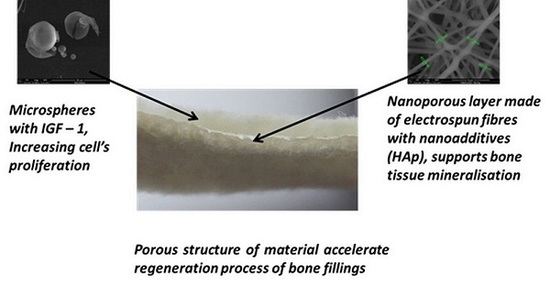
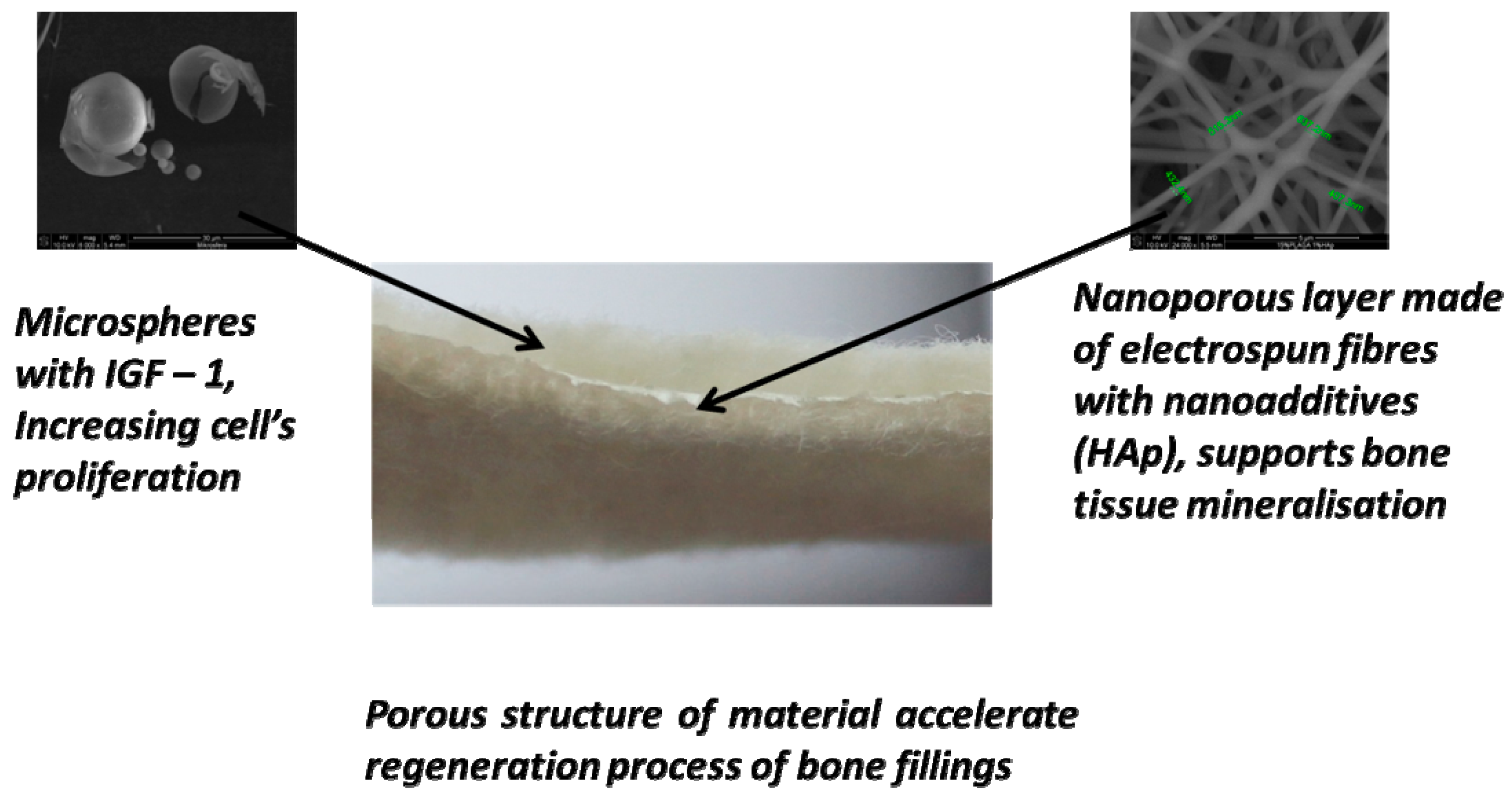
Biological Properties of Low-Toxicity PLGA and PLGA/PHB Fibrous Nanocomposite Implants for Osseous Tissue Regeneration. Part I: Evaluation of Potential Biotoxicity
Abstract
:1. Introduction
2. Materials
2.1. Structure of the Designed Bone Implant
2.2. Polymers Characteristic
2.3. Fibres Characteristic
2.4. Characteristic of the Macroporous Layer
3. Methodology
3.1. Assessment of Technical Properties
3.2. Biological Studies
3.2.1. Cytotoxicity Test
3.2.2. Genotoxicity Test
3.2.3. Chronic Toxicity Studies after Implantation
Surgical Procedures
Blood Test
Macroscopic Post-MortemExaminations
Histological Evaluation
4. Results
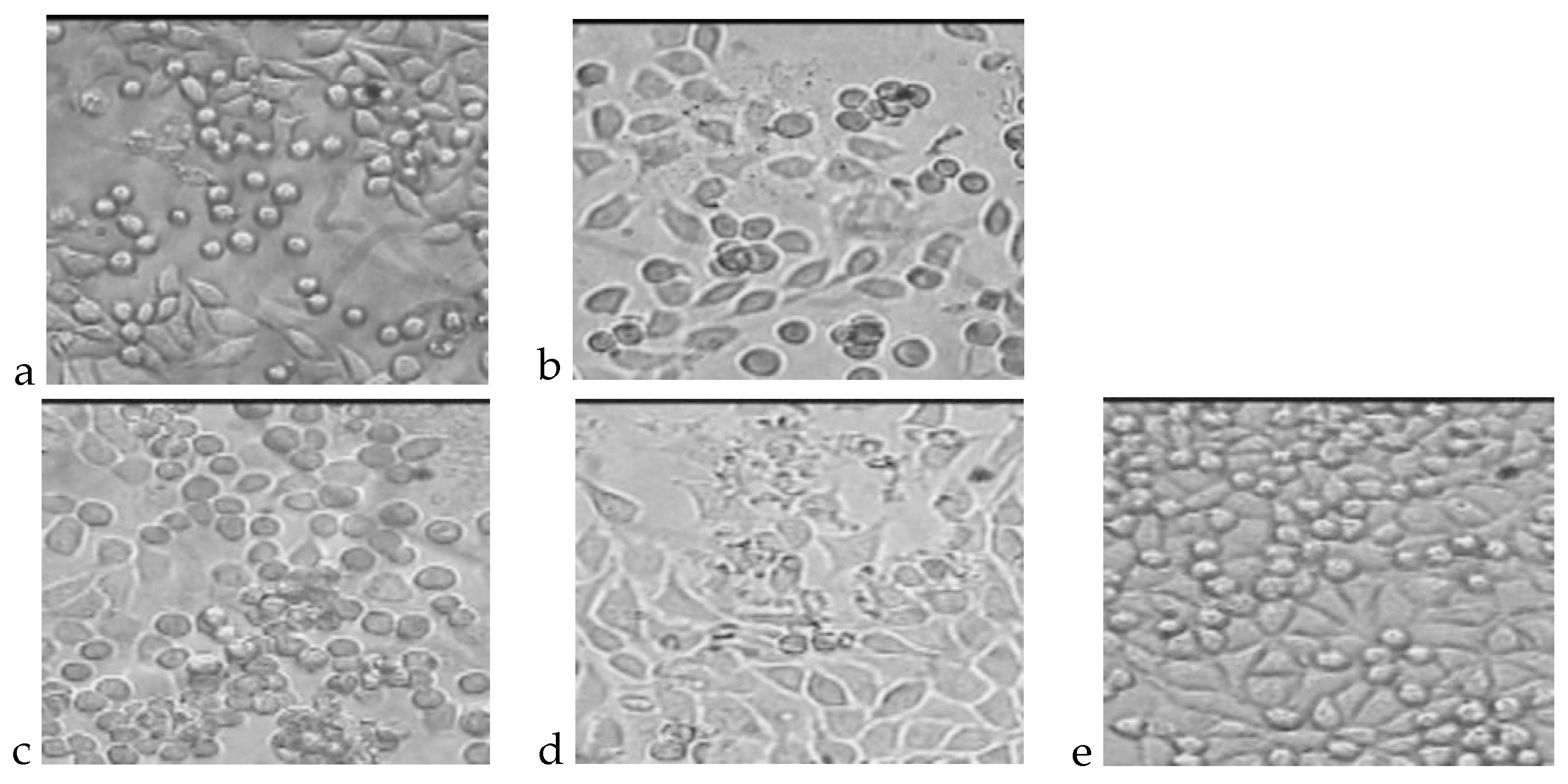
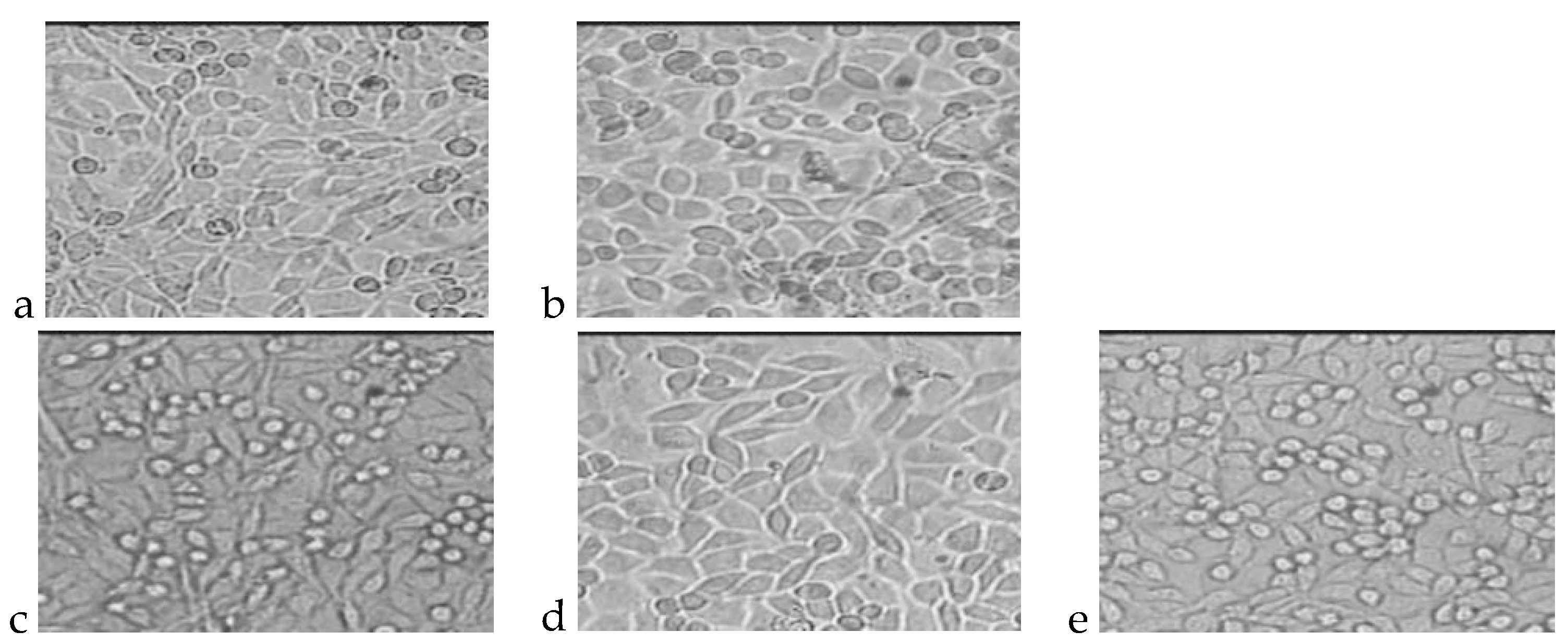
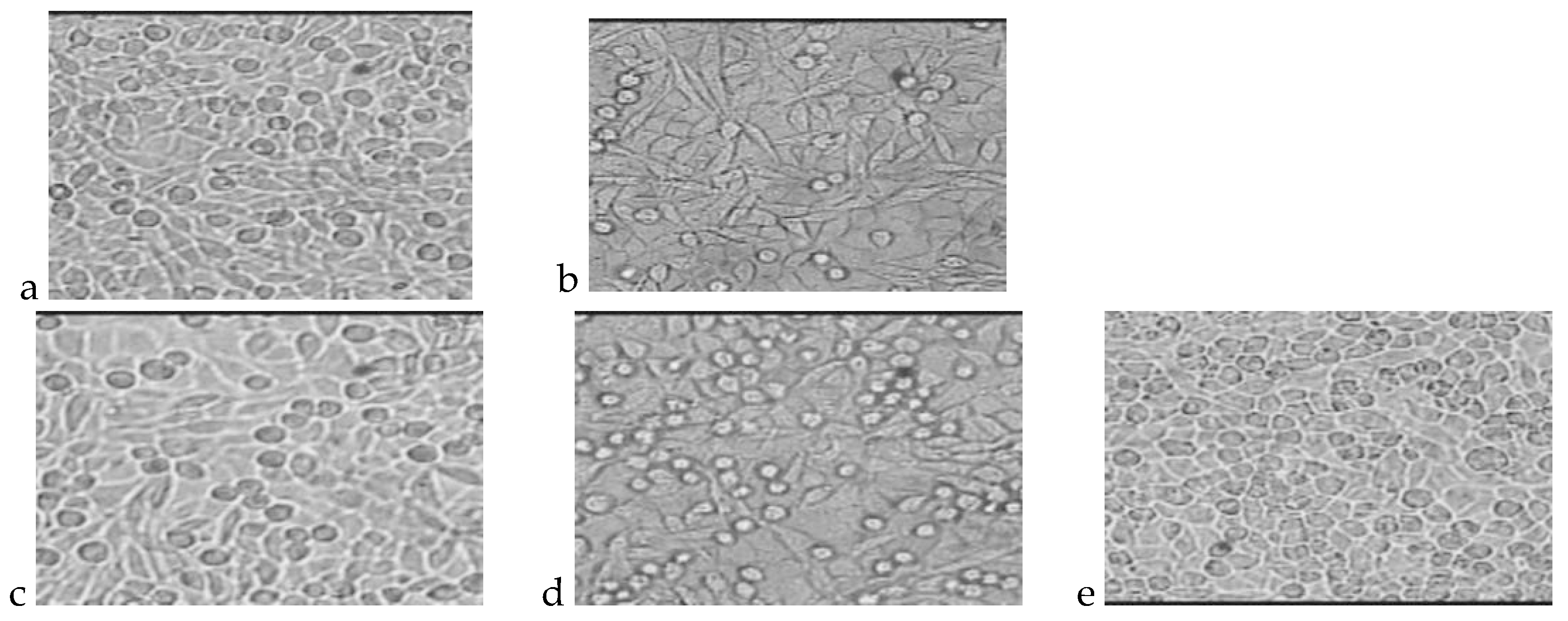
4.1. Cytotoxicity Test
4.2. Genotoxicity Test
4.3. Chronic toxicity Studies after Implantation
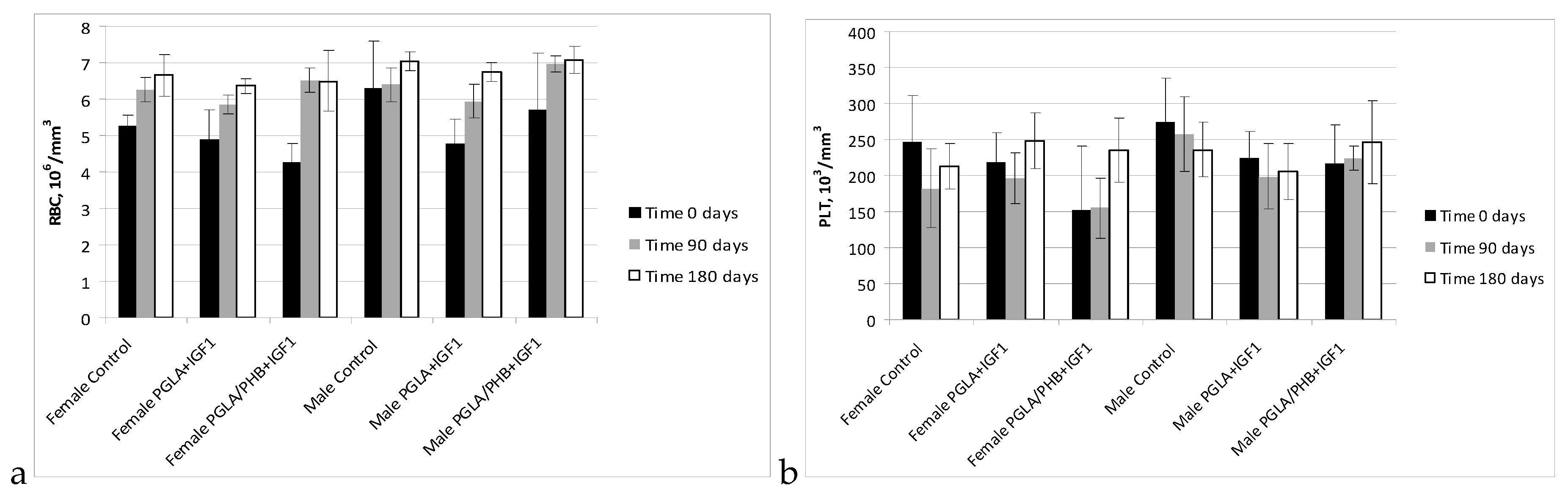
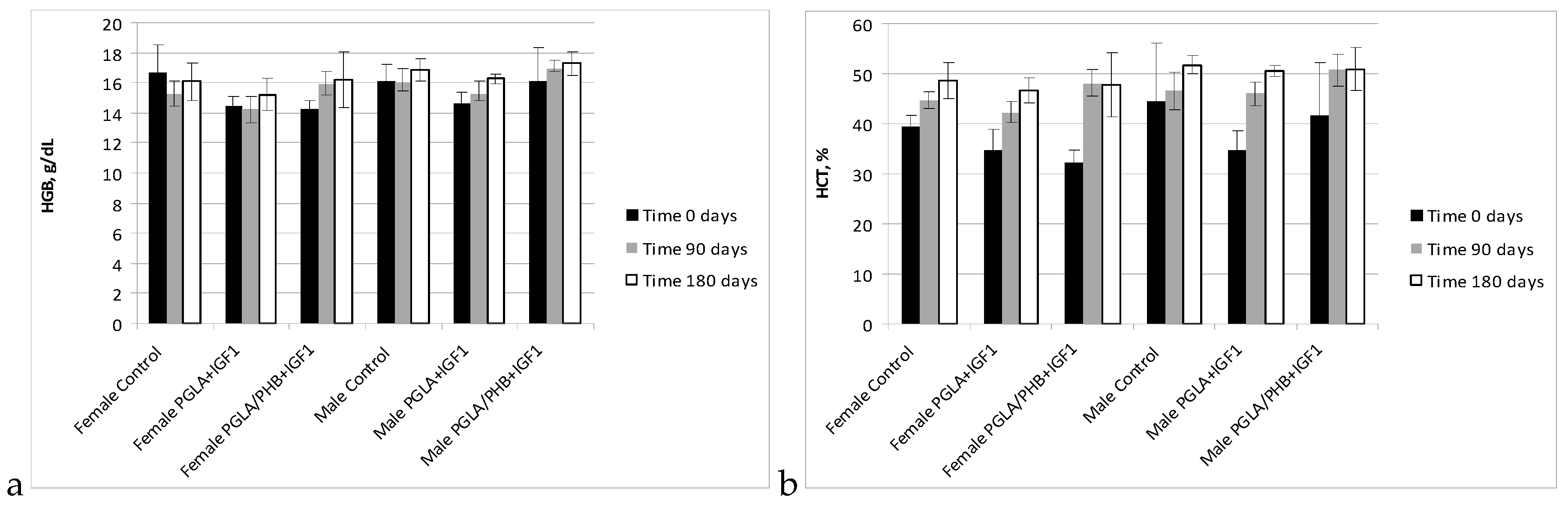
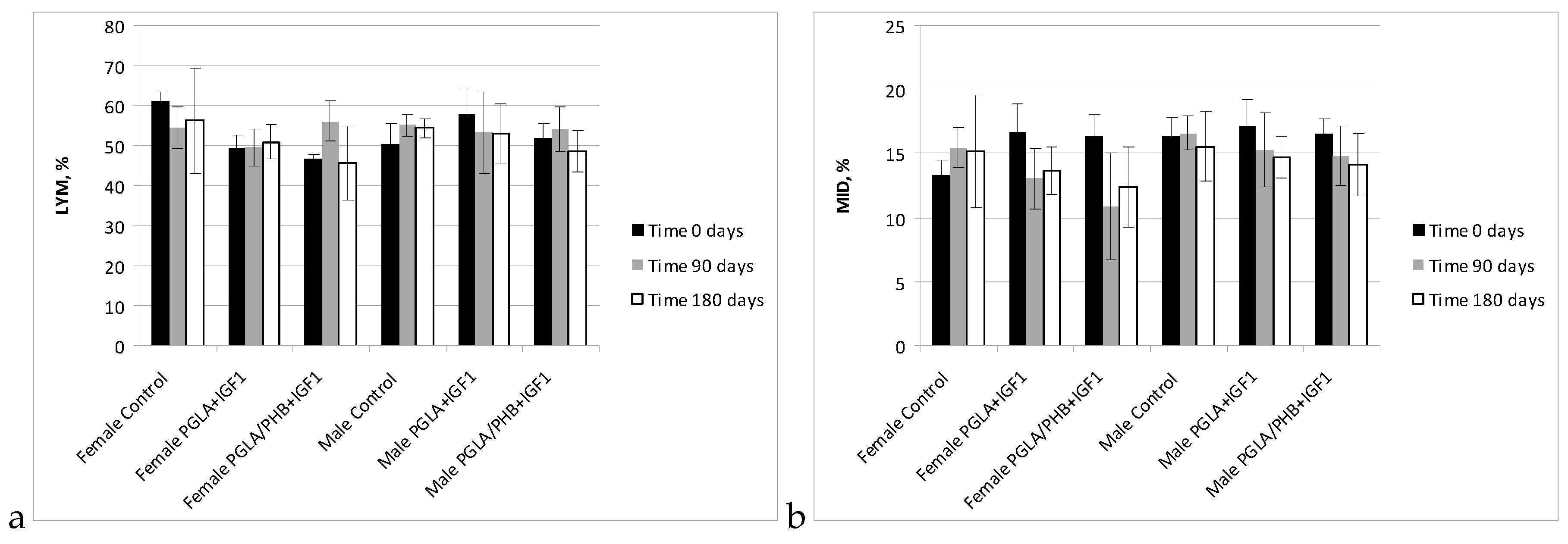
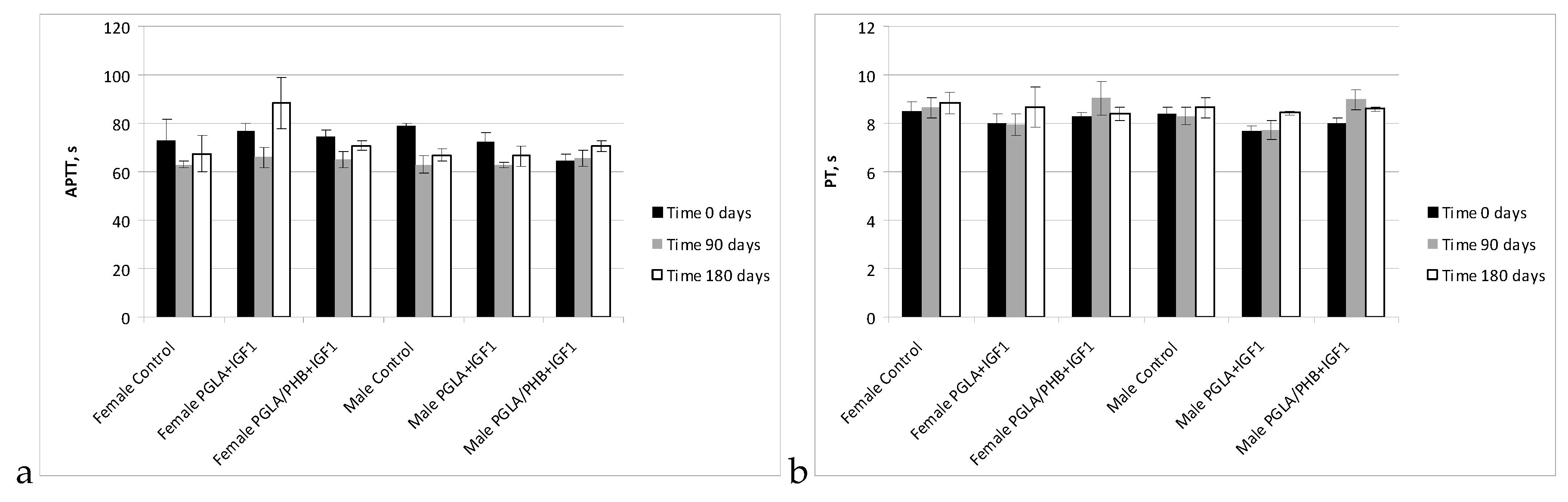
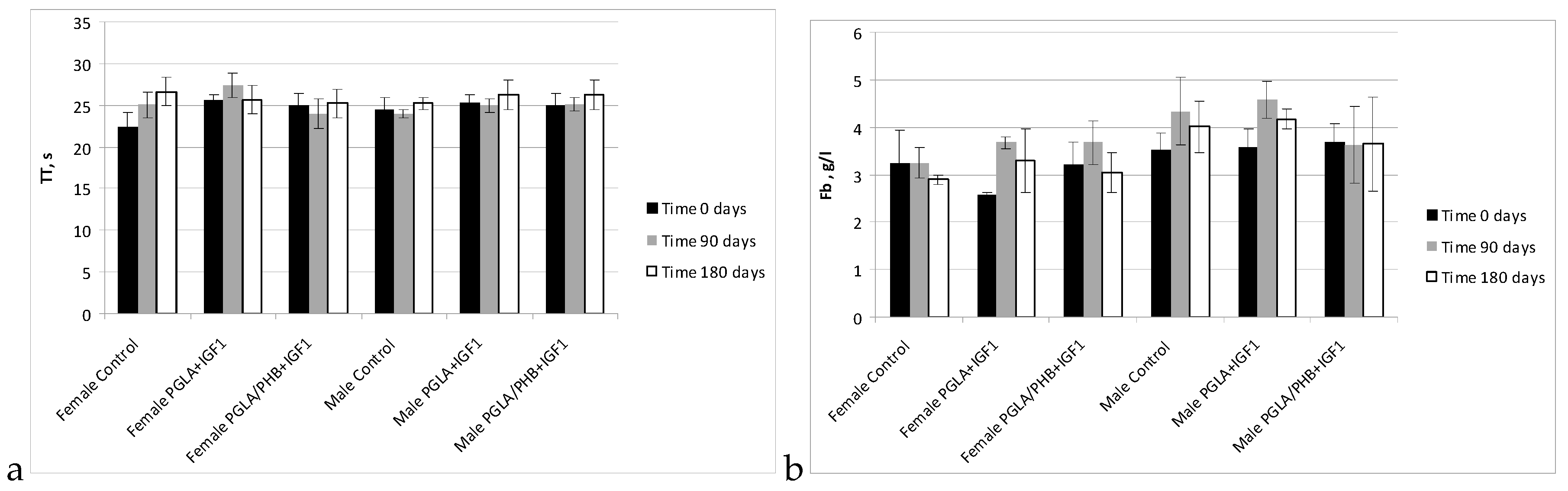
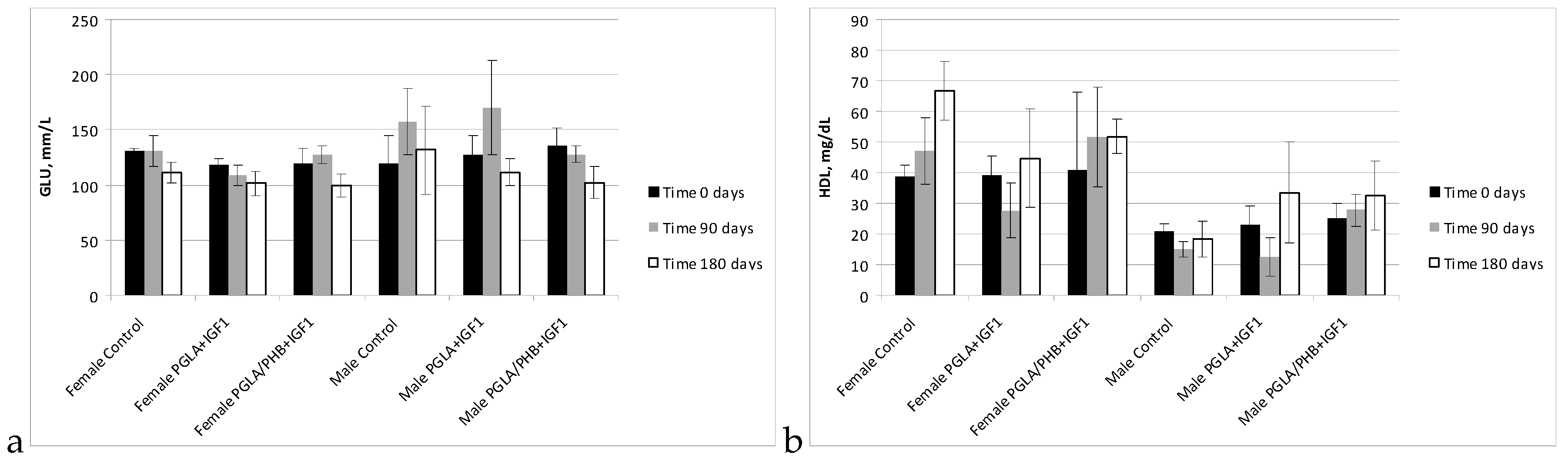
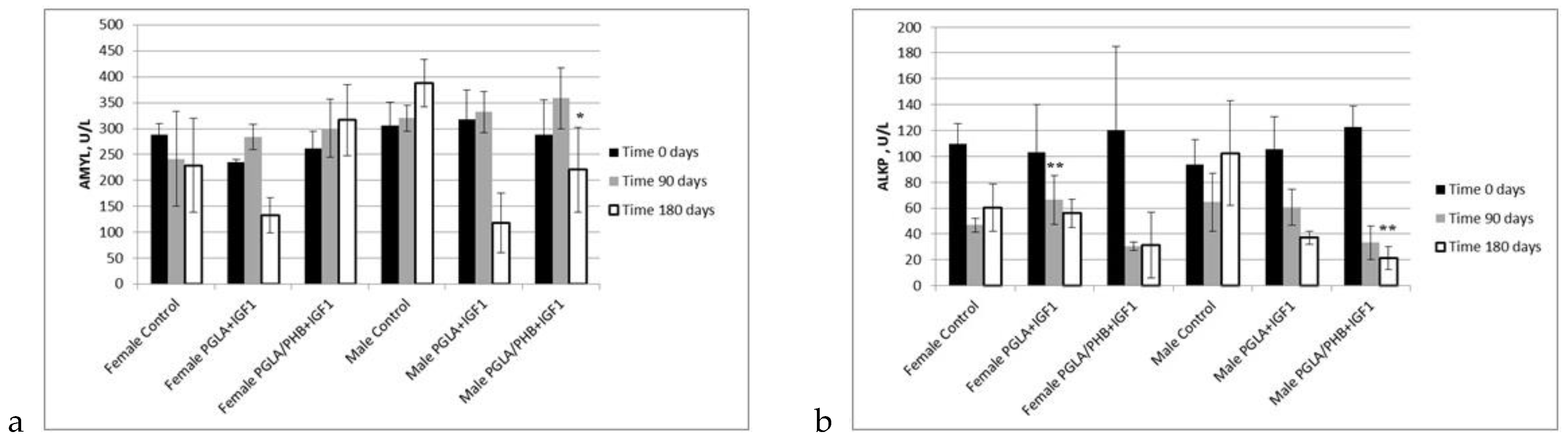
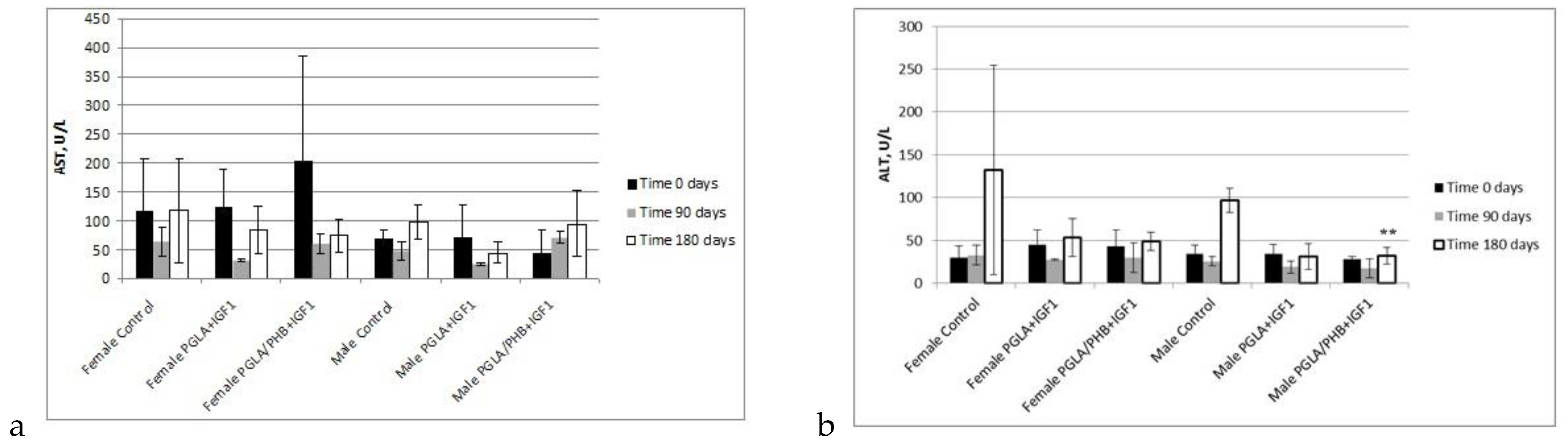
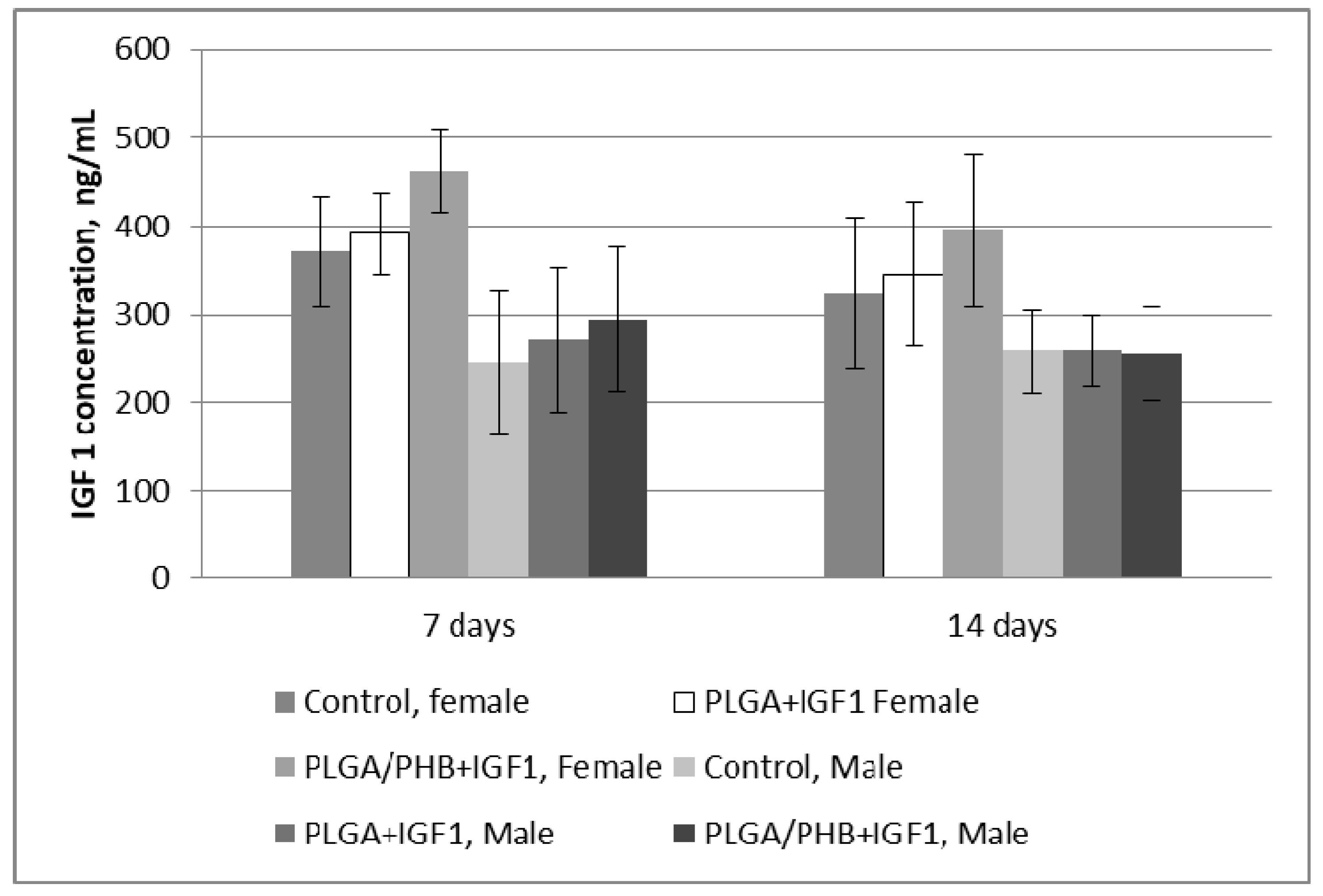
Blood Parameters
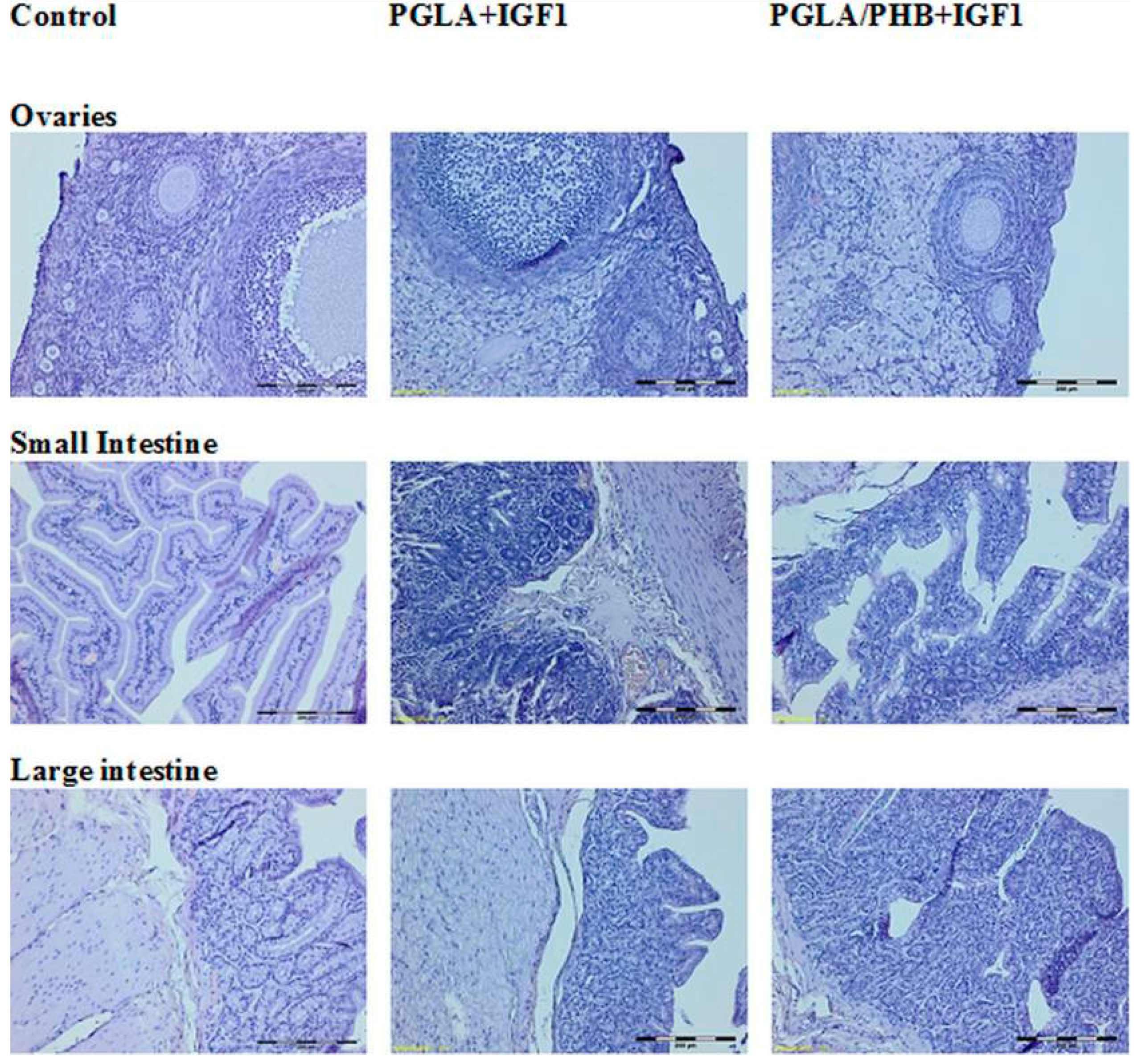
4.4. Macroscopic and Histological Research
5. Discussion
6. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bastioli, C. Handbook of Biodegradable Polymers; Rapra Technology Limited: Shawbury, UK, 2005. [Google Scholar]
