Platinum-Catalyzed Allylation of 2,3-Disubstituted Indoles with Allylic Acetates
Abstract
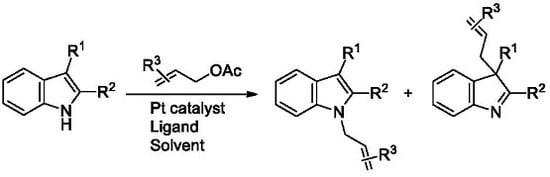
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Considerations
3.2. General Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Higuchi, K. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2005, 22, 761–793. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Maincent, P.; Le Verge, R.; Sado, P.; Couvreur, P.; Devissaguet, J.P. Disposition kinetics and oral bioavailability of vincamine-loaded polyalkyl cyanoacrylate nanoparticles. J. Pharm. Sci. 1986, 75, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Rahbæk, L.; Christophersen, C. Marine alkaloids. 19. Three new alkaloids, securamines e−g, from the marine bryozoan securiflustra securifrons. J. Nat. Prod. 1997, 60, 175–177. [Google Scholar] [CrossRef]
- Vepsäläinen, J.J.; Auriola, S.; Tukiainen, M.; Ropponen, N.; Callaway, J.C. Isolation and characterization of yuremamine, a new phytoindole. Planta Med. 2005, 71, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.S.; Buchanan, M.S.; Carroll, A.R.; Feng, Y.J.; Quinn, R.J.; Avery, V.M. Flinderoles a−c: Antimalarial bis-indole alkaloids from flindersia species. Org. Lett. 2009, 11, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Vallakati, R.; Smuts, J.P.; Armstrong, D.W.; May, J.A. On the biosynthesis and optical activity of the flinderoles. Tetrahedron Lett. 2013, 54, 5892–5894. [Google Scholar] [CrossRef]
- Kimura, M.; Futamata, M.; Mukai, R.; Tamaru, Y. Pd-catalyzed c3-selective allylation of indoles with allyl alcohols promoted by triethylborane. J. Am. Chem. Soc. 2005, 127, 4592–4593. [Google Scholar] [CrossRef] [PubMed]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Sundberg, R.J. Indoles; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Bandini, M.; Melloni, A.; Tommasi, S.; Umani-Ronchi, A. A journey across recent advances in catalytic and stereoselective alkylation of indoles. Synlett 2005, 2005, 1199–1222. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G. Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2005, 105, 2873–2920. [Google Scholar] [CrossRef] [PubMed]
- Lakhdar, S.; Westermaier, M.; Terrier, F.; Goumont, R.; Boubaker, T.; Ofial, A.R.; Mayr, H. Nucleophilic reactivities of indoles. J. Org. Chem. 2006, 71, 9088–9095. [Google Scholar] [CrossRef] [PubMed]
- Otero, N.; Mandado, M.; Mosquera, R.A. Nucleophilicity of indole derivatives: Activating and deactivating effects based on proton affinities and electron density properties. J. Phys. Chem. A 2007, 111, 5557–5562. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, N.; Malerich, J.P.; Rawal, V.H. Palladium-catalyzed β-allylation of 2,3-disubstituted indoles. Org. Lett. 2008, 10, 2381–2384. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chan, C.; Heimann, A.C.; Danishefsky, S.J. On the rearrangement of an azaspiroindolenine to a precursor to phalarine: Mechanistic insights. Angew. Chem. Int. Ed. 2007, 46, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Sapi, J.; Dridi, S.; Laronze, J.; Sigaut, F.; Patigny, D.; Laronze, J.-Y.; Lévy, J.; Toupet, L. Indole as a tool in synthesis. Indolenine approach to 4,5-epoxy-10-normorphinans. Tetrahedron 1996, 52, 8209–8222. [Google Scholar] [CrossRef]
