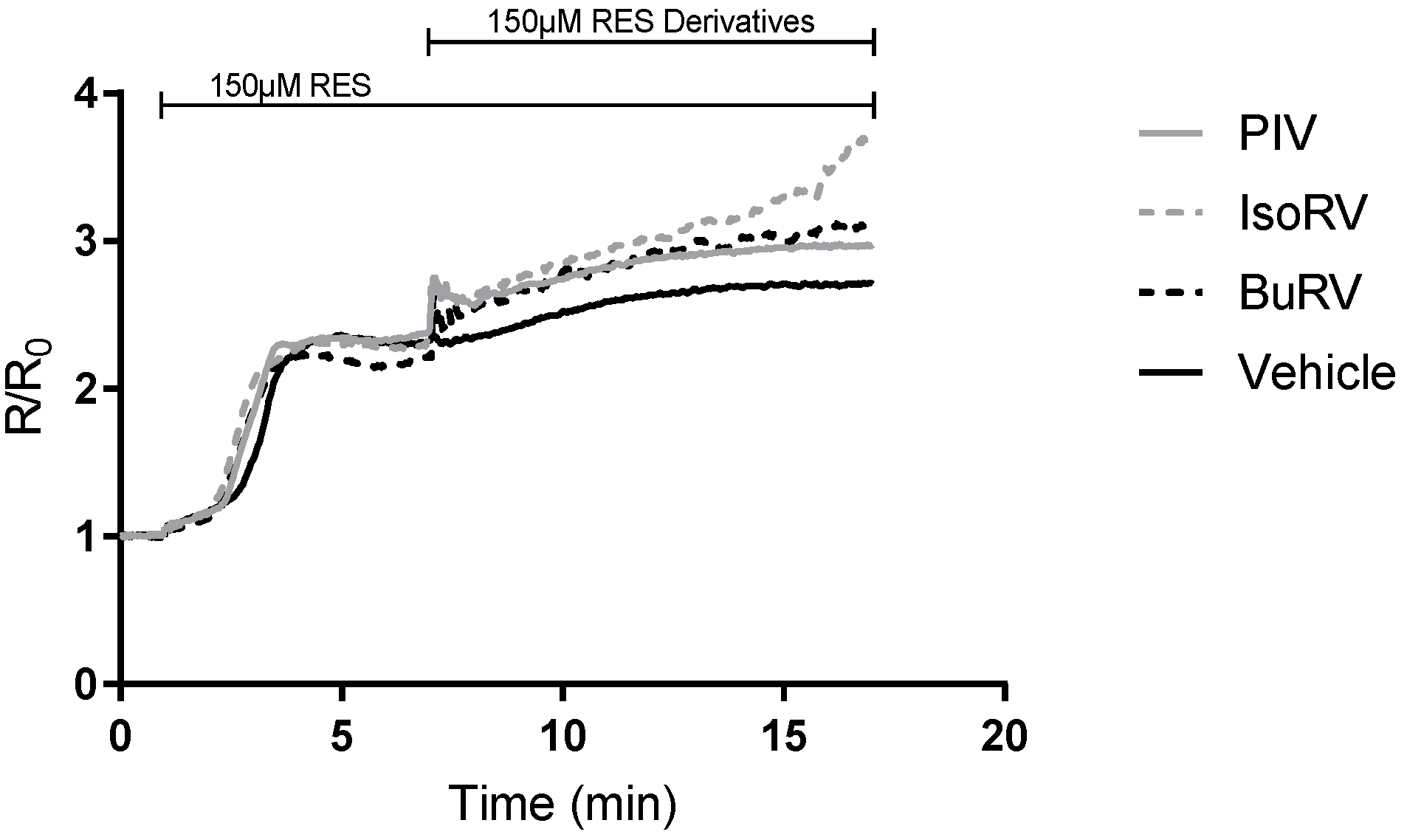
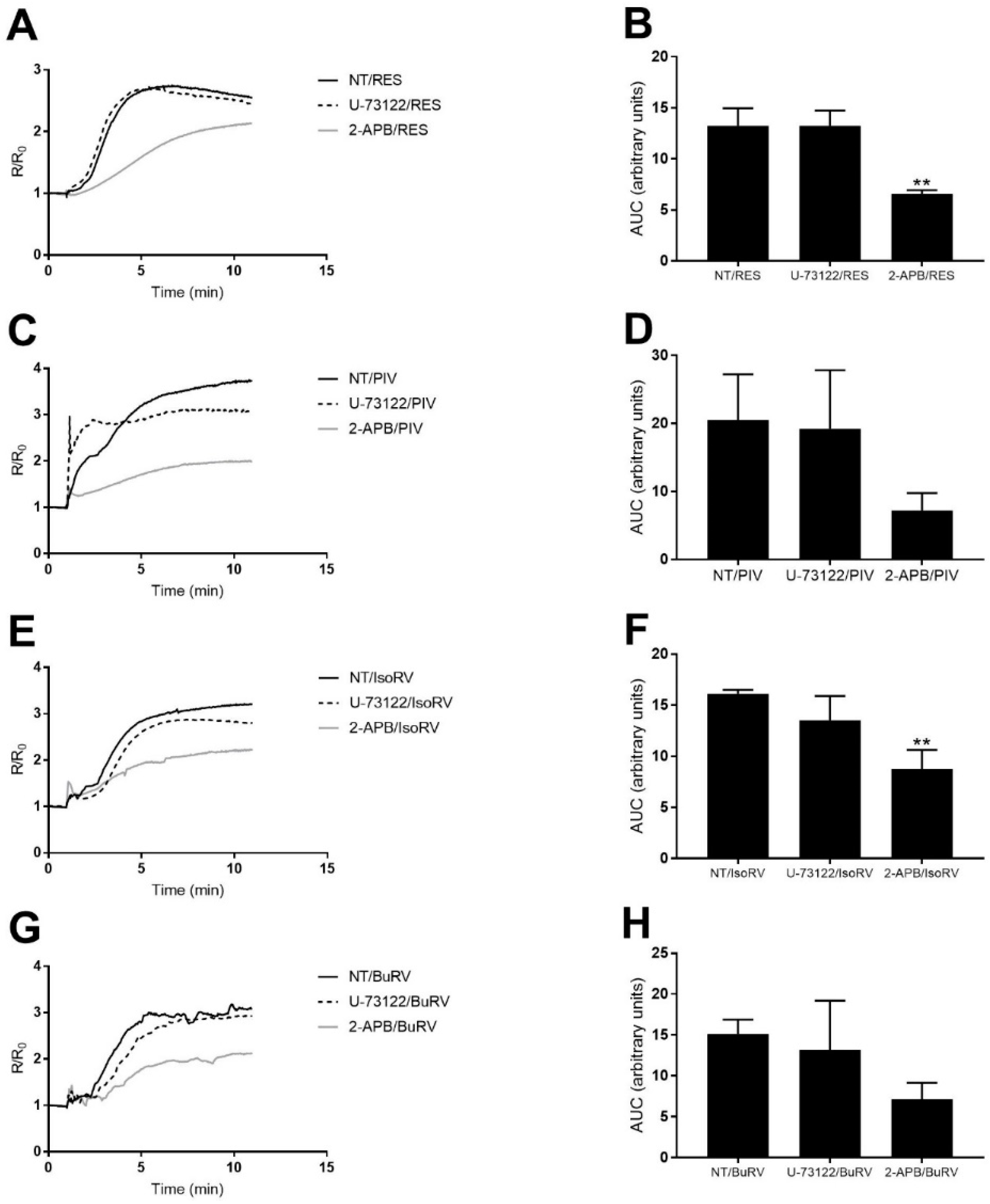
2.5. The Effect of RES and Derivatives on ER Calcium Signaling
2.6. RES and Derivatives Decrease Cell Viability through an Additional, Non-p53-Dependent Pathway
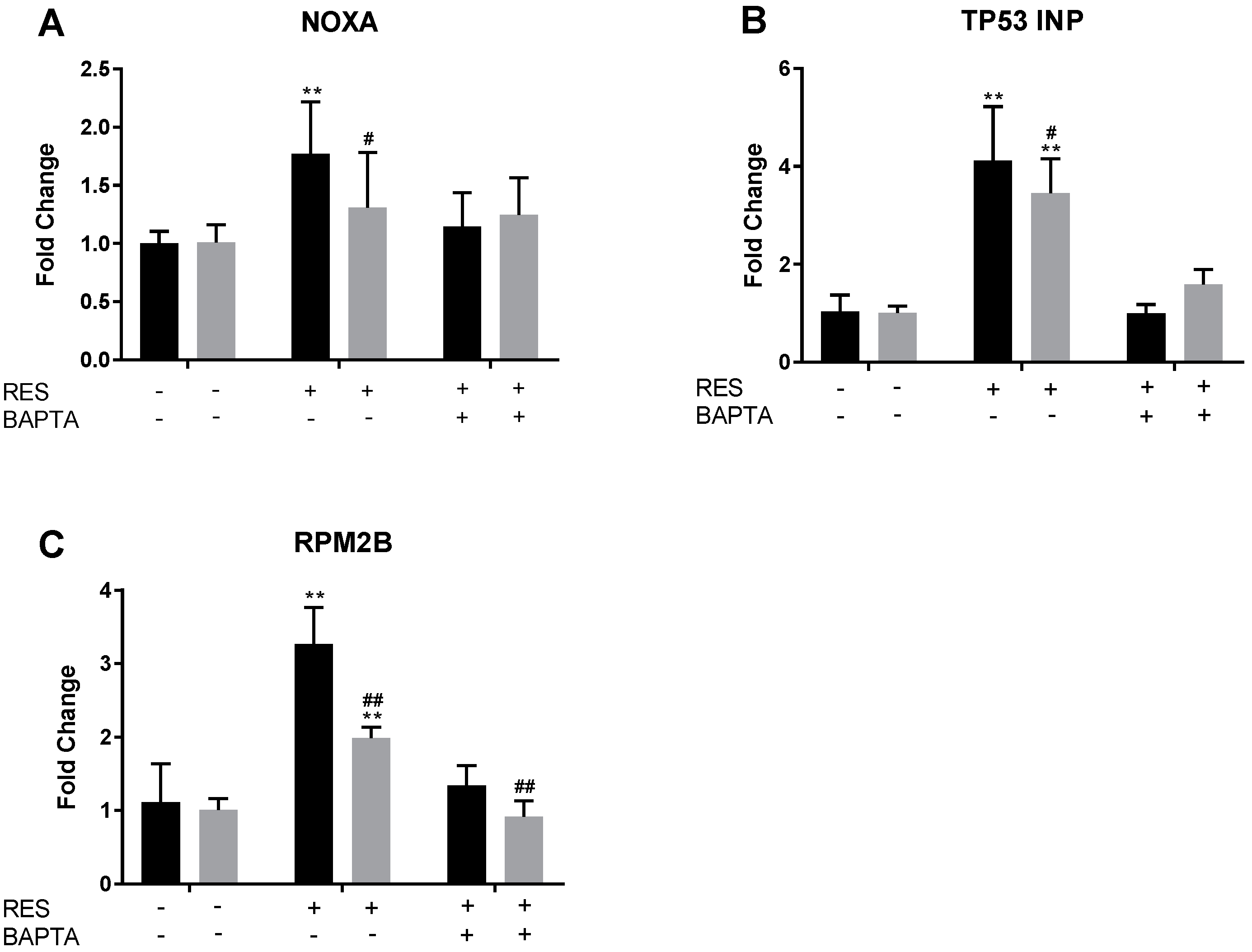
2.7. RES Induces Upregulation of NOXA, TP53INP, and RPM2B Gene Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability
4.4. Intracellular Calcium Imaging
4.5. siRNA-Mediated Knockdown of p53 Gene Expression
4.6. PCR
4.7. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Azimi, I.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium influx pathways in breast cancer: Opportunities for pharmacological intervention. Br. J. Pharmacol. 2014, 171, 945–960. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. The AM and FM of calcium signalling. Nature 1997, 386, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Collins, T.J.; Peppiatt, C.M.; Prothero, L.S.; MacKenzie, L.; De Smet, P.; Travers, M.; Tovey, S.C.; Seo, J.T.; Berridge, M.J.; et al. Calcium signalling—An overview. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Kadio, B.; Yaya, S.; Basak, A.; Dje, K.; Gomes, J.; Mesenge, C. Calcium role in human carcinogenesis: A comprehensive analysis and critical review of literature. Cancer Metastasis Rev. 2016, 35, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Renaud, S.D.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Hsieh, T.Ä. Resveratrol: A cardioprotective substance. Ann. N. Y. Acad. Sci. 2011, 1215, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [PubMed]