- Li, Y.; Yang, S. Effects of Three-dimensional Scaffolds on Cell Organization and Tissue Development. Biotechnol. Bioprocess Eng. 2001, 6, 311–325. [Google Scholar] [CrossRef]
- Pietrucha, K.; Safandowska, M. Effect of fish collagen modification on its thermal and rheological properties. Int. J. Biol. Macromol. 2013, 53, 32–37. [Google Scholar]
- Draczyński, Z. Synthesis and solubility properties of chitin acetate/butyrate copolymers. J. Appl. Polym. Sci. 2011, 122, 175–182. [Google Scholar] [CrossRef]
- Boguń, M.; Krucińska, I.; Komisarczyk, A.; Mikołajczyk, T.; Błażewicz, M.; Stodolak-Zych, E.; Menaszek, E.; Ścisłowska-Czarnecka, A. Fibrous Polymeric Composites Based on Alginate Fibres and Fibres Made of Poly-ε-caprolactone and Dibutyryl Chitin for Use in Regenerative Medicine. Molecules 2013, 18, 3118–3136. [Google Scholar] [CrossRef] [PubMed]
- Pamuła, E. Biomaterials for Tissue Engineering. Studies of the Evolution of the Structure and Biological Properties of Aliphatic Polyesters; Prace Monograficzne AGH Kraków, Wydział Inżynierii Materiałowej i Ceramiki: Krakow, Poland, 2008; Volume 1. (In Polish) [Google Scholar]
- Auras, R.A.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons: Etobicoke, ON, Canada, 2011. [Google Scholar]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Dobrzyński, P.; Bero, M.; Kasperczyk, J. A Process for Preparing Bioresorbable Polymers. Patent No. PL 191 846 B1, 2000. [Google Scholar]
- Smola, A.; Dobrzyński, P.; Pastusiak, M.; Sobota, M.; Kasperczyk, J. New semicrystallinebioresorbable materials with shape memory properties. Eng. Biomater. 2009, 12, 82–87. [Google Scholar]