- Vercauteren, J.; Massiot, G.; Levy, J. Methyleneindolines, indolenines, and indoleniniums. 19. A new entry into the hexahydropyrrolidino[2,3-d]carbazole system. J. Org. Chem. 1984, 49, 3230–3231. [Google Scholar] [CrossRef]
- Bramely, R.K.; Caldwell, J.; Grigg, R. Site specificity of [3,3] sigmatropic rearrangements of 3-allyl- and 3-(prop-2-ynyl)-3h-indoles. J. Chem. Soc. Perkin Trans. 1 1973, 17, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Suginome, H. 41. Experiments on the synthesis of physostigmine (eserine). Part i. Some indolenine derivatives. J. Chem. Soc. (Resumed) 1932, 298–304. [Google Scholar] [CrossRef]
- Zaitsev, A.B.; Gruber, S.; Plüss, P.A.; Pregosin, P.S.; Veiros, L.F.; Wörle, M. Fast and highly regioselective allylation of indole and pyrrole compounds by allyl alcohols using ru-sulfonate catalysts. J. Am. Chem. Soc. 2008, 130, 11604–11605. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-B.; He, H.; Dai, L.-X.; You, S.-L. Ir-catalyzed regio- and enantioselective friedel-crafts-type allylic alkylation of indoles. Org. Lett. 2008, 10, 1815–1818. [Google Scholar] [CrossRef] [PubMed]
- Sundararaju, B.; Achard, M.; Demerseman, B.; Toupet, L.; Sharma, G.V.M.; Bruneau, C. Ruthenium(iv) complexes featuring p,o-chelating ligands: Regioselective substitution directly from allylic alcohols. Angew. Chem. Int. Ed. 2010, 49, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Herdtweck, E.; Bach, T. Pd(II)-catalyzed regioselective 2-alkylation of indoles via a norbornene-mediated C–H activation: Mechanism and applications. J. Am. Chem. Soc. 2012, 134, 14563–14572. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.D.; Recio, A.; Grenning, A.J.; Tunge, J.A. Transition metal-catalyzed decarboxylative allylation and benzylation reactions. Chem. Rev. 2011, 111, 1846–1913. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Miyake, Y.; Nishibayashi, Y. Cooperative catalytic reactions using organocatalysts and transition metal catalysts: Propargylic allylation of propargylic alcohols with α,β-unsaturated aldehydes. Organometallics 2012, 31, 3810–3813. [Google Scholar] [CrossRef]
- Schwarz, K.J.; Amos, J.L.; Klein, J.C.; Do, D.T.; Snaddon, T.N. Uniting C1-ammonium enolates and transition metal electrophiles via cooperative catalysis: The direct asymmetric α-allylation of aryl acetic acid esters. J. Am. Chem. Soc. 2016, 138, 5214–5217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Vemula, S.R.; Balasubramanian, N.; Cook, G.R. Indium-mediated stereoselective allylation. Acc. Chem. Res. 2016, 49, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Finke, R. Principles and Applications of Organotransition Metal Chemistry; University Science Book: Mill Valley, CA, USA, 1987. [Google Scholar]
- Balasubramanian, N.; Mandal, T.; Cook, G.R. Highly diastereoselective palladium-catalyzed indium-mediated allylation of chiral hydrazones. Org. Lett. 2015, 17, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Nishikawa, T.; Nakazaki, A. Palladium-catalyzed cascade wacker/allylation sequence with allylic alcohols leading to allylated dihydropyrones. ACS Omega 2017, 2, 487–495. [Google Scholar] [CrossRef]
