New Metabolites of Coumarin Detected in Human Urine Using Ultra Performance Liquid Chromatography/Quadrupole-Time-of-Flight Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. UPLC-MS Method Development
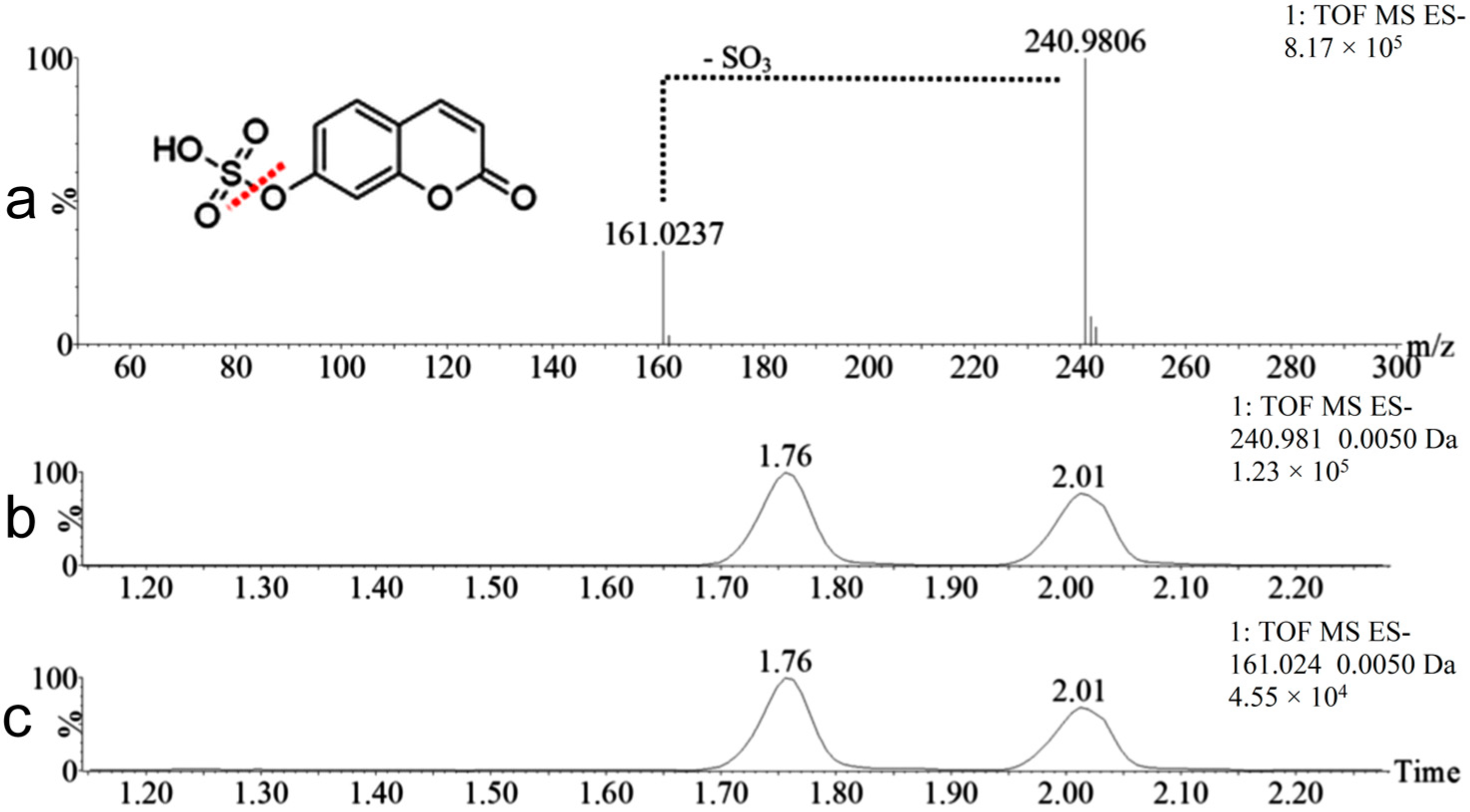
2.2. Coumarin Metabolites in Human Urine
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Apparatus and Conditions
4.3. Ethics Statement, Study Design and Urine Collection
4.4. Urine Sample Preparation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soine, T.O. Naturally occurring coumarins and related physiological activities. J. Pharm. Sci. 1964, 53, 231–264. [Google Scholar] [CrossRef] [PubMed]
- Feuer, G. The metabolism and biological actions of coumarins. Prog. Med. Chem. 1974, 10, 85–158. [Google Scholar] [PubMed]
- Gasparetto, J.C.; de Francisco, T.M.G.; Pontarolo, R. Chemical constituents of Mikania glomerata Spreng and Mikania laevigata Sch. Bip. ex baker. J. Med. Plants Res. 2013, 7, 753–765. [Google Scholar]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Opdyke, D.L.J. Monographs on fragrance raw materials: Coumarin. Food Cosmet. Toxicol. 1974, 12, 385–388. [Google Scholar] [CrossRef]
- Cohen, A.J. Critical review of the toxicology of coumarin with special reference to interspecies differences in metabolism and hepatotoxic response and their significance to man. Food Cosmet. Toxicol. 1979, 17, 277–289. [Google Scholar] [CrossRef]