- McCalley, A.E.; Kaja, S.; Payne, A.J.; Koulen, P. Resveratrol and calcium signaling: Molecular mechanisms and clinical relevance. Molecules 2014, 19, 7327–7340. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Osmond, G.; Brewer, K.I.; Tyler, D.S.; Andrus, M.B. Synthesis of 4′-ester analogs of resveratrol and their evaluation in malignant melanoma and pancreatic cell lines. Bioorg. Med. Chem. Lett. 2010, 20, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Acerson, M.J.; Fabick, K.M.; Wong, Y.; Blake, C.; Lephart, E.D.; Andrus, M.B. A new synthesis of 4′-resveratrol esters and evaluation of the potential for anti-depressant activity. Bioorg. Med. Chem. Lett. 2013, 23, 2941–2944. [Google Scholar] [CrossRef] [PubMed]
- Gwak, H.; Kim, S.; Dhanasekaran, D.N.; Song, Y.S. Resveratrol triggers ER stress-mediated apoptosis by disrupting N-linked glycosylation of proteins in ovarian cancer cells. Cancer Lett. 2016, 371, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, S.; Dou, M.; Chen, Y.; Zhang, J.; Zhao, X. Synergic effects of artemisinin and resveratrol in cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, X.; Huang, X.; Yan, F.; Qiao, D. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca2+ and mCICR-mediated MPT activation in HepG2 cells. Mol. Cell. Biochem. 2007, 302, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Sareen, D.; Darjatmoko, S.R.; Albert, D.M.; Polans, A.S. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol. Pharmacol. 