- Shimizu, M.; Kimura, M.; Watanabe, T.; Tamaru, Y. Palladium-catalyzed allylation of imines with allyl alcohols. Org. Lett. 2005, 7, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, T.D.; Zhu, Y.; Kagawa, N.; Rawal, V.H. Palladium-catalyzed decarboxylative allylation and benzylation of n-alloc and n-cbz indoles. Org. Lett. 2013, 15, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Hikawa, H.; Yokoyama, Y. Palladium-catalyzed mono-n-allylation of unprotected anthranilic acids with allylic alcohols in aqueous media. J. Org. Chem. 2011, 76, 8433–8439. [Google Scholar] [CrossRef] [PubMed]
- Hossian, A.; Singha, S.; Jana, R. Palladium(0)-catalyzed intramolecular decarboxylative allylation of ortho nitrobenzoic esters. Org. Lett. 2014, 16, 3934–3937. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, J. Palladium Reagents and Catalysts; Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Trost, B.M. Cyclizations via palladium-catalyzed allylic alkylations [new synthetic methods (79)]. Angew. Chem. Int. Ed. Engl. 1989, 28, 1173–1192. [Google Scholar] [CrossRef]
- Oppolzer, W. Intramolecular, stoichiometric (Li, Mg, Zn) and catalytic (Ni, Pd, Pt) metallo-ene reactions in organic synthesis [new synthetic methods (75)]. Angew. Chem. Int. Ed. Engl. 1989, 28, 38–52. [Google Scholar] [CrossRef]
- Tsuji, J. Expanding industrial applications of palladium catalysts. Synthesis 1990, 1990, 739–749. [Google Scholar] [CrossRef]
- Uozumi, Y.; Danjo, H.; Hayashi, T. Cross-coupling of aryl halides and allyl acetates with arylboron reagents in water using an amphiphilic resin-supported palladium catalyst. J. Org. Chem. 1999, 64, 3384–3388. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Banerji, B.; Iqbal, J. Palladium(0)-catalyzed regioselective synthesis of α-dehydro-β-amino esters from amines and allyl acetates: Synthesis of a α-dehydro-β-amino acid derived cyclic peptide as a constrained β-turn mimic. J. Org. Chem. 2002, 67, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Wallner, O.A.; Szabó, K.J. Palladium-catalyzed electrophilic substitution of allyl chlorides and acetates via bis-allylpalladium intermediates. J. Org. Chem. 2003, 68, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, D.R.; Savin, K.A.; Justman, C.J.; Karanjawala, Z.E.; Sheppeck, J.E.; Hager, D.C.; Aydin, N. Conversion of allylic alcohols into allylic nitromethyl compounds via a palladium-catalyzed solvolysis: An enantioselective synthesis of an advanced carbocyclic nucleoside precursor1. J. Org. Chem. 1996, 61, 3616–3622. [Google Scholar] [CrossRef] [PubMed]
- Kadota, J.; Katsuragi, H.; Fukumoto, Y.; Murai, S. Platinum and palladium complex-catalyzed regioselective nucleophilic substitutions with two different nucleophiles at the central and terminal carbon atoms of the π-allyl ligand. Organomet. 2000, 19, 979–983. [Google Scholar] [CrossRef]
- Kamijo, S.; Jin, T.; Yamamoto, Y. Novel synthetic route to allyl cyanamides: Palladium-catalyzed coupling of isocyanides, allyl carbonate, and trimethylsilyl azide. J. Am. Chem. Soc. 2001, 123, 9453–9454. [Google Scholar] [CrossRef] [PubMed]
- Minami, I.; Yuhara, M.; Tsuji, J. 1-Isopropylallyloxycarbonyl (ipaoc) as a protective group of amines and its deprotection catalysed by palladium-phosphine complex. Tetrahedron Lett. 1987, 28, 2737–2740. [Google Scholar] [CrossRef]
- Kawabata, T.; Itoh, K.; Hiyama, T. Regio- and chemoselective epimerization of cis-3-amino-β-lactams to the trans-isomers: A new synthesis of aztreonam. Tetrahedron Lett. 1989, 30, 4837–4840. [Google Scholar] [CrossRef]