- Gasparetto, J.C.; Peccinini, R.G.; de Francisco, T.M.; Cerqueira, L.B.; Campos, F.R.; Pontarolo, R. A kinetic study of the main guaco metabolites using syrup formulation and the identification of an alternative route of coumarin metabolism in humans. PLoS ONE 2015, 10, e0118922. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- Shilling, W.H.; Crampton, R.F.; Longland, R.C. Metabolism of coumarin in man. Nature 1969, 221, 664–665. [Google Scholar] [CrossRef] [PubMed]
- Ritschel, W.A.; Brady, M.E.; Tan, H.S.; Hoffmann, K.A.; Yiu, I.M.; Grummich, K.W. Pharmacokinetics of coumarin and its 7-hydroxy-metabolites upon intravenous and peroral administration of coumarin in man. Eur. J. Clin. Pharmacol. 1977, 12, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Ritschel, W.A.; Brady, M.E.; Tan, H.S. First-pass effect of coumarin in man. Int. J. Clin. Pharmacol. Biopharm. 1979, 17, 99–103. [Google Scholar] [PubMed]
- Ritschel, W.A.; Hoffmann, K.A. Pilot study on bioavailability of coumarin and 7-hydroxycoumarin upon peroral administration of coumarin in a sustained-release dosage form. J. Clin. Pharmacol. 1981, 21, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.; O’Kennedy, R.; Thornes, R.D. Analysis of coumarin and its urinary metabolites by high-performance liquid chromatography. J. Chromatogr. 1987, 416, 165–169. [Google Scholar] [CrossRef]
