Metabolites Produced by an Endophytic Phomopsis sp. and Their Anti-TMV Activity
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
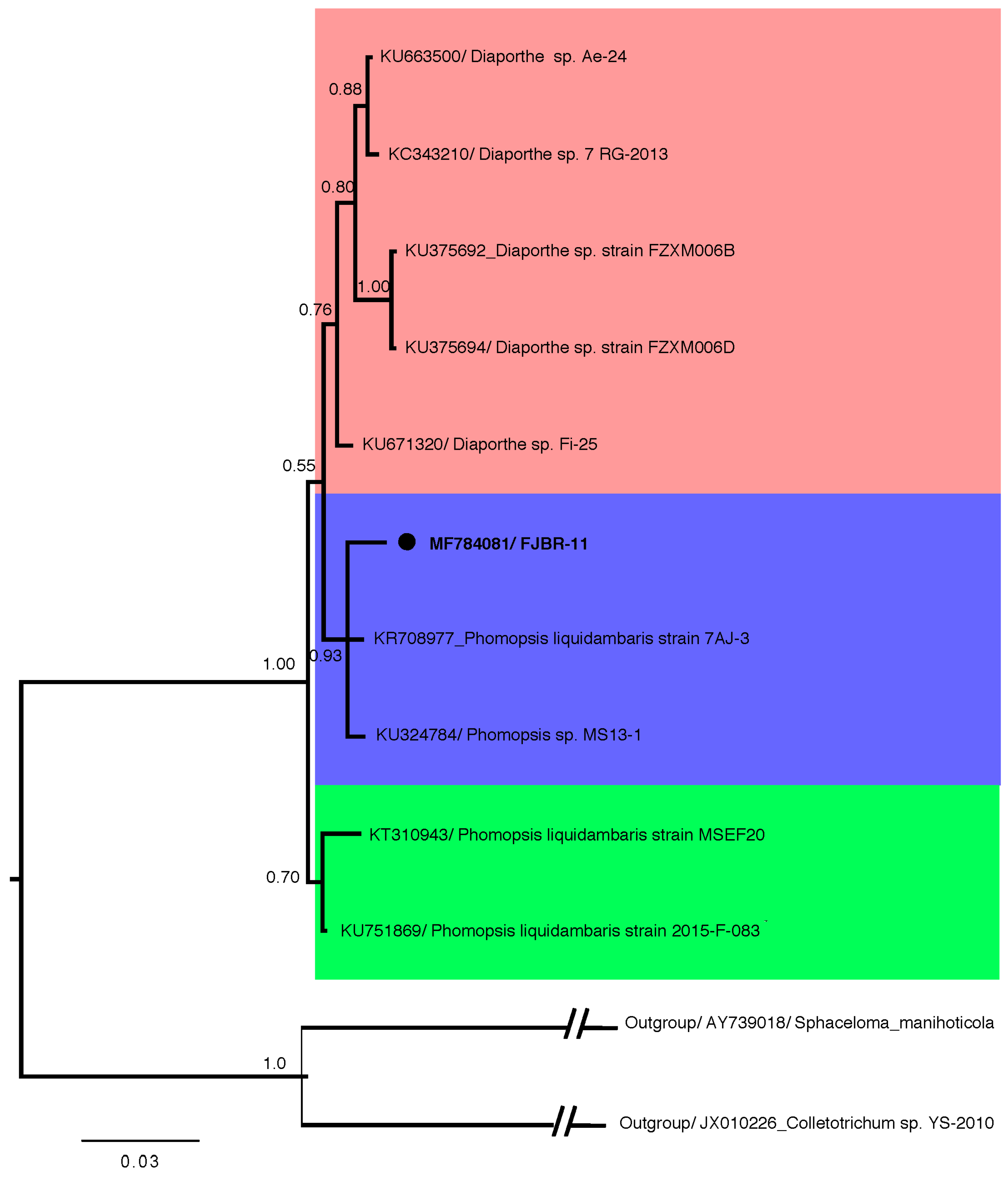
3.2. Fungal Strain
3.3. Fermentation, Extraction, and Isolation
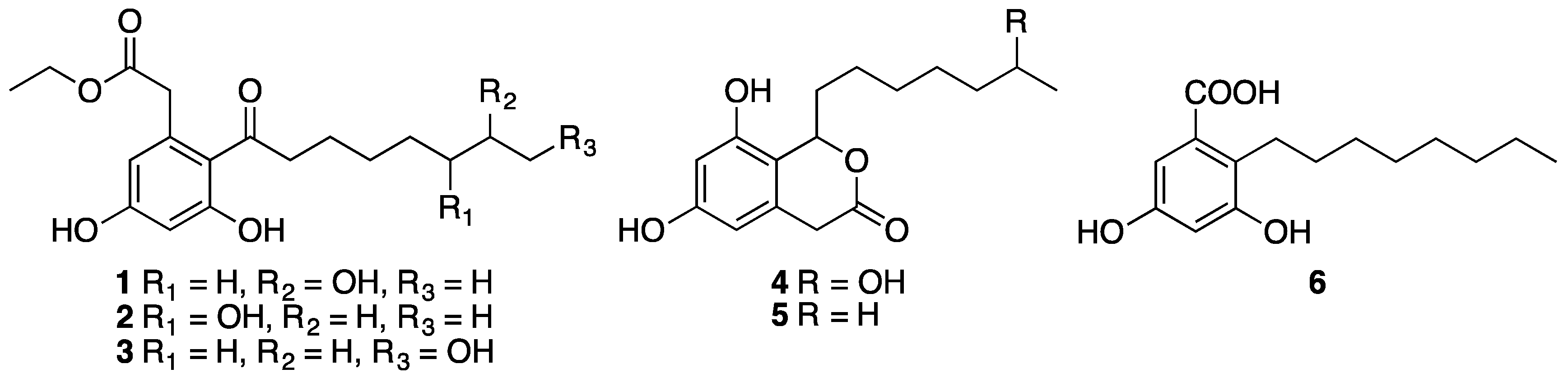
3.3.1. Dothiorelone A (1)
3.3.2. Dothiorelone B (2)
3.3.3. Dothiorelone C (3)
3.3.4. Dothiorelone H (4)
3.3.5. Cytosporone C (5)
3.3.6. Cytosporone U (6)
3.4. Determination of the Molecular Weight of the Polysaccharide
3.5. Anti-TMV Assay
3.5.1. Virus and Host Plant
3.5.2. Local-Lesion Assay
3.5.3. Leaf-Disc Method
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, L.; Zhang, L.; Li, D.; Wang, F.; Yu, D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, E1963–E1971. [Google Scholar] [PubMed]
- Ma, Y.; Mao, X.Y.; Huang, L.J.; Fan, Y.M.; Gu, W.; Yan, C.; Huang, T.; Zhang, J.X.; Yuan, C.M.; Hao, X.J. Diterpene alkaloids and diterpenes from Spiraea japonica and their anti-Tobacco mosaic virus activity. Fitoterapia 2016, 109, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Chen, F.; Wang, L.; Wang, Q. Design, synthesis, antiviral activity, and structure-activity relationships (SARs) of two types of structurally novel phenanthroindo/quinolizidine analogues. J. Agric. Food Chem. 2014, 62, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, P.; Li, Z.; Yin, J.; He, M.; Xue, W.; Chen, Z.; Song, B. Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(p-trifluoromethoxy)anilino]-4(3H)-quinazolinone moiety. J. Agric. Food Chem. 2013, 61, 9575–9582. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.W.; Gao, F.L.; Wang, F.R.; Chen, Q.J. Anti-TMV activity of malformin A1, a cyclic penta-peptide produced by an endophytic fungus Aspergillus Tubingensis FJBJ11. Int. J. Mol. Sci. 2015, 16, 5750–5761. [Google Scholar] [CrossRef] [PubMed]
