How Secondary and Tertiary Amide Moieties are Molecular Stations for Dibenzo-24-crown-8 in [2]Rotaxane Molecular Shuttles?
Abstract
:1. Introduction
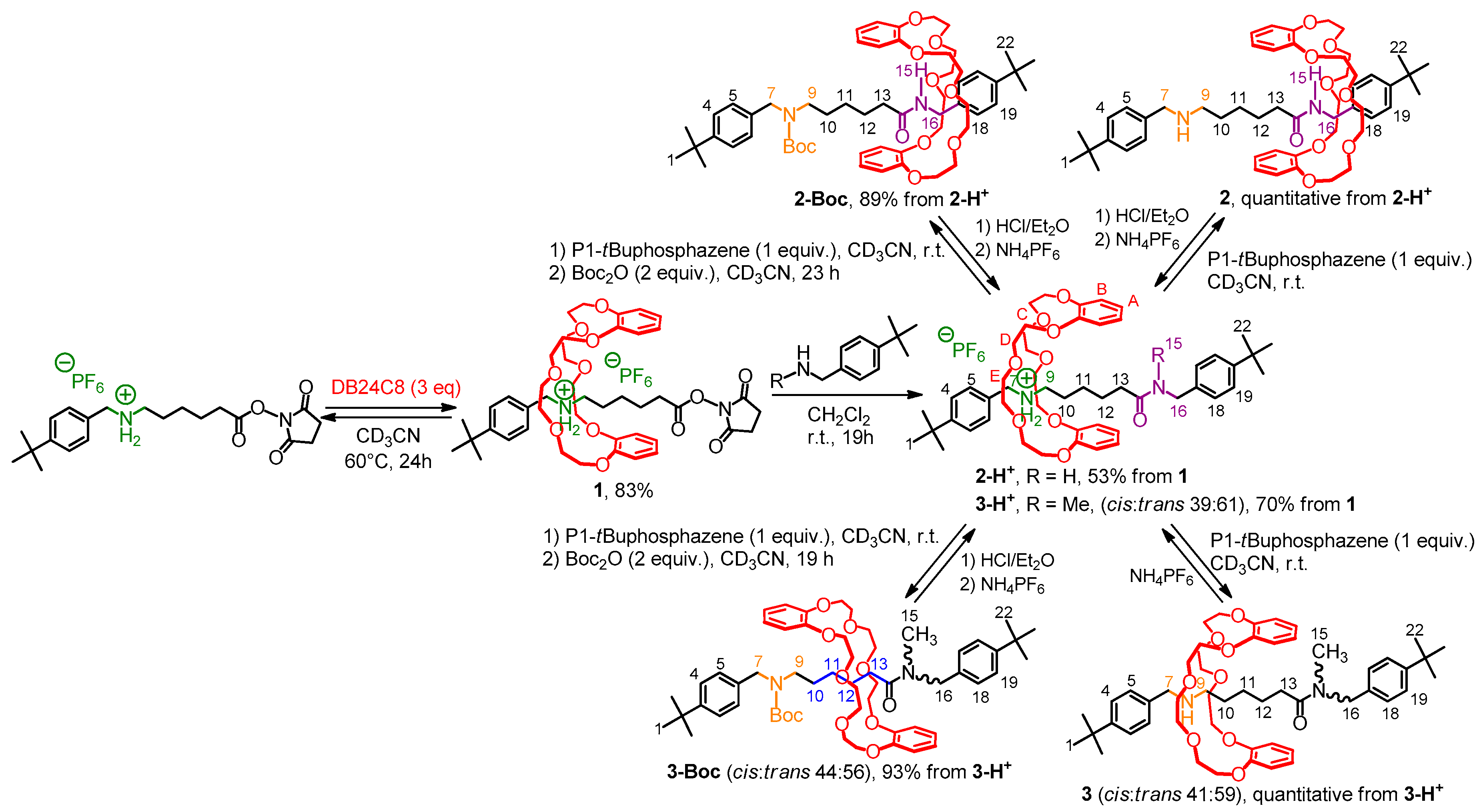
2. Results
2.1. Synthetic Access to Molecular Shuttles
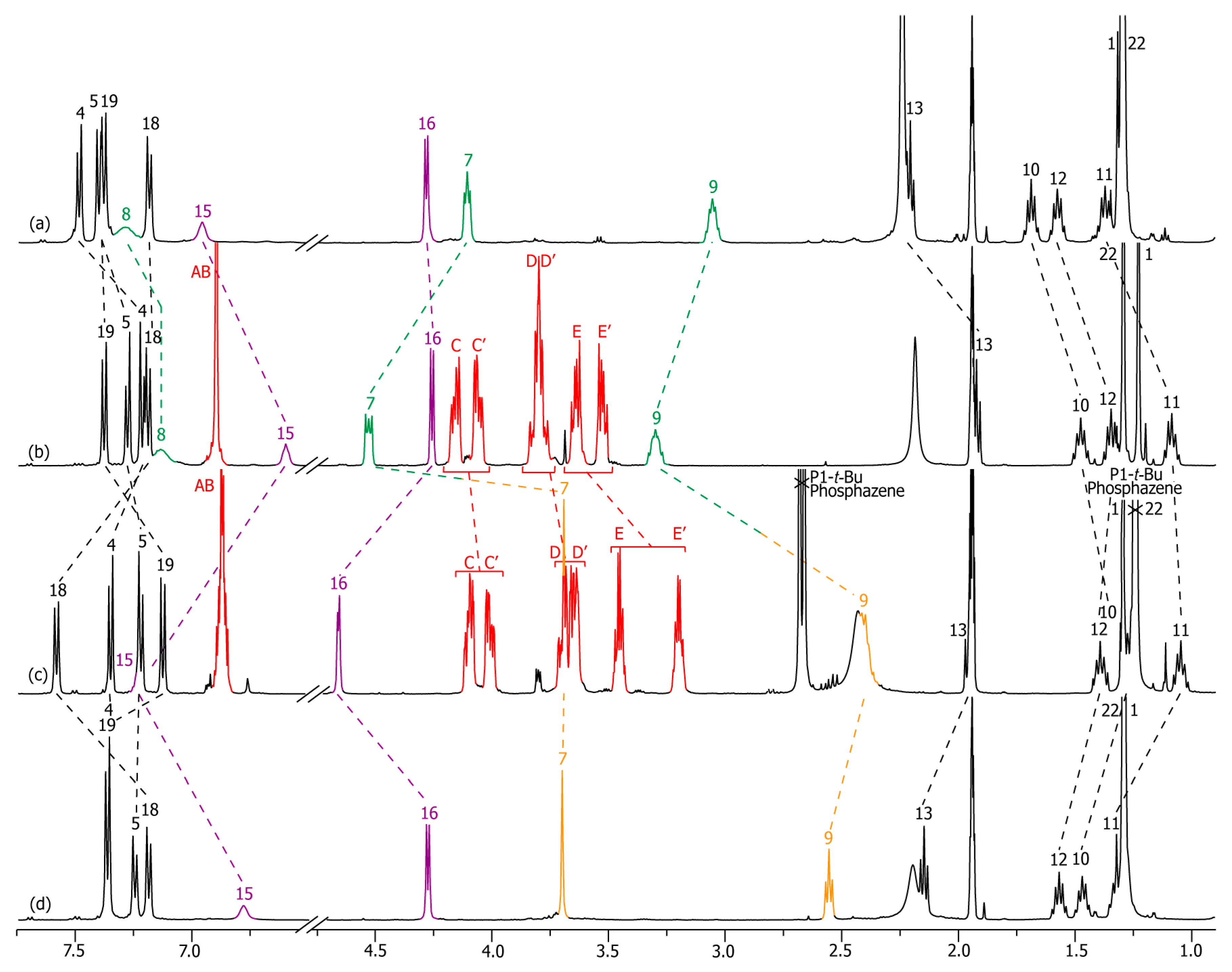
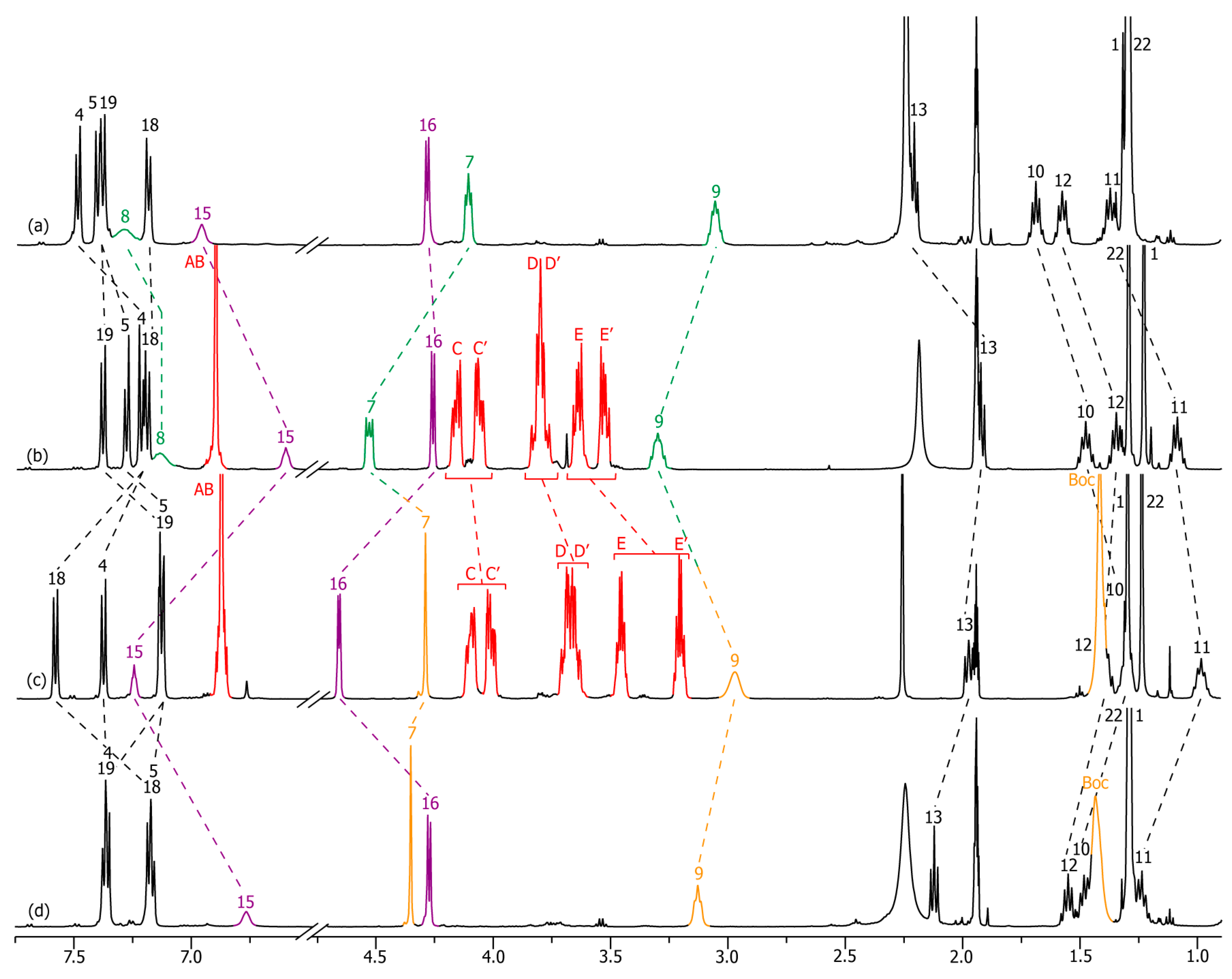
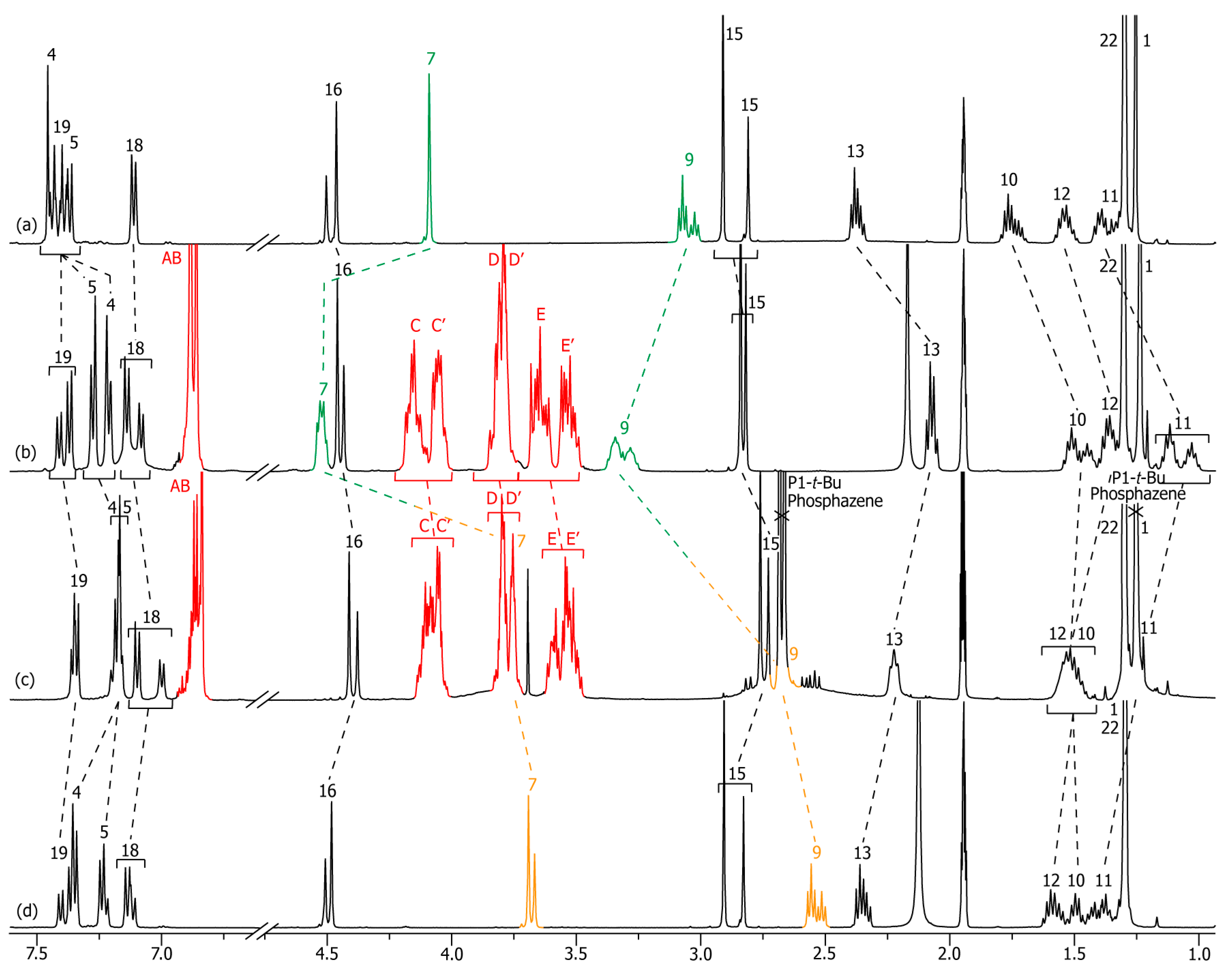
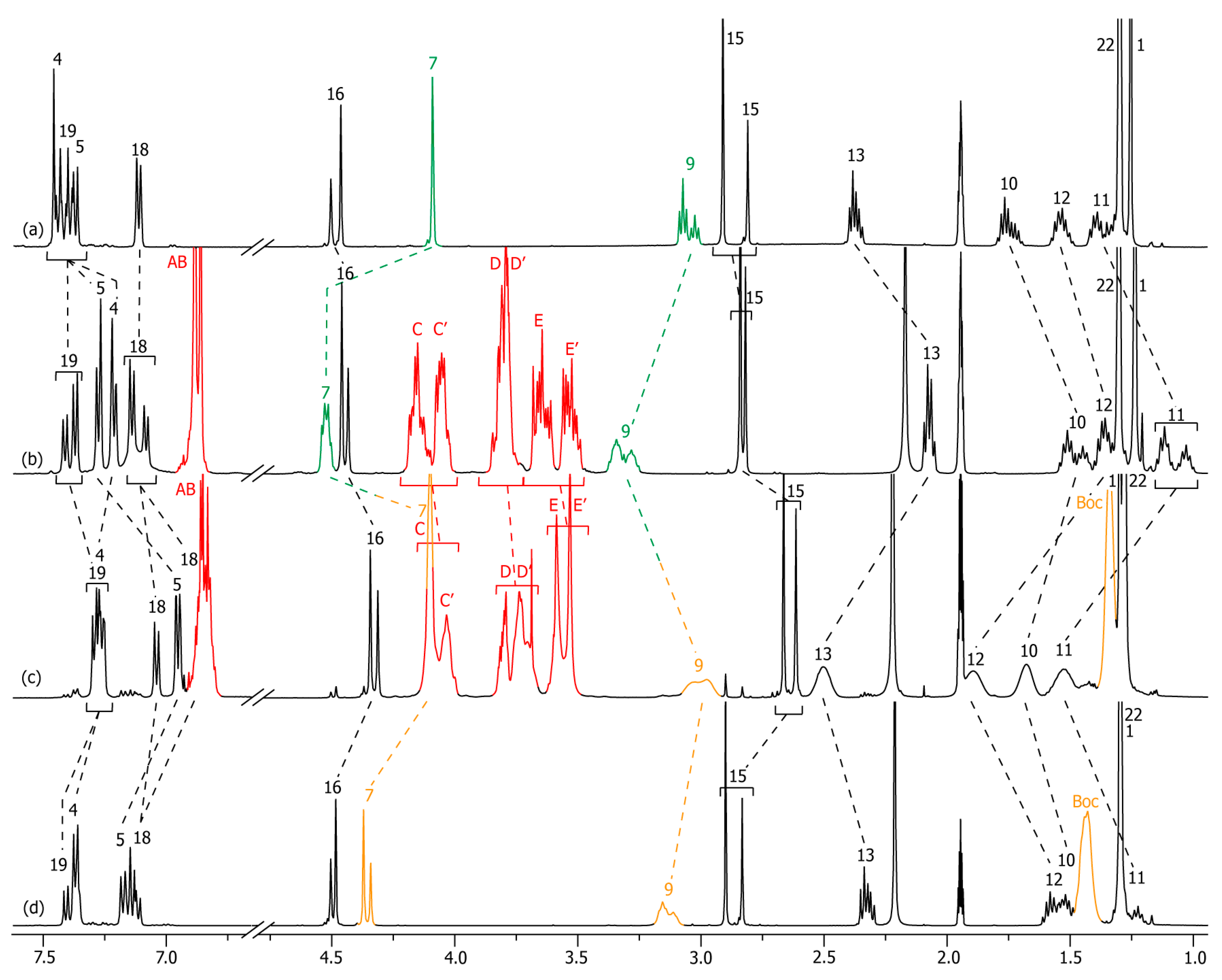
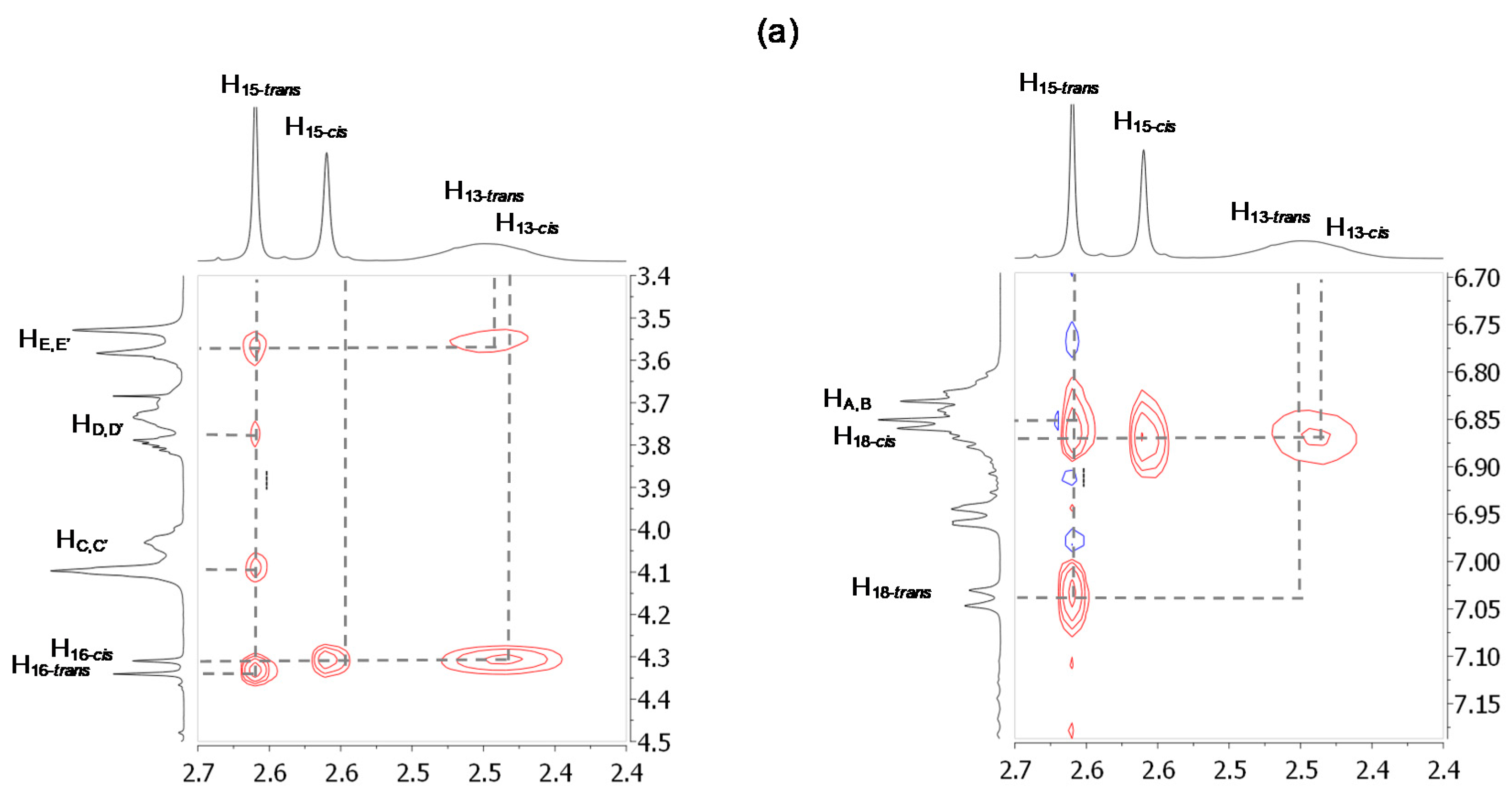
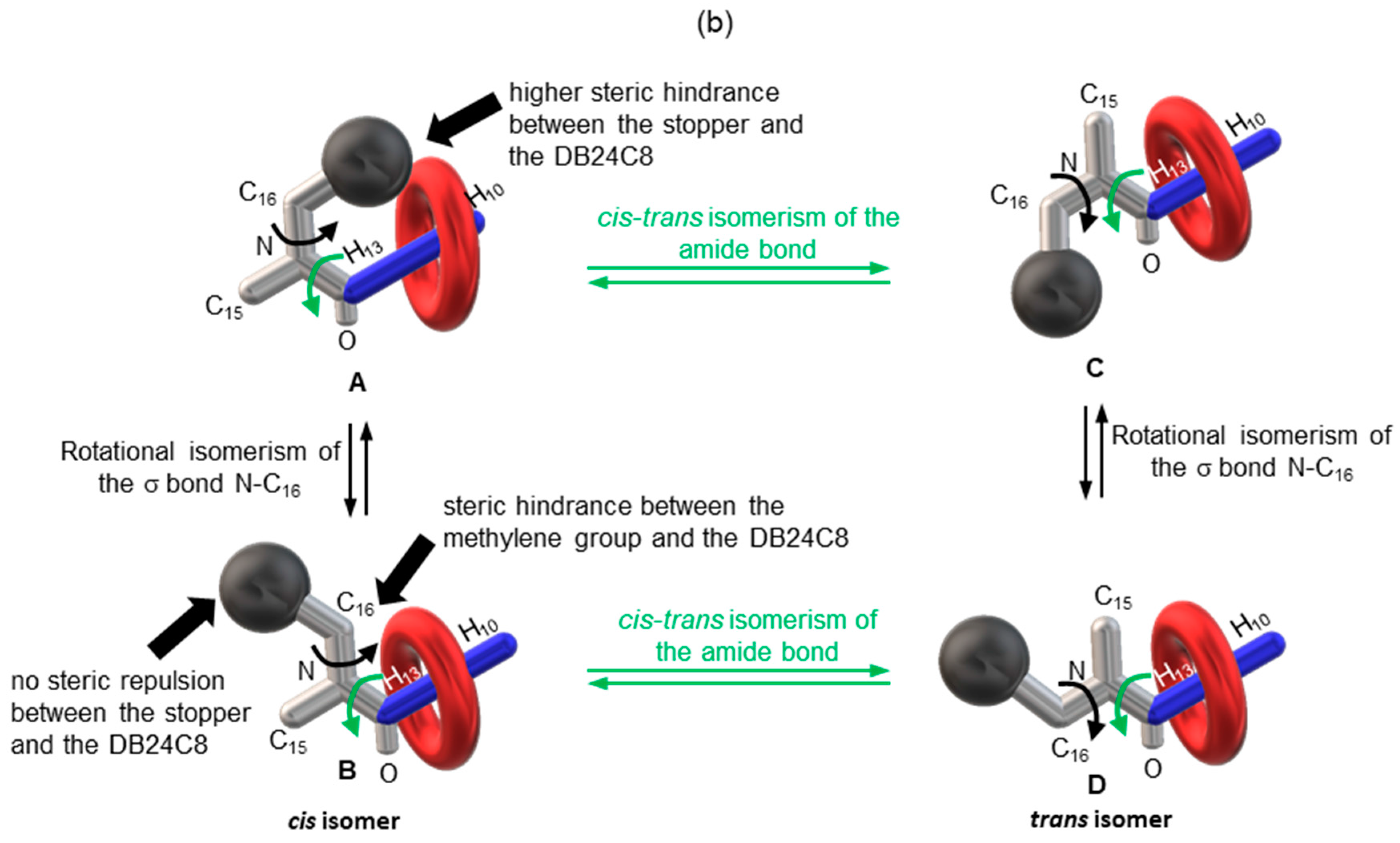
2.2. Characterizations of the Molecular Shuttles
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Livoreil, A.; Dietrich-Buchecker, C.O.; Sauvage, J.-P. Electrochemically Triggerred Swinging of a [2]-Catenate. J. Am. Chem. Soc. 1994, 116, 9399–9400. [Google Scholar] [CrossRef] [PubMed]
- Bissel, R.A.; Cordova, E.; Kaifer, A.E.; Stoddart, J.F. A chemically and electrochemically switchable molecular shuttle. Nature 1994, 369, 133–137. [Google Scholar] [CrossRef]
- Kolchinski, A.G.; Busch, D.H.; Alcock, N.W. Gaining control over molecular threading: Benefits of second coordination sites and aqueous-organic interfaces in rotaxane synthesis. J. Chem. Soc. Chem. Commun. 1995, 1289–1291. [Google Scholar] [CrossRef]
- Martinez-Diaz, M.-V.; Spencer, N.; Stoddart, J.F. The Self-Assembly of a Switchable [2]Rotaxane. Angew. Chem. Int. Ed. Engl. 1997, 36, 1904–1907. [Google Scholar] [CrossRef]
- Gasa, T.; Spruell, J.M.; Dichtel, W.R.; Sorensen, T.J.; Philp, D.; Stoddart, J.F.; Kuzmic, P. Complexation between Methyl Viologen (Paraquat) Bis (Hexafluorophosphate) and Dibenzo[24]Crown-8 Revisited. Chem. Eur. J. 2009, 15, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Álvaro, M.; Ferrer, B.; Garcia, H.; Palomares, E.J.; Balzani, V.; Credi, A.; Venturi, M.; Stoddart, J.F.; Wenger, S. Photochemistry of a dumbbell-shaped multicomponent system hosted inside the mesopores of Al/MCM-41 aluminosilicate. Generation of long-lived viologen radicals. J. Phys. Chem. B 2003, 107, 14319–14325. [Google Scholar] [CrossRef]