- Rautio, A.; Kraul, H.; Kojo, A.; Salmela, E.; Pelkonen, O. Interindividual variability of coumarin 7-hydroxylation in healthy volunteers. Pharmacogenetics 1992, 2, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Iscan, M.; Rostami, H.; Guray, T.; Pelkonen, O.; Rautio, A. Interindividual variability of coumarin 7-hydroxylation in a turkish population. Eur. J. Clin. Pharmacol. 1994, 47, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.; Pfister, M.; Wohrlin, F.; Lampen, A. Relative bioavailability of coumarin from cinnamon and cinnamon-containing foods compared to isolated coumarin: A four-way crossover study in human volunteers. Mol. Nutr. Food Res. 2011, 55, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.A.; Hawkins, D.R.; Mayo, B.C.; Api, A.M. The in vivo dermal absorption and metabolism of [4-14c] coumarin by rats and by human volunteers under simulated conditions of use in fragrances. Food Chem. Toxicol. 2001, 39, 153–162. [Google Scholar] [CrossRef]
- Kaighen, M.; Williams, R.T. The metabolism of [3-14c]coumarin. J. Med. Pharm. Chem. 1961, 3, 25–43. [Google Scholar] [CrossRef]
- Fentem, J.H.; Fry, J.R.; Whiting, D.A. O-hydroxyphenylacetaldehyde: A major novel metabolite of coumarin formed by rat, gerbil and human liver microsomes. Biochem. Biophys. Res. Commun. 1991, 179, 197–203. [Google Scholar] [CrossRef]
- Fernando Aguilar, H.N.A.; Barlow, S.; Castle, L.; Crebelli, R.; Wolfgang Dekant, K.-H.E.; Gontard, N.; Gott, D.M.; Grilli, S.; Rainer Gürtler, J.C.L.; Leclercq, C.; et al. Coumarin in flavourings and other food ingredients with flavouring properties. EFSA 2008, 793, 1–15. [Google Scholar]