- Abreu, L.M.; Costa, S.S.; Pfenning, L.H.; Takahashi, J.A.; Larsen, T.O.; Andersen, B. Chemical and molecular characterization of Phomopsis and Cytospora-like endophytes from different host plants in Brazil. Fungal Biol. 2012, 116, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, M.M.; Clardy, J. Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungus Phomopsis longicolla isolated from an endangered mint. J. Nat. Prod. 2001, 64, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Sterner, O.; Anke, T.; Gorzalczancy, S.; Martino, V.; Acevedo, C. Phomol, a new anti-inflammatory metabolite from an endophyte of the medicinal plant Erythrina crista-galli. J. Antibiot. 2004, 57, 559–563. [Google Scholar] [PubMed]
- Dai, J.; Krohn, K.; Flörke, U.; Gehle, D.; Aust, H.; Draeger, S.; Schulz, B.; Rheinheimer, J. Novel highly substituted biraryl ethers, phomosines D–G, isolated from the endophytic fungus Phomopsis sp. from Adenocarpus foliolosus. Eur. J. Org. Chem. 2005, 23, 5100–5105. [Google Scholar]
- Dai, J.; Krohn, K.; Gehle, D.; Kock, I.; Flörke, U.; Aust, H.; Draeger, S.; Schulz, B.; Rheinheimer, J. New oblongolides isolated from the endophytic fungus Phomopsis sp. from Melilotus dentate from the shores of the Baltic Sea. Eur. J. Org. Chem. 2005, 18, 4009–4016. [Google Scholar]
- Pornpakakul, S.; Roengsumran, S.; Deechangvipart, S.; Petsom, A.; Muangsin, N.; Ngamrojnavanich, N.; Sriubolmas, N.; Chaichit, N.; Ohta, T. Diaporthichalasin, a novel CYP3A4 inhibitor from an endophytic Diaporthe sp. Tetrahedron Lett. 2007, 48, 651–655. [Google Scholar]