- Braunschweig, A.B.; Ronconi, C.M.; Han, J.Y.; Arico, F.; Cantrill, S.J.; Stoddart, J.F.; Khan, S.I.; White, A.J.P.; Williams, D.J. Pseudorotaxanes and rotaxanes formed by viologen derivatives. Eur. J. Org. Chem. 2006, 1857–1866. [Google Scholar] [CrossRef]
- Allwood, B.L.; Shahriari-Zavareh, H.; Stoddart, J.F.; Williams, D.J. Complexation of Paraquat and Diquat by a bismetaphenylene-32-crown-10 derivative. J. Chem. Soc. Chem. Commun. 1987, 1058–1061. [Google Scholar] [CrossRef]
- Allwood, B.L.; Spencer, N.; Shahriari-Zavareh, H.; Stoddart, J.F.; Williams, D.J. Complexation of Paraquat by a bisparaphenylene-34-crown-10 derivative. J. Chem. Soc. Chem. Commun. 1987, 1064–1066. [Google Scholar] [CrossRef]
- Huang, F.; Zakharov, L.N.; Rheingold, A.L.; Ashraf-Khorassani, M.; Gibson, H.W. Synthesis of a symmetric cylindrical bis (crown ether) host and its complexation with paraquat. J. Org. Chem. 2005, 70, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.J. Rotaxanes as ligands: From molecules to materials. Chem. Soc. Rev. 2007, 36, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.J.; Tiburcio, J.; Vella, S.J.; Wisner, J.A. A versatile template for the formation of [2]pseudorotaxanes. 1, 2-Bis (pyridinium) ethane axles and 24-crown-8 ether wheels. Org. Biomol. Chem. 2006, 4, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.J.; Wisner, J.A. [2]Rotaxane molecular shuttles employing 1,2-bis(pyridinium)ethane binding sites and dibenzo-24-crown-8 ethers. Chem. Commun. 2000, 1939–1940. [Google Scholar] [CrossRef]
- Hoffart, D.J.; Tiburcio, J.; De la Torre, A.; Knight, L.K.; Loeb, S. Cooperative Ion–Ion Interactions in the Formation of Interpenetrated Molecules. Angew. Chem. Int. Ed. 2008, 47, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.J.; Wisner, J.A. A New Motif for the Self-Assembly of [2]Pseudorotaxanes; 1, 2-Bis (pyridinium) ethane Axles and [24] Crown-8 Ether Wheels. Angew. Chem. Int. Ed. 1998, 37, 2838–2840. [Google Scholar] [CrossRef]