- Gilding, D.K.; Reed, A.M. Biodegradable polymers for use in surgery. Polyglycolic/poly (lactic acid) homo and copolymers. Polymer 1979, 20, 1459–1464. [Google Scholar] [CrossRef]
- Kreiser-Saunders, I.; Kricheldorf, H.R. Polylactones. Part 39. Zn lactate-catalyzed copolymerization of l-lactide with glycolide or ε-caprolactone. Macromol. Chem. Phys. 1998, 199, 1081–1087. [Google Scholar]
- Winship, K.A. Toxicity of tin and its compounds. Adv. Drug React. Acute Poisoning Rev. 1988, 7, 19–38. [Google Scholar]
- Dobrzyński, P.; Kasperczyk, J.; Bero, M. Application of calcium acetylacetonate to the polymerization of glycolide and copolymerization of glycolide with ε-caprolactone and l-lactide. Macromolecules 1999, 32, 4735–4737. [Google Scholar] [CrossRef]
- Dobrzyński, P.; Kasperczyk, J.; Janeczek, H.; Bero, M. Synthesis of Biodegradable Copolymers with the Use of Low Toxic Zirconium Compounds. 1. Copolymerization of Glycolide with l-Lactide Initiated by Zr(AcAc)4. Macromolecules 2001, 34, 5090–5098. [Google Scholar] [CrossRef]
- Dobrzyński, P. Initiation of l-lactide polymerization carried out with zirconium (IV) acetylacetonate. J. Polym. Sci. Part A 2004, 42, 1886–1900. [Google Scholar] [CrossRef]
- Dobrzyński, P. Synthesis of Biodegradable Copolymers with Low-Toxicity Zirconium Compounds. II. Copolymerization of Glycolide with ε-Caprolactone Initiated by Zirconium (IV) Acetylacetonate and Zirconium (IV) Chloride. J. Polym. Sci. Part A 2002, 40, 1379–1394. [Google Scholar] [CrossRef]