2007, 72, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Oblad, R.V.; Mecham, J.C.; Kenealey, J.D. Resveratrol inhibits plasma membrane Ca2+-ATPase inducing an increase in cytoplasmic calcium. Biochem. Biophys. Rep. 2016, 7, 253–258. [Google Scholar] [PubMed]
- Van Ginkel, P.R.; Yan, M.B.; Bhattacharya, S.; Polans, A.S.; Kenealey, J.D. Natural products induce a G protein-mediated calcium pathway activating p53 in cancer cells. Toxicol. Appl. Pharmacol. 2015, 288, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; James, A.; Wong, J.; Mankad, P.; Whitehouse, J.; Patel, W.; Alves-Simoes, M.; Siriwardena, A.K.; Bruce, J.I. Insulin protects pancreatic acinar cells from palmitoleic acid-induced cellular injury. J. Biol. Chem. 2014, 289, 23582–23595. [Google Scholar] [CrossRef] [PubMed]
- Foskett, J.K.; White, C.; Cheung, K.H.; Mak, D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007, 87, 593–658. [Google Scholar] [CrossRef] [PubMed]
- Genka, S.; Deutsch, J.; Shetty, U.H.; Stahle, P.L.; John, V.; Lieberburg, I.M.; Ali-Osman, F.; Rapoport, S.I.; Greig, N.H. Development of lipophilic anticancer agents for the treatment of brain tumors by the esterification of water-soluble chlorambucil. Clin. Exp. Metastasis 1993, 11, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.A.; Milevskiy, M.J.G.; Lee, W.C.; Curry, M.C.; Smart, C.E.; Sannus, J.M.; Reid, L.; da Silva, L.; Marcial, D.L.; Dray, E.; et al. The calcium pump plasma membrane Ca2+-ATPase 2 (PMCA2) regulates breast cancer cell proliferation and sensitivity to doxorubicin. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Hanna, W.M.; Trudeau, M.; Rawlinson, E.; Sun, P.; Narod, S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res. Treat. 2009, 115, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [PubMed]