- Loeb, S.J.; Tiburcio, J.; Vella, S.J. A mechanical “flip-switch”. Interconversion between co-conformations of a [2]rotaxane with a single recognition site. Chem. Commun. 2006, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.J.; Vella, S.J.; Guertin, L.; Suhan, N.D.; Tiburcio, J.; Vukotic, V.N.; Wisner, J.A.; Loeb, S.J. Rotaxanes Based on the 1, 2-Bis (pyridinio) ethane–24-Crown-8 Templating Motif. Eur. J. Org. Chem. 2011, 9, 1763–1770. [Google Scholar] [CrossRef]
- Tramontozzi, D.A.; Suhan, N.D.; Eichhorn, S.H.; Loeb, S.J. The effect of incorporating Fréchet dendrons into rotaxanes and molecular shuttles containing the 1,2-bis(pyridinium) ethane⊂[24]crown-8 templating motif. Chem. Eur. J. 2010, 16, 4466–4476. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.A.; Tiburcio, J.; Loeb, S.J. Characterization of a slippage stopper for the 1,2-bis (pyridinium)ethane–[24]crown-8 ether [2]pseudorotaxane motif. Tetrahedron 2008, 64, 8423–8428. [Google Scholar] [CrossRef]
- Hoffart, D.J.; Loeb, S.J. Metal–organic rotaxane frameworks: Three-dimensional polyrotaxanes from Lanthanide-ion nodes, pyridinium N-oxide axles, and crown-ether wheels. Angew. Chem. Int. Ed. Engl. 2005, 44, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.J.E.; Loeb, S.J. Channels and cavities lined with interlocked components: Metal-based polyrotaxanes that utilize pyridinium axles and crown ether wheels as ligands. Angew. Chem. Int. Ed. Engl. 2003, 42, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Coutrot, F. A Focus on Triazolium as a Multipurpose Molecular Station for pH-Sensitive Interlocked Crown Ether-Based Molecular Machines. ChemistryOpen 2015, 4, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Waelès, P.; Riss-Yaw, B.; Coutrot, F. Synthesis of a pH-Sensitive Hetero[4]Rotaxane Molecular Machine that Combines [c2]Daisy and [2]Rotaxane Arrangements. Chem. Eur. J. 2016, 22, 6837–6845. [Google Scholar] [CrossRef] [PubMed]