- Sommart, U.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Towatana, N.H.; Graidist, P.; Hajiwangoh, Z.; Sakayaroj, J. A cyclohexenone derivative from diaporthaceous fungus PSU-H2. Arch. Pharm. Res. 2009, 32, 1227–1231. [Google Scholar] [PubMed]
- Takahashi, S.; Akita, Y.; Nakamura, T.; Koshino, H. Total synthesis of curvulone B and a proposed structure for dothiorelone B; determination of the configuration of curvulone B and structural revision of phomopsin A. Tetrahedron Asymmetry 2012, 23, 952–958. [Google Scholar]
- Du, X.P.; Su, W.J. Two new polyketides from mangrove endophytic fungus Dothiorella sp. Chem. Nat. Compd. 2014, 50, 214–216. [Google Scholar]
- Brady, S.F.; Wagenaar, M.M.; Singh, M.P.; Janso, J.E.; Clardy, J. The cytosporones, new octaketide antibiotics isolated from an endophytic fungus. Org. Lett. 2000, 2, 4043–4046. [Google Scholar] [PubMed]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Guan, H.; Edrada, R.; Müller, W.E.G.; Bayer, M.; Lin, W.; Wu, J.; et al. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorg. Med. Chem. 2009, 17, 7362–7367. [Google Scholar] [PubMed]
- Wu, Q.; Guo, Y.; Guo, Z.K.; Chu, Y.L.; Wang, T.; Tan, R.X. Two new cytosporones from the culture of endophytic Phomopsis sp. Chem. Nat. Compd. 2013, 48, 938–941. [Google Scholar]
- Leal, J.A.; Jiménez-Barbero, J.; Gómez-Miranda, B.; Parra, E.; Prieto, A.; Bernabé, M. Structural investigation of cell-wall polysaccharides from Neosartorya: Relationships with their putative anamorphs of Aspergillus. Carbohydr. Res. 1995, 273, 255–262. [Google Scholar] [PubMed]