- Barbara, J.E.; Buckley, D.B.; Horrigan, M.J. Exploring the utility of high-resolution ms with post-acquisition data mining for simultaneous exogenous and endogenous metabolite profiling. Bioanalysis 2013, 5, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Kutney, J.P.; Eigendor, G.; Inaba, T.; Dreyer, D.L. Mass spectral fragmentation studies in monomeric and dimeric coumarins. Org. Mass Spetrom. 1971, 5, 249–263. [Google Scholar] [CrossRef]
- Lerman, L.; Klonkay, A.C.; Filipović, A.; Elenkov, I. Collision-induced dissociation of a series of coumarins studied by positive- and negativeion electrospray triple quadrupole tandem mass spectrometry. Eur. J. Mass Spectrom. 2004, 10, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, J.C.; Pontarolo, R.; de Francisco, T.M.G.; Campos, F.R. Mikania glomerata and m. Laevigata: Clinical and toxicological advances. In Toxicity and Drug Testing; Acree, B., Ed.; In Tech: Rijeka, Croatia, 2012; Volume 1, pp. 297–320. [Google Scholar]
- Booth, A.N.; Masri, M.S.; Robbins, D.J.; Emerson, O.H.; Jones, F.T.; Deeds, F. Urinary metabolites of coumarin and ortho-coumaric acid. J. Biol. Chem. 1959, 234, 946–948. [Google Scholar] [PubMed]
- Huwer, T.; Altmann, H.J.; Grunow, W.; Lenhardt, S.; Przybylski, M.; Eisenbrand, G. Coumarin mercapturic acid isolated from rat urine indicates metabolic formation of coumarin 3,4-epoxide. Chem. Res. Toxicol. 1991, 4, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Lake, B.G.; Gaudin, H.; Price, R.J.; Walters, D.G. Metabolism of [3-14C] coumarin to polar and covalently bound products by hepatic microsomes from the rat, Syrian hamster, gerbil, and humans. Food Chem. Toxicol. 1992, 30, 105–115. [Google Scholar] [CrossRef]