- Legigan, T.; Riss-Yaw, B.; Clavel, C.; Coutrot, F. Active Esters as Pseudo-Stoppers for Slippage Synthesis of [2]Pseudorotaxane Building-Blocks: A Straightforward Route to Multi-Interlocked Molecular Machines. Chem. Eur. J. 2016, 22, 8835–8847. [Google Scholar] [CrossRef] [PubMed]
- Waelès, P.; Fournel-Marotte, K.; Coutrot, F. Distinguishing two Ammonium and a Triazolium Sites of Interactions in a Three-Station [2]Rotaxane Molecular Shuttle. Chem. Eur. J. 2017, 23, 11529–11539. [Google Scholar] [CrossRef] [PubMed]
- Waelès, P.; Clavel, C.; Fournel-Marotte, K.; Coutrot, F. Synthesis of Triazolium-Based Mono- and Tris-branched [1]Rotaxanes Using a Molecular Transporter of Dibenzo-24-Crown-8. Chem. Sci. 2015, 6, 4828–4836. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Han, Y.; Wang, L.-N.; Xiang, J.-F.; He, S.-G.; Chen, C.F. Stepwise Motion in a Multivalent [2](3) catenane. J. Am. Chem. Soc. 2015, 137, 9739–9745. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Fu, X.; Zheng, X.-L.; Cao, Z.Q.; Qu, D.-H. Two functional [2]rotaxanes featuring efficient intercomponent interactions between chromophores. Dyes Pigments 2015, 121, 13–20. [Google Scholar] [CrossRef]
- Alvarez, C.M.; Barbero, H.; Miguel, D. Multivalent Molecular Shuttles–Effect of Increasing the Number of Centers in Switchable Catalysts. Eur. J. Org. Chem. 2015, 2015, 6631–6640. [Google Scholar] [CrossRef]
- Ragazzon, G.; Credi, A.; Colasson, B. Thermodynamic Insights on a Bistable Acid–BaseSwitchable Molecular Shuttle with Strongly Shifted Co-conformational Equilibria. Chem. Eur. J. 2017, 23, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Slebodnick, C.; Ratliff, A.E.; Gibson, H.W. Bis (m-phenylene)-32-crown-10/monopyridinium [2]pseudorotaxanes. Tetrahedron Lett. 2005, 46, 6019–6022. [Google Scholar] [CrossRef]