- Ziegler, F.E.; Wester, R.T. Regiochemical control in the hemiacetalization of a dihydroxydialdehyde. An application of the use of homochiral 3-methyl-δ-butyrolactones to the construction of homochiral tripropionate units. Tetrahedron Lett. 1986, 27, 1225–1228. [Google Scholar] [CrossRef]
- Ziegler, F.E.; Cain, W.T.; Kneisley, A.; Stirchak, E.P.; Wester, R.T. Applications of the 3-methyl-.Gamma.-butyrolactone strategy to the synthesis of polypropionates: The prelog-djerassi lactonic ester, ent-invictolide, and the c19-c27 fragment of rifamycin s. J. Am. Chem. Soc. 1988, 110, 5442–5452. [Google Scholar] [CrossRef]
- Schenck, T.G.; Bosnich, B. Homogeneous catalysis. Transition-metal-catalyzed claisen rearrangements. J. Am. Chem. Soc. 1985, 107, 2058–2066. [Google Scholar] [CrossRef]
- Auburn, P.R.; Whelan, J.; Bosnich, B. Homogeneous catalysis. Production of allyl alkyl sulphides by palladium mediated allylation. J. Chem. Soc. Chem. Commun. 1986, 17, 146–147. [Google Scholar] [CrossRef]
- Tamura, R.; Kamimura, A.; Ono, N. Displacement of aliphatic nitro groups by carbon and heteroatom nucleophiles. Synthesis 1991, 1991, 423–434. [Google Scholar] [CrossRef]
- Trost, B.M.; Schmuff, N.R.; Miller, M.J. Allyl sulfones as synthons for 1,1- and 1,3-dipoles via organopalladium chemistry. J. Am. Chem. Soc. 1980, 102, 5979–5981. [Google Scholar] [CrossRef]
- Yasushi, T.; Jun, S.; Ryo, T.; Yoshihisa, W. The platinum complex catalyzed transformation of primary amine to secondary amine. Chem. Lett. 1984, 13, 889–890. [Google Scholar]
- Yasushi, T.; Ryo, T.; Hiroshi, O.; Yoshihisa, W. Platinum complex catalyzed transformation of amine. N-alkylation and N-allylation using primary alcohols. Chem. Lett. 1986, 15, 293–294. [Google Scholar]
- Yang, S.-C.; Tsai, Y.-C.; Shue, Y.-J. Direct platinum-catalyzed allylation of anilines using allylic alcohols. Organometallics 2001, 20, 5326–5330. [Google Scholar] [CrossRef]
- Huynh, K.Q.; Seizert, C.A.; Ozumerzifon, T.J.; Allegretti, P.A.; Ferreira, E.M. Platinum-catalyzed α,β-unsaturated carbene formation in the formal syntheses of frondosin b and liphagal. Org. Lett. 2017, 19, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Labinger, J.A. Platinum-catalyzed C–H functionalization. Chem. Rev. 2017, 117, 8483–8496. [Google Scholar] [CrossRef] [PubMed]
- Gan, K.-H.; Jhong, C.-J.; Shue, Y.-J.; Yang, S.-C. Platinum-catalyzed allylation of aminonaphthalenes with allylic acetates in water. Tetrahedron 2008, 64, 9625–9629. [Google Scholar] [CrossRef]
- Yang, S.-C.; Feng, W.-H.; Gan, K.-H. Platinum-catalyzed allylation of aminonaphthalenes with allylic acetates. Tetrahedron 2006, 62, 3752–3760. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.-P.; Liu, C.; You, S.-L. Ru-catalyzed intermolecular dearomatization reaction of indoles with allylic alcohols. Chem. Sci. 2013, 4, 3239–3243. [Google Scholar] [CrossRef]
- Motoki, Y.; Yuko, K.; Koichi, N. Palladium-catalyzed carboacylation of alkenes by using acylchromates as acyl donors. Bull. Chem. Soc. Jpn. 2005, 78, 331–340. [Google Scholar]
- Masao, N. Alkylation of 2,3-dimethylindole in liquid ammonia. Bull. Chem. Soc. Jpn. 1959, 32, 838–840. [Google Scholar]
Sample Availability: Samples of the compounds 3a, 4a, 3b, 4b, 3c, 4c, 3d, 4d, 3e, 4e, 3f, and 4f are available from the authors. |