- Van Iersel, M.L.; Henderson, C.J.; Walters, D.G.; Price, R.J.; Wolf, C.R.; Lake, B.G. Metabolism of [3-14C] coumarin by human liver microsomes. Xenobiotica 1994, 24, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yamazaki, H.; Guengerich, F.P. Ethnic-related differences in coumarin 7-hydroxylation activities catalyzed by cytochromes P4502A6 in liver microsomes of Japanese and Caucasian populations. Xenobiotica 1996, 26, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Gu, J.; Zhang, Q.-Y.; Spink, D.C.; Kaminsky, L.S.; Ding, X. Biotransformation of coumarin by rodent and human cytochromes P450: Metabolic basis of tissue-selective toxicity in olfactory mucosa of rats and mice. J. Pharmacol. Exp. Ther. 1999, 288, 463–471. [Google Scholar] [PubMed]
- Pelkonen, O.; Rautio, A.; Raunio, H.; Pasanen, M. CYP2A6: A human coumarin 7-hydroxylase. Toxicology 2000, 144, 139–147. [Google Scholar] [CrossRef]
- Born, S.L.; Caudill, D.; Fliter, K.L.; Purdon, M.P. Identification of the cytochromes P450 that catalyze coumarin 3,4-epoxidation and 3-hydroxylation. Drug Metab. Dispos. 2002, 30, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.F.V.; Ito, Y.; Lake, B.G. Metabolism of coumarin by human P450s: A molecular modelling study. Toxicol. In Vitro 2006, 20, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, J.; Chowbay, B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab. Rev. 2009, 41, 89–295. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds coumarin, 7-hydroxycoumarin, o-hydroxyphenylacetic acid and o-coumaric acid are available from the authors. |
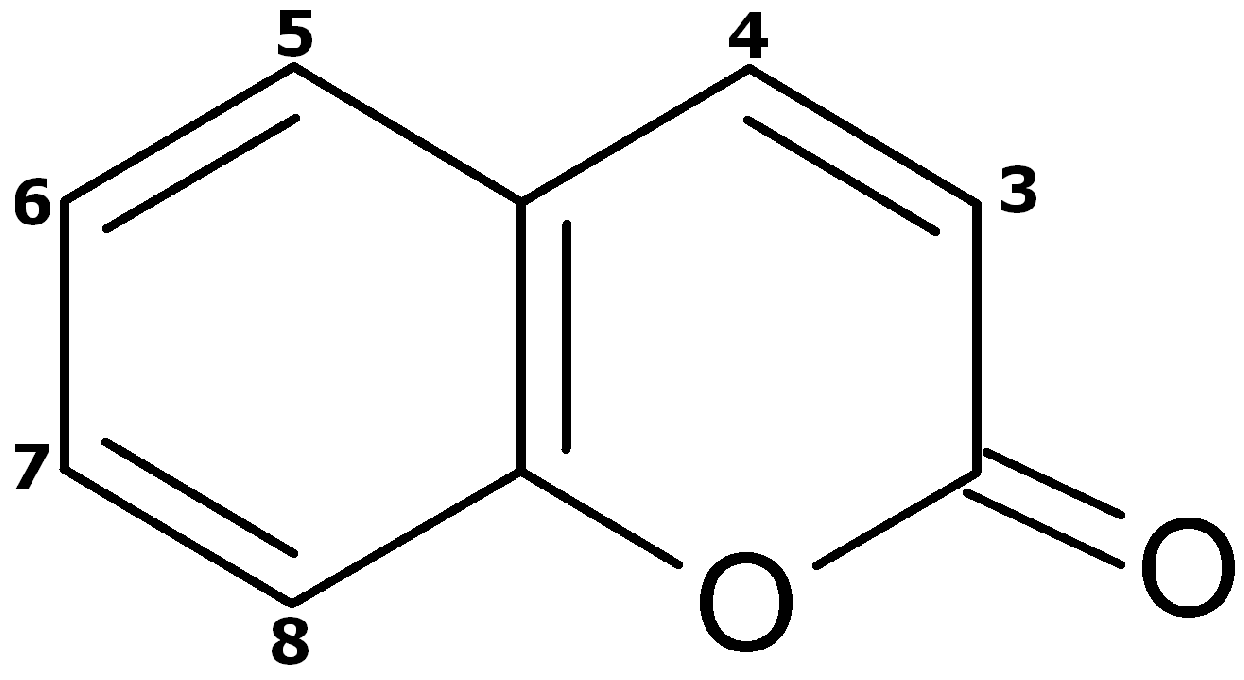

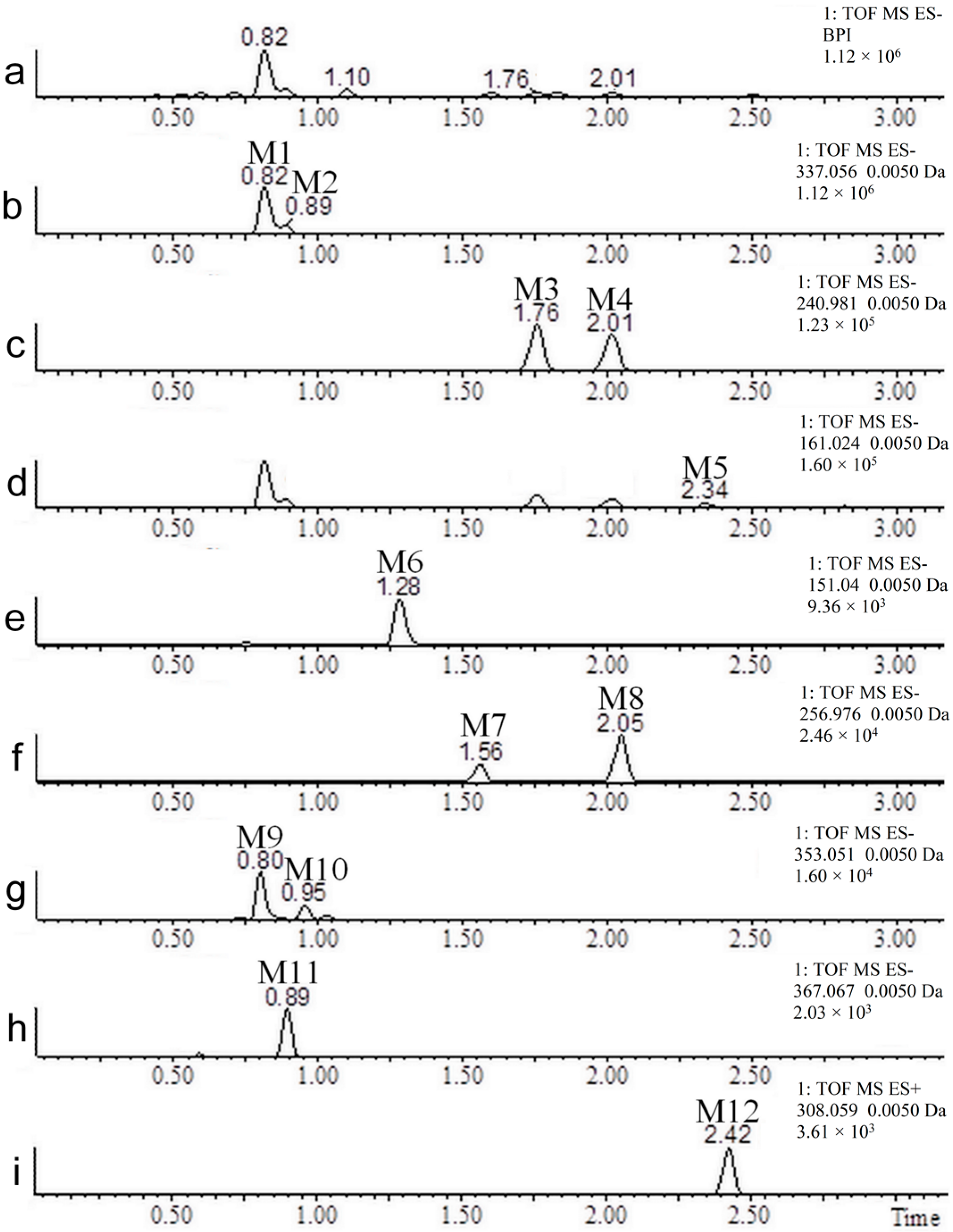
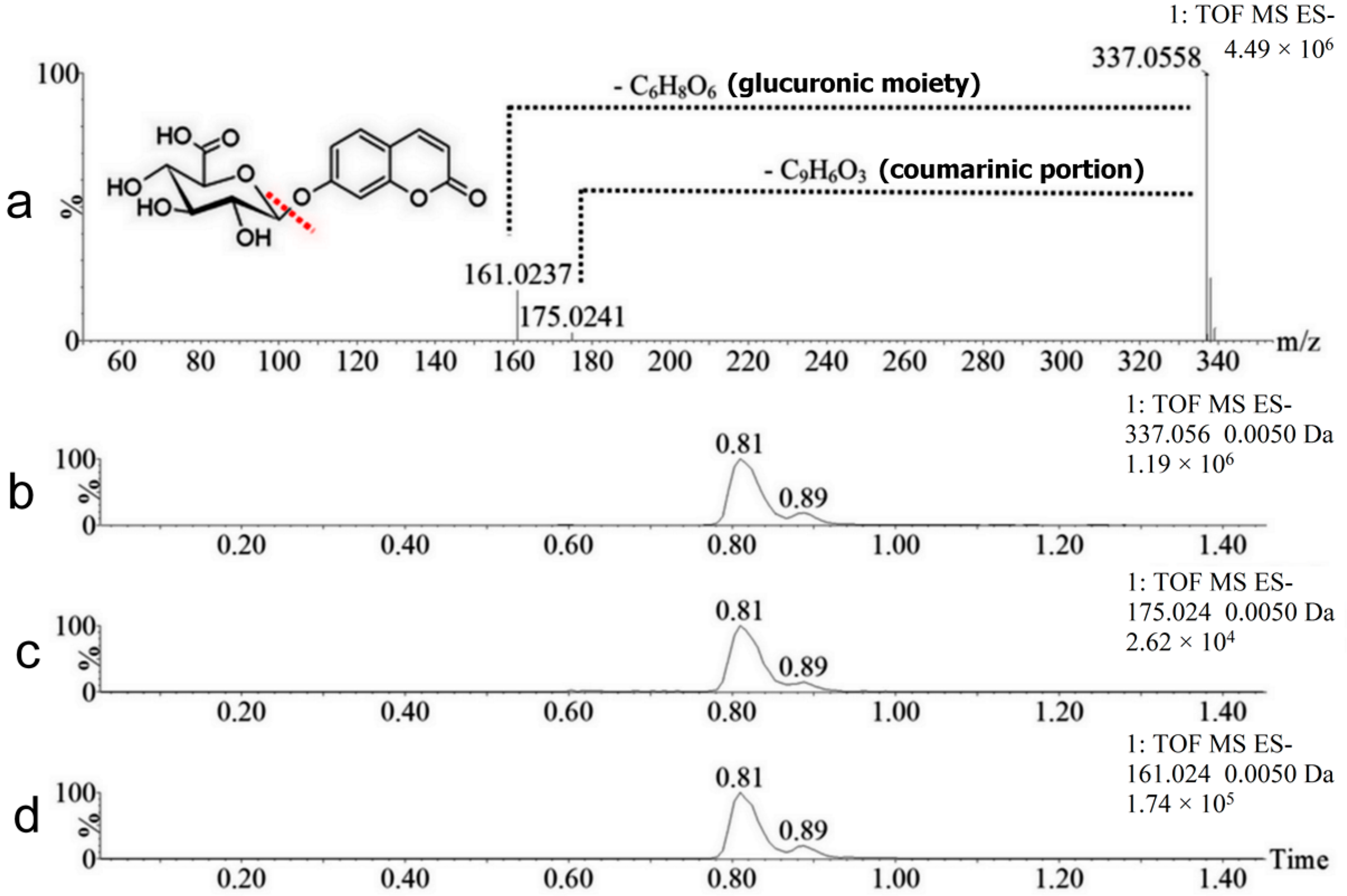
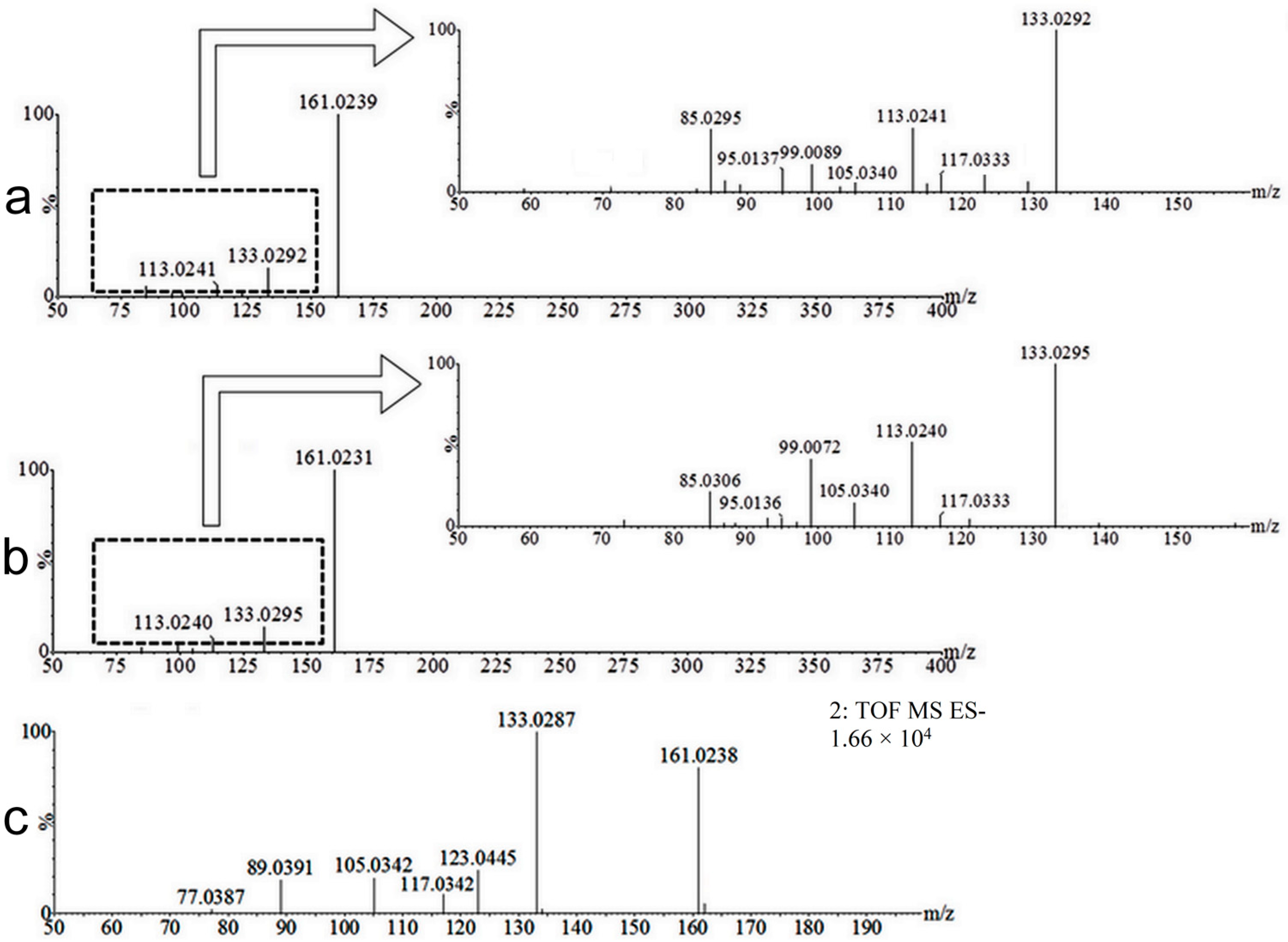
| No. | Neutral Mass (Da) | Ionization Mode | RT (min) | Biotransformation | Mass Difference to Coumarin 1 | Known Metabolite |
|---|---|---|---|---|---|---|
| M1 | 338.0638 | Positive and negative | 0.81 | Hydroxylation + conjugation with glucuronic acid | 192.0270 Da | Yes |
| M2 | 338.0638 | Positive and negative | 0.89 | Hydroxylation + conjugation with glucuronic acid | 192.0270 Da | No |
| M3 | 241.9885 | Positive and negative | 1.76 | Hydroxylation + sulfation | 95.9517 Da | Yes |
| M4 | 241.9885 | Positive and negative | 2.02 | Hydroxylation + sulfation | 95.9517 Da | No |
| M5 | 162.0317 | Negative | 2.34 | Hydroxylation | 15.9949 Da | Yes |
| M6 | 152.0473 | Negative | 1.28 | (Demethylation + hydroxylation) + two reductions | 6.0106 Da | Yes |
| M7 | 257.9834 | Negative | 1.57 | Two hydroxylations + sulfation | 111.9466 Da | No |
| M8 | 257.9834 | Negative | 2.05 | Two hydroxylations + sulfation | 111.9466 Da | No |
| M9 | 354.0587 | Negative | 0.82 | Two hydroxylations + conjugation with glucuronic acid | 208.0219 Da | No |