- Żywicka, B.; Zaczyńska, E.; Czarny, A.; Dobrzyński, P.; Kowalczuk, M.; Struszczyk, M.H. Cytotoxicity of new biodegradable fibrous-forming copolymers with low-toxicity zirconium compounds for tissue repair and regeneration. In Proceedings of the 18th European Conference on Orthopaedics “The Summit of European Othopaedic Research”, Davos, Switzerland, 30 June–2 July 2010. [Google Scholar]
- Sharma, B.; Elisseeff, J.H. Engineering Structurally Organized Cartilage and Bone Tissues. Ann. Biomed. Eng. 2004, 32, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Limmahakhuna, S.; Oloyedea, A.; Sitthiseripratipb, K.; Xiaoa, Y.; Yan, C. 3D-printed cellular structures for bone biomimetic implants. Addit. Manuf. 2017, 15, 93–101. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Waller, M.A.; Yannas, I.V.; Gibson, L.J.; Prendergast, P.J. The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol. Health Care 2007, 15, 3–17. [Google Scholar] [PubMed]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric control of cell life and death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E.; Folkman, J. Mechanical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: Role of extracellular matrix. J. Cell Biol. 1989, 109, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Regulation of cell functions by micropattern-immobilized biosignal molecules. Nanotechnology 1998, 9, 200–204. [Google Scholar] [CrossRef]
- Campbell, P.; Miller, E.; Fisher, G.; Walker, L.; Weiss, L. Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell organization. Biomaterials 2005, 26, 6762–6770. [Google Scholar] [CrossRef] [PubMed]