- Cheng, P.-N.; Lin, C.-F.; Liu, Y.H.; Lai, C.-C.; Peng, S.M.; Chiu, S.-H. [3]Pseudorotaxane-like complexes formed between bipyridinium dications and bis-p-xylyl [26]crown-6. Org. Lett. 2006, 8, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Lin, C.-F.; Cheng, P.-N.; Lai, C.-C.; Liu, Y.-H.; Peng, S.-M.; Chiu, S.-H. Bis-p-xylyl [26]crown-6/pyridinium ion recognition: One-pot synthesis of molecular shuttles. Tetrahedron Lett. 2008, 49, 1665–1669. [Google Scholar] [CrossRef]
- Coutrot, F.; Busseron, E. Controlling the Chair Conformation of a Mannopyranose in a Large-Amplitude [2]Rotaxane Molecular Machine. Chem. Eur. J. 2009, 15, 5186–5190. [Google Scholar]
- Riss-Yaw, B.; Waeles, P.; Coutrot, F. Reverse Anomeric Effect in Large-Amplitude Pyridinium Amide-Containing Mannosyl [2]Rotaxane Molecular Shuttles. ChemPhysChem 2016, 17, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Busseron, E.; Romuald, C.; Coutrot, F. Very Contracted to Extended co-Conformations with or without Oscillations in Two- and Three-Station [c2]Daisy Chains. J. Org. Chem. 2010, 75, 6516–6531. [Google Scholar]
- Busseron, E.; Romuald, C.; Coutrot, F. Bistable or Oscillating State Depending on Station and Temperature in Three-Station Glycorotaxane Molecular Machines. Chem. Eur. J. 2010, 16, 10062–10073. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Vukotic, V.N.; Loeb, S.J. Acid-Base Switchable [2]- and [3]Rotaxane Molecular Shuttles with Benzimidazolium and Bis(pyridinium) Recognition Sites. Chem. Asian J. 2016, 11, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Clarkson, G.J. New Bis(benzimidazole) Cations for Threading through Dibenzo-24-crown-8. Org. Lett. 2007, 9, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.; Astudillo, P.; Mares, J.; González, F.J.; Vela, A.; Tiburcio, J. Chemically controlled self-assembly of [2]pseudorotaxanes based on 1,2-bis(benzimidazolium)ethane cations and 24-crown-8 macrocycles. Org. Biomol. Chem. 2007, 5, 2252–2256. [Google Scholar] [CrossRef] [PubMed]