- Corsaro, M.M.; De Castro, C.; Evidente, A.; Lanzetta, R.; Molinaro, A.; Mugnai, L.; Parrilli, M.; Surico, G. Chemical structure of two phytotoxic exopolysaccharides produced by Phomopsis foeniculi. Carbohydr. Res. 1998, 308, 349–357. [Google Scholar] [PubMed]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005, 21, 2649–2659. [Google Scholar] [PubMed]
- Zhao, L.; Hao, X.; Wu, Y. Inhibitory effect of polysaccharide peptide (PSP) against Tobacco mosaic virus (TMV). Int. J. Biol. Macromol. 2015, 75, 474–478. [Google Scholar] [PubMed]
- Keen, N.T.; Wang, M.C.; Bartnicki-Garcia, S.; Zentmyer, G.A. Phytotoxicity of mycolaminarans—β-1,3-glucans from Phytophthora spp. Physiol. Plant Pathol. 1975, 7, 91–97. [Google Scholar]
- Woodward, J.R.; Keane, P.J.; Stone, B.A. Structures and properties of wilt-inducing polysaccharides from Phytophthora species. Physiol. Plant Pathol. 1980, 16, 439–454. [Google Scholar]
- Edgar, R.C. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [PubMed]
- Xia, X. DAMBE6: New tools for microbial genomics, phylogenetics, and molecular evolution. J. Hered. 2017, 108, 431–437. [Google Scholar] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Method 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, X.H.; Dong, J.H.; Sang, P.; Fang, X.; Di, Y.T.; Zhang, Z.K.; Hao, X.J. Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides Benn. J. Agric. Food Chem. 2009, 57, 6590–6595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Fan, H.J.; Li, Y.; Shi, Z.L.; Pan, Y.; Lu, C.P. Development of a multi-mimotope peptide as a vaccine immunogen for infectious bursal disease virus. Vaccine 2007, 25, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–6 and the exopolysaccharide are available from the authors. |