- Ker, E.; Nain, A.; Weiss, L.; Wang, J.; Suhan, J.; Amon, C.; Campbell, P. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials 2011, 32, 8097–8107. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Fisher, G.; Weiss, L.; Walker, L.; Campbell, P. Dose-dependent cell growth in response to concentration modulated patterns of FGF-2 printed on fibrin. Biomaterials 2006, 27, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Jang, J.H.; Jeon, E.; Kang, W.; Lee, S.; Won, J.E.; Kim, H.W.; Wall, I. Administration of growth factors for bone regeneration. Regen. Med. 2012, 7, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, Q.; Shao, X.; Zhang, T.; Xue, C.; Shi, S.; Zhao, D.; Lin, Y. IGF-1 promotes angiogenesis in endothelial cells/adipose-derived stem cells co-culture system with activation of PI3K/Akt signal pathway. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zou, X.; Zhang, R.X.; Pi, C.J.; Wu, N.; Yin, L.J.; Deng, Z.L. IGF1 potentiates BMP9-induced osteogenic differentiation in mesenchymal stem cells through the enhancement of BMP/Smad signaling. BMB Rep. 2016, 49, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, M.; Korczyński, M.; Dobrzyński, M.; Zawisza, K.; Mikulewicz, M.; Karuga-Kuźniewska, E.; Żywicka, B.; Rybak, Z.; Wiglusz, R.J. Cytotoxicity evaluation of high-temperature annealed nanohydroxyapatite in contact with fibroblast cells. Materials 2017, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- CEN. PN-EN 10993–3:2008 Biological Evaluation of Medical Devices—Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity; CEN-CENELEC Management Centre: Brussels, Belgium, 2008. [Google Scholar]
- ISO. PN-EN ISO 10993–1:2010. Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Biodegradable Fibrous Product; Krucinska, I. (Ed.) Lodz University of Technology: Lodz, Poland, 2014. (In Polish) [Google Scholar]