- Curry, M.C.; Luk, N.A.; Kenny, P.A.; Roberts-Thomson, S.J.; Monteith, G.R. Distinct regulation of cytoplasmic calcium signals and cell death pathways by different plasma membrane calcium ATPase isoforms in MDA-MB-231 breast cancer cells. J. Biol. Chem. 2012, 287, 28598–28608. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the resveratrol derivatives tested are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, J.A.; Doughty, H.P.; Eells, A.J.; Johnson, T.A.; Hastings, J.P.; Crowther, C.M.; Andrus, M.B.; Kenealey, J.D. The Effects of 4′-Esterified Resveratrol Derivatives on Calcium Dynamics in Breast Cancer Cells. Molecules 2017, 22, 1968. https://doi.org/10.3390/molecules22111968
Peterson JA, Doughty HP, Eells AJ, Johnson TA, Hastings JP, Crowther CM, Andrus MB, Kenealey JD. The Effects of 4′-Esterified Resveratrol Derivatives on Calcium Dynamics in Breast Cancer Cells. Molecules. 2017; 22(11):1968. https://doi.org/10.3390/molecules22111968
Chicago/Turabian StylePeterson, Joshua A., Hayden P. Doughty, Austin J. Eells, Trent A. Johnson, Jordan P. Hastings, Colton M. Crowther, Merritt B. Andrus, and Jason D. Kenealey. 2017. "The Effects of 4′-Esterified Resveratrol Derivatives on Calcium Dynamics in Breast Cancer Cells" Molecules 22, no. 11: 1968. https://doi.org/10.3390/molecules22111968