| Compound | Inhibition Rate (%) | |
|---|---|---|
| Local Lesion Assay | Leaf-Disc Method | |
| 1 | <10 | <10 |
| 2 | <10 | 10.9 ± 1.1 |
| 3 | <10 | 12.7 ± 1.0 |
| 4 | 11.3 ± 1.2 | 29.6 ± 2.1 |
| 5 | 22.9 ± 2.1 | 31.9 ± 2.0 |
| 6 | 39.2 ± 2.2 | 80.0 ± 3.0 |
| Ningnanmycin | 90.3 ± 3.4 | 91.4 ± 3.8 |
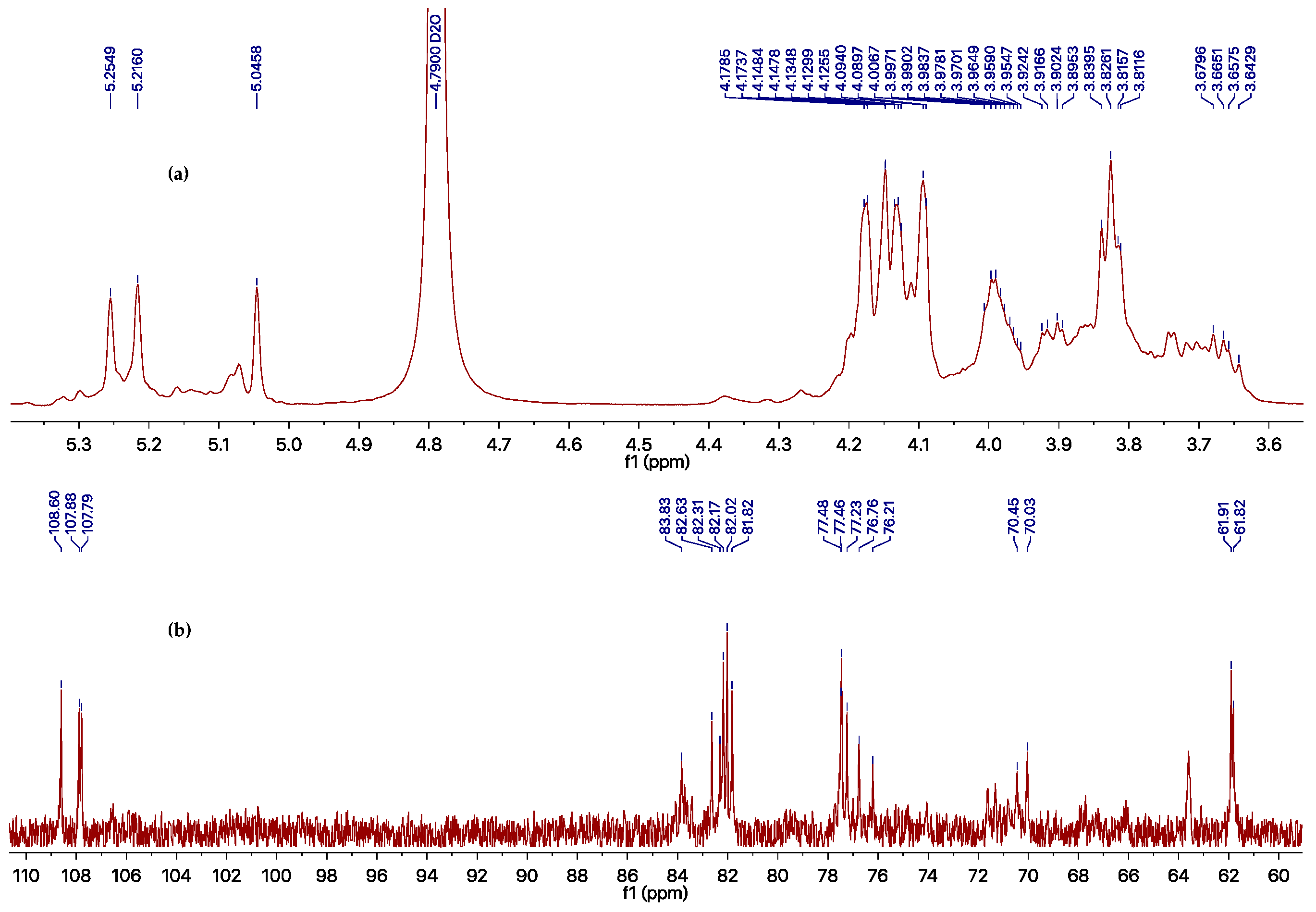
| Position | Unit A | Unit B | Unit C | |||
|---|---|---|---|---|---|---|
| δC (ppm) | δH (ppm) | δC (ppm) | δH (ppm) | δC (ppm) | δH (ppm) | |
| 1 | 107.8 | 5.25 | 107.9 | 5.22 | 108.6 | 5.05 |
| 2 | 82.2 | 4.17 | 82.0 | 4.18 | 81.8 | 4.15 |
| 3 | 77.5 | 4.09 | 77.5 | 4.13 | 77.2 | 4.13 |
| 4 | 83.8 | 4.09 | 82.3 | 4.15 | 82.6 | 4.13 |
| 5 | 70.4 | 4.02–3.95 | 76.2 | 4.02–3.95 | 76.8 | 4.02–3.95 |
| 6 | 70.0 | 3.93–3.89, 3.69–3.64 | 61.9 | 3.85–3.80 | 61.8 | 3.85–3.80 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Q.-W.; Fang, P.-H.; Ni, J.-C.; Gao, F.; Chen, Q.-J. Metabolites Produced by an Endophytic Phomopsis sp. and Their Anti-TMV Activity. Molecules 2017, 22, 2073. https://doi.org/10.3390/molecules22122073
Tan Q-W, Fang P-H, Ni J-C, Gao F, Chen Q-J. Metabolites Produced by an Endophytic Phomopsis sp. and Their Anti-TMV Activity. Molecules. 2017; 22(12):2073. https://doi.org/10.3390/molecules22122073
Chicago/Turabian StyleTan, Qing-Wei, Pei-Hua Fang, Jian-Cheng Ni, Fangluan Gao, and Qi-Jian Chen. 2017. "Metabolites Produced by an Endophytic Phomopsis sp. and Their Anti-TMV Activity" Molecules 22, no. 12: 2073. https://doi.org/10.3390/molecules22122073