- Kowalczuk, M. Anionic Ring-Opening Polymerization for Synthetic Analogues of Aliphatic Biopolyesters. Polym. Sci. Ser. A 2009, 51, 1229–1232. [Google Scholar] [CrossRef]
- Packaging Materials from Compostable Polymers; Kowalczuk, M. (Ed.) Packaging Research Institute: Warsaw, Poland, 2012. (In Polish) [Google Scholar]
- Krucińska, I.; Chrzanowska, O.; Boguń, M.; Kowalczuk, M.; Dobrzański, P. Fabrication of PLGA/HAP and PLGA/PHB/HAP fibrous nanocomposite materials for osseous tissue regeneration. AUTEX Res. J. 2014, 14, 2014. [Google Scholar] [CrossRef]
- ISO. PN-EN ISO 11137–2 Sterilization of Health Care Products. Radiation. Establishing the Sterilization Dose; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- ISO. PN-ISO 1973:2011 Textile Fibres—Determination of Linear Density—Gravimetric Method and Vibroscope Method; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- ISO. EN ISO 5079:1999 Textile Fibres—Determination of Breaking Force and Elongation at Break of Individual Fibres; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- PN-87/P-04761.05 Testing Methods of Textile Raw Materials—Chemical Fibers—Determination of Linear Shrinkage; Polish Committee for Standardization: Warsaw, Poland, 1987. (In Polish)
- Rabiej, M.; Rabiej, S. Analiza rentgenowskich krzywych dyfrakcyjnych polimerów za pomocą programu komputerowego WAXSFIT; ATH: Bielsko-Biała, Poland, 2006. (In Polish) [Google Scholar]
- EN 29073–1:1994 Textile—Test Methods for Nonwovens—Determination of Surface Mass; CEN-CENELEC Management Centre: Brussels, Belgium, 1994.