- Noujeim, N.; Zhu, K.; Vukotic, V.N.; Loeb, S.J. [2]Pseudorotaxanes from T-shaped benzimidazolium axles and [24]Crown-8 wheels. Org. Lett. 2012, 14, 2484–2487. [Google Scholar] [CrossRef] [PubMed]
- Kihara, N.; Hashimoto, M.; Takata, T. Behavior of Ferrocene-Containing Rotaxane: Transposition of the Rotaxane Wheel by Redox Reaction of a Ferrocene Moiety Tethered at the End of the Axle. Org. Lett. 2004, 6, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, S.-Y.; Kuo, C.-T.; Lu, T.-W.; Lai, C.-C.; Liu, Y.-H.; Hsu, H.-F.; Peng, S.-M.; Chen, C.-H.; Chiu, S.-H. Acid/Base- and Anion-Controllable Organogels Formed From a Urea-Based Molecular Switch. Angew. Chem. Int. Ed. 2010, 49, 9170–9173. [Google Scholar] [CrossRef] [PubMed]
- Kwan, C.-S.; Chan, A.S.C.; Leung, K.C.-F. A Fluorescent and Switchable Rotaxane Dual Organocatalyst. Org. Lett. 2016, 18, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Valentina, S.; Ogawa, T.; Nakazono, K.; Aoki, D.; Takata, T. Efficient Synthesis of Cyclic Block Copolymers by Rotaxane Protocol by Linear/Cyclic Topology Transformation. Chem. Eur. J. 2016, 22, 8759–8762. [Google Scholar] [CrossRef] [PubMed]
- Aoki, D.; Takata, T. Mechanically linked supramolecular polymer architectures derived from macromolecular [2]rotaxanes: Synthesis and topology transformation. Polymer 2017, 128, 276–296. [Google Scholar] [CrossRef]
- Sawada, J.; Aoki, D.; Takta, T. Vinylic rotaxane cross-linker comprising different axle length for the characterization of rotaxane cross-linked polymers. Macromol. Symp. 2017, 372, 115–119. [Google Scholar] [CrossRef]
- Ogawa, T.; Nakazono, K.; Aoki, D.; Uchida, S.; Takata, T. Effective approach to cyclic polymer from linear polymer: Synthesis and transformation of macromolecular [1]rotaxane. ACS Macro Lett. 2015, 4, 343–347. [Google Scholar] [CrossRef]