| M10 | 354.0587 | Negative | 0.95 | Two hydroxylations + conjugation with glucuronic acid | 208.0219 Da | No |
| M11 | 368.0743 | Negative | 0.91 | (Hydroxylation + methylation) + (hydroxylation + conjugation with glucuronic acid) | 222.0375 Da | No |
| M12 | 307.0514 | Positive | 2.47 | Conjugation with N-acetylcysteine | 161.0146 Da | No |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonart, L.P.; Gasparetto, J.C.; Pontes, F.L.D.; Cerqueira, L.B.; De Francisco, T.M.G.; Pontarolo, R. New Metabolites of Coumarin Detected in Human Urine Using Ultra Performance Liquid Chromatography/Quadrupole-Time-of-Flight Tandem Mass Spectrometry. Molecules 2017, 22, 2031. https://doi.org/10.3390/molecules22112031
Leonart LP, Gasparetto JC, Pontes FLD, Cerqueira LB, De Francisco TMG, Pontarolo R. New Metabolites of Coumarin Detected in Human Urine Using Ultra Performance Liquid Chromatography/Quadrupole-Time-of-Flight Tandem Mass Spectrometry. Molecules. 2017; 22(11):2031. https://doi.org/10.3390/molecules22112031
Chicago/Turabian StyleLeonart, Letícia Paula, João Cleverson Gasparetto, Flávia Lada Degaut Pontes, Letícia Bonancio Cerqueira, Thais Martins Guimarães De Francisco, and Roberto Pontarolo. 2017. "New Metabolites of Coumarin Detected in Human Urine Using Ultra Performance Liquid Chromatography/Quadrupole-Time-of-Flight Tandem Mass Spectrometry" Molecules 22, no. 11: 2031. https://doi.org/10.3390/molecules22112031