- EN ISO 9073–2: 2002 Textile—Test Methods for Nonwovens—Part 2: Determination of Thickness; ISO: Geneva, Switzerland, 2002.
- EN ISO 9237:1998 Textiles—Determination of the Permeability of Fabrics to Air; ISO: Geneva, Switzerland, 1998.
- EN ISO 139:2005 Textiles—Standard Atmospheres for Conditioning and Testing; ISO: Geneva, Switzerland, 2005.
- AutoPore Manual; Micromeritics: Aachen, Germany, 2005.
- PN-EN ISO 10993–5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009.
- PN-EN ISO 10993–12:2009 Biological Evaluation of Medical Devices. Sample Preparation and Reference Materials; ISO: Geneva, Switzerland, 2009.
- PN-EN 10993–11:2009 Biological Evaluation of Medical Devices—Part 11: Tests for Systemic Toxicity; ISO: Geneva, Switzerland, 2009.
- PN-EN ISO 10993–2:2006 Biological Evaluation of Medical Devices—Part 2: Animal Welfare Requirements; ISO: Geneva, Switzerland, 2006.
- PN-EN ISO 10993–4:2006 Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood; ISO: Geneva, Switzerland, 2006.
- Manual of Automatic Haematology Analyzer for the Veterinary PE-6800; CHS Medica: Gdynia, Poland, 2005. (In Polish)
- Methodology of ALPHA DIAGNOSTICS®; Alpha Diagnostics Sp.z.o.o.: Warszawa, Poland, 2011. (In Polish)
- Dembińska-Kieć, A.; Naskalski, J.W. Laboratory Diagnostic with Elements of Clinical Biochemistry; Elsevier Urban &Partner: Amsterdam, The Netherlands, 2009. (In Polish) [Google Scholar]
- Manual of CoagulometerCoagChrom 3003 (CC-3003); Bio-Ksel Sp.zo.o: Grudziądz, Poland, 2011. (In Polish)
- Raszeja-Specht, A. The Research of the Hemostasis in Laboratory Practice; Bio-Ksel: Grudziądz, Poland, 2008; pp. 1–87. (In Polish) [Google Scholar]
- Vieira, I.L.B.F.; Souza, D.C.P.; Coelho, L.S.; Chen, L.; Guillo, L.A. In vitro mutagenicity and blood compatibility of paclitaxel and curcumin in poly (dl-lactide-co-glicolide) films. Toxicol. In Vitro 2013, 27, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Gábelová, A.; El Yamani, N.; Alonso, T.I.; Buliaková, B.; Srančíková, A.; Bábelová, A.; Pran, E.R.; Fjellsbø, L.M.; Elje, E.; Yazdani, M.; et al. Fibrous shape underlies the mutagenic and carcinogenic potential of nanosilver while surface chemistry affects the biosafety of iron oxide nanoparticles. Mutagenesis 2017, 32, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Semete, B.; Booysen, L.; Lemmer, Y.; Kalombo, L.; Katata, L.; Verschoor, J.S.; Swai, H.S. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine 2010, 6, 662–671. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the multilayer materials made from PLGA or PLGA/PHB are available at Lodz University of Technology, Department of Material and Commodity Sciences and Textile Metrology, Żeromskiego 116, 90-924 Lodz, Poland. Contact person: Prof Izabella Krucinska, e-mail: [email protected]. |






| No. | Polymer | Linear Mass, dtex | Tenacity, cN/tex | Elongation, % | Shrinkage, % | Crystallinity Degree, % |
|---|---|---|---|---|---|---|
| 1 | PLGA | 6.02 | 24.1 | 40.4 | 5.0 | 14.0 |
| 2 | PLGA/PHB | 5.80 | 26.8 | 41.9 | 16.4 | 13.0 |
| Polymer | Surface Mass, g/m2 | Thickness, mm | Apparent Density, kg/m3 | Air Permeability, lm−2 s−1 | Average Pore Diameter, nm | Total Pore Area, m2/g |
|---|---|---|---|---|---|---|
| PGLA | 333.76 | 2.6 | 128.08 | 331 | 148,523.10 | 0.116 |
| PGLA/PHB | 318.13 | 2.2 | 144.55 | 409 | 158,719.30 | 0.085 |
| No. | Polymer | Surface Mass, g/m2 | Thickness, mm | Apparent Density, kg/m3 | Average Pore Diameter, nm | Total Pore Area, m2/g |
|---|---|---|---|---|---|---|
| 1 | PLGA | 4.5 | 0.03 | 150.00 | 9.80 | 1.49 |
| 2 | PLGA/PHB | 20.4 | 0.07 | 219.43 | 16.90 | 0.56 |
| No. | Polymer | Surface Mass, g/m2 | Thickness, mm | Apparent Density, kg/m3 | Air Permeability lm−2 s−1 |
|---|---|---|---|---|---|
| 1 | PLGA | 602.36 | 2.8 | 215.13 | 205 |
| 2 | PLGA/PHB | 613.24 | 2.8 | 219.01 | 202 |
| 3 | PLGA + IGF1 | 665.95 | 2.8 | 237.84 | 198 |
| 4 | PLGA/PHB + IGF1 | 692.34 | 2.7 | 256.42 | 178 |
| No. | Polymer | Average Pore Diameter, nm | Total Pore Area, m2/g |
|---|---|---|---|
| 1 | PLGA | 168,236.2 | 0.246 |
| 2 | PLGA/PHB | 171,522.9 | 0.205 |
| 3 | PLGA + IGF1 | 172,877.1 | 0.139 |
| 4 | PLGA/PHB + IGF1 | 181,042.3 | 0.133 |
| No. | Polymer | Dead Cells, % | Living Cells, % | Total Number of Cells, ×105 | Level of Cytotoxicity |
|---|---|---|---|---|---|
| 1 | PLGA–control | 0 | 100 | 4.1 | 0 |
| 2 | PLGA + IGF1 | 0 | 100 | 5.6 | 0 |
| 3 | PLGA/PHB–control | 1 | 99 | 3.8 | 0 |
| 4 | PLGA/PHB + IGF1 | 0 | 100 | 4.7 | 0 |
| 5 | L929 culture | 0 | 100 | 6.8 | 0 |
| No. | Polymer | Dead Cells, % | Living Cells, % | Total Number of Cells, ×105 | Level of Cytotoxicity |
|---|---|---|---|---|---|