- Aoki, D.; Aibara, G.; Uchida, S.; Takata, T. A rational entry to cyclic polymers via selective cyclization by self-assembly and topology transformation of linear polymers. J. Am. Chem. Soc. 2017, 139, 6791–6794. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Aoki, D.; Takata, T. Synthesis and Star/Linear Topology Transformation of a Mechanically Linked ABC Terpolymer. ACS Macro Lett. 2016, 5, 699–703. [Google Scholar] [CrossRef]
- Matsumura, T.; Ishiwari, F.; Koyama, Y.; Takata, T.C.-C. Bond-Forming Click Synthesis of Rotaxanes Exploiting Nitrile N-Oxide. Org. Lett. 2010, 12, 3828–3831. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Kawasaki, H.; Kihara, N.; Takata, T. Sequential O- and N-acylation protocol for high-yield preparation and modification of rotaxanes: Synthesis, functionalization, structure, and intercomponent interaction of rotaxanes. J. Org. Chem. 2006, 71, 5093–5104. [Google Scholar] [CrossRef] [PubMed]
- Smukste, I.; House, B.E.; Smithrud, D.B. Host-[2]rotaxane: Advantage of converging functional groups for guest recognition. J. Org. Chem. 2003, 68, 2559–2571. [Google Scholar] [CrossRef] [PubMed]
- Dvornikovs, V.; House, B.E.; Kaetzel, M.; Dedman, J.R.; Smithrud, D.B. Host-[2]rotaxanes as cellular transport agents. J. Am. Chem. Soc. 2003, 125, 8290–8301. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.W.; Smithrud, D.B. Facile synthesis of rotaxanes through condensation reactions of DCC-[2]rotaxanes. Org. Lett. 2001, 3, 2485–2487. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Isaacsohn, I.; Drew, A.F.; Smithrud, D.B. Determining the intracellular transport mechanism of a cleft-[2]rotaxane. J. Am. Chem. Soc. 2006, 128, 12229–12238. [Google Scholar] [CrossRef] [PubMed]
- Smukste, I.; Smithrud, D.B. Structure-function relationship of amino acid-[2]rotaxanes. J. Org. Chem. 2003, 68, 2547–2558. [Google Scholar] [CrossRef] [PubMed]