| 1 | PLGA–control | 0 | 100 | 5.2 | 0 |
| 2 | PLGA + IGF1 | 0 | 100 | 4.5 | 0 |
| 3 | PLGA/PHB–control | 0 | 100 | 4.6 | 0 |
| 4 | PLGA/PHB + IGF1 | 1 | 99 | 4.7 | 0 |
| 5 | L929 culture | 0 | 100 | 6.4 | 0 |
| No. | Polymer | Dead Cells, % | Living Cells, % | Total Number of Cells, ×105 | Level of Cytotoxicity |
|---|---|---|---|---|---|
| 1 | PLGA–control | 0 | 100 | 7.9 | 0 |
| 2 | PLGA + IGF1 | 2 | 98 | 5.9 | 0 |
| 3 | PLGA/PHB–control | 0 | 100 | 6.1 | 0 |
| 4 | PLGA/PHB + IGF1 | 0 | 100 | 5.4 | 0 |
| 5 | L929 culture | 0 | 100 | 7.2 | 0 |
| Dose | TA 98 | TA 100 | TA 1535 | TA 1537 | E. coli wp2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| cm2/mL | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 |
| Spontaneous reversion | 0.56 ± 0.53 | 1.67 ± 2.08 | 0.33 ± 0.58 | 0.67 ± 0.58 | 0.33 ± 0.58 | 2.00 ± 1.73 | 1.67 ± 1.53 | 4.33 ± 2.31 | 6.67 ± 1.53 | 5.00 ± 2.65 |
| 0.09375 | 0.33 ± 0.58 | 0.67 ± 1.15 | 0.33 ± 0.58 | 2.33 ± 0.58 | 1.67 ± 1.15 | 1.70 ± 1.53 | 0.00 ± 0.00 | 3.00 ± 1.00 | 10.00 ± 4.58 | 5.00 ± 1.00 |
| 0.1875 | 1.67 ± 1.15 | 1.33 ± 0.58 | 0.00 ± 0.00 | 1.67 ± 1.53 | 0.67 ± 1.15 | 1.67 ± 0.58 | 1.33 ± 1.53 | 2.00 ± 1.00 | 8.33 ± 5.13 | 3.67 ± 5.51 |
| 0.375 | 0.33 ± 0.58 | 2.67 ± 2.31 | 0.33 ± 0.58 | 1.33 ± 0.58 | 0.00 ± 0.00 | 1.33 ± 0.58 | 1.00 ± 1.00 | 3.67 ± 0.58 | 8.33 ± 2.52 | 5.00 ± 0.00 |
| 0.75 | 0.67 ± 1.15 | 5.00 ± 1.00 | 0.00 ± 0.00 | 2.00 ± 1.00 | 1.00 ± 0.00 | 2.67 ± 0.58 | 0.67 ± 0.58 | 2.33 ± 1.53 | 8.00 ± 3.46 | 7.67 ± 8.02 |
| 1.5 | 0.00 ± 0.00 | 2.67 ± 3.06 | 1.00 ± 1.00 | 2.00 ± 1.00 | 0.67 ± 0.58 | 1.00 ± 1.00 | 0.67 ± 0.58 | 3.00 ± 0.00 | 9.67 ± 4.62 | 8.00 ± 7.00 |
| 3 | 2.00 ± 1.00 | 3.00 ± 0.00 | 1.67 ± 1.53 | 0.67 ± 0.58 | 0.33 ± 0.58 | 1.67 ± 1.15 | 0.67 ± 0.58 | 2.33 ± 0.58 | 6.67 ± 1.53 | 9.33 ± 3.51 |
| Positive control | 40.67 ± 2.08 | 47.67 ± 0.58 | 36.33 ± 1.53 | 46.33 ± 2.08 | 48.00 ± 0.00 | 27.67 ± 2.08 | 48.00 ± 0.00 | 32.67 ± 7.37 | 45.33 ± 1.15 | 43.00 ± 1.00 |
| Dose | TA 98 | TA 100 | TA 1535 | TA 1537 | E. coli wp2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| cm2/mL | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 |
| Spontaneous reversion | 0.56 ± 0.53 | 1.67 ± 2.08 | 0.33 ± 0.58 | 0.67 ± 0.58 | 0.33 ± 0.58 | 2.00 ± 1.73 | 1.67 ± 1.53 | 4.33 ± 2.31 | 6.67 ± 1.53 | 5.00 ± 2.65 |
| 0.09375 | 1.67 ± 1.53 | 1.33 ± 1.15 | 0.33 ± 0.58 | 1.33 ± 0.58 | 0.33 ± 0.58 | 1.67 ± 2.08 | 2.33 ± 2.52 | 2.00 ± 2.65 | 4.00 ± 3.61 | 6.67 ± 3.06 |
| 0.1875 | 0.33 ± 0.58 | 1.67 ± 0.58 | 0.67 ± 0.58 | 0.33 ± 0.58 | 0.67 ± 0.58 | 2.67 ± 2.08 | 1.33 ± 1.53 | 2.33 ± 1.53 | 6.33 ± 3.51 | 1.67 ± 1.15 |
| 0.375 | 1.67 ± 2.89 | 3.33 ± 0.58 | 0.67 ± 1.15 | 2.33 ± 2.52 | 1.00 ± 1.73 | 2.00 ± 1.00 | 1.33 ± 2.31 | 1.33 ± 1.53 | 13.00 ± 2.65 | 5.67 ± 4.73 |
| 0.75 | 1.00 ± 1.00 | 1.67 ± 1.53 | 0.33 ± 0.58 | 2.00 ± 3.46 | 0.33 ± 0.58 | 2.00 ± 1.00 | 1.00 ± 1.00 | 4.00 ± 3.00 | 9.67 ± 2.52 | 3.00 ± 1.73 |
| 1.5 | 1.00 ± 1.00 | 2.00 ± 1.73 | 0.33 ± 0.58 | 1.00 ± 0.00 | 0.67 ± 0.58 | 1.33 ± 1.53 | 2.00 ± 1.00 | 1.67 ± 0.58 | 8.33 ± 1.53 | 2.00 ± 2.00 |
| 3 | 1.67 ± 1.15 | 2.33 ± 2.52 | 0.67 ± 0.58 | 1.33 ± 1.53 | 0.33 ± 0.58 | 2.00 ± 1.00 | 1.67 ± 0.58 | 2.33 ± 0.58 | 6.00 ± 1.00 | 5.33 ± 1.53 |
| Positive control | 40.67 ± 2.08 | 47.67 ± 0.58 | 36.33 ± 1.53 | 46.33 ± 2.08 | 48.00 ± 0.00 | 27.67 ± 2.08 | 48.00 ± 0.00 | 32.67 ± 7.37 | 45.33 ± 1.15 | 43.00 ± 1.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krucińska, I.; Żywicka, B.; Komisarczyk, A.; Szymonowicz, M.; Kowalska, S.; Zaczyńska, E.; Struszczyk, M.; Czarny, A.; Jadczyk, P.; Umińska-Wasiluk, B.; et al. Biological Properties of Low-Toxicity PLGA and PLGA/PHB Fibrous Nanocomposite Implants for Osseous Tissue Regeneration. Part I: Evaluation of Potential Biotoxicity. Molecules 2017, 22, 2092. https://doi.org/10.3390/molecules22122092
Krucińska I, Żywicka B, Komisarczyk A, Szymonowicz M, Kowalska S, Zaczyńska E, Struszczyk M, Czarny A, Jadczyk P, Umińska-Wasiluk B, et al. Biological Properties of Low-Toxicity PLGA and PLGA/PHB Fibrous Nanocomposite Implants for Osseous Tissue Regeneration. Part I: Evaluation of Potential Biotoxicity. Molecules. 2017; 22(12):2092. https://doi.org/10.3390/molecules22122092
Chicago/Turabian StyleKrucińska, Izabella, Bogusława Żywicka, Agnieszka Komisarczyk, Maria Szymonowicz, Stanisława Kowalska, Ewa Zaczyńska, Marcin Struszczyk, Anna Czarny, Piotr Jadczyk, Barbara Umińska-Wasiluk, and et al. 2017. "Biological Properties of Low-Toxicity PLGA and PLGA/PHB Fibrous Nanocomposite Implants for Osseous Tissue Regeneration. Part I: Evaluation of Potential Biotoxicity" Molecules 22, no. 12: 2092. https://doi.org/10.3390/molecules22122092