- Romuald, C.; Cazal, G.; Enjalbal, C.; Coutrot, F. Straightforward Synthesis of a Double-Lasso Macrocycle from a Nonsymmetrical [c2]Daisy Chain. Org. Lett. 2013, 15, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Schwesinger, R.; Willaredt, J.; Schlemper, H.; Keller, M.; Schmitt, D.; Fritz, H. Novel, Very Strong, Uncharged Auxiliary Bases; Design and Synthesis of Monomeric and Polymer-Bound Triaminoiminophosphorane Bases of Broadly Varied Steric Demand. Chem. Ber. 1994, 127, 2435–2454. [Google Scholar] [CrossRef]
- Nakazono, K.; Takata, T. Neutralization of a sec-Ammonium Group Unusually Stabilized by the “Rotaxane Effect”: Synthesis, Structure, and Dynamic Nature of a “Free” sec-Amine/Crown Ether-Type Rotaxane. Chem. Eur. J. 2010, 16, 13783–13794. [Google Scholar] [CrossRef] [PubMed]
- Riss-Yaw, B.; Clavel, C.; Laurent, P.; Coutrot, F. The relationship between the conformational degree of freedom of template-containing threads and slippage in the formation of [2]rotaxane building blocks. Chem. Commun. 2017, 53, 10874–10877. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riss-Yaw, B.; Morin, J.; Clavel, C.; Coutrot, F. How Secondary and Tertiary Amide Moieties are Molecular Stations for Dibenzo-24-crown-8 in [2]Rotaxane Molecular Shuttles? Molecules 2017, 22, 2017. https://doi.org/10.3390/molecules22112017
Riss-Yaw B, Morin J, Clavel C, Coutrot F. How Secondary and Tertiary Amide Moieties are Molecular Stations for Dibenzo-24-crown-8 in [2]Rotaxane Molecular Shuttles? Molecules. 2017; 22(11):2017. https://doi.org/10.3390/molecules22112017
Chicago/Turabian StyleRiss-Yaw, Benjamin, Justine Morin, Caroline Clavel, and Frédéric Coutrot. 2017. "How Secondary and Tertiary Amide Moieties are Molecular Stations for Dibenzo-24-crown-8 in [2]Rotaxane Molecular Shuttles?" Molecules 22, no. 11: 2017. https://doi.org/10.3390/molecules22112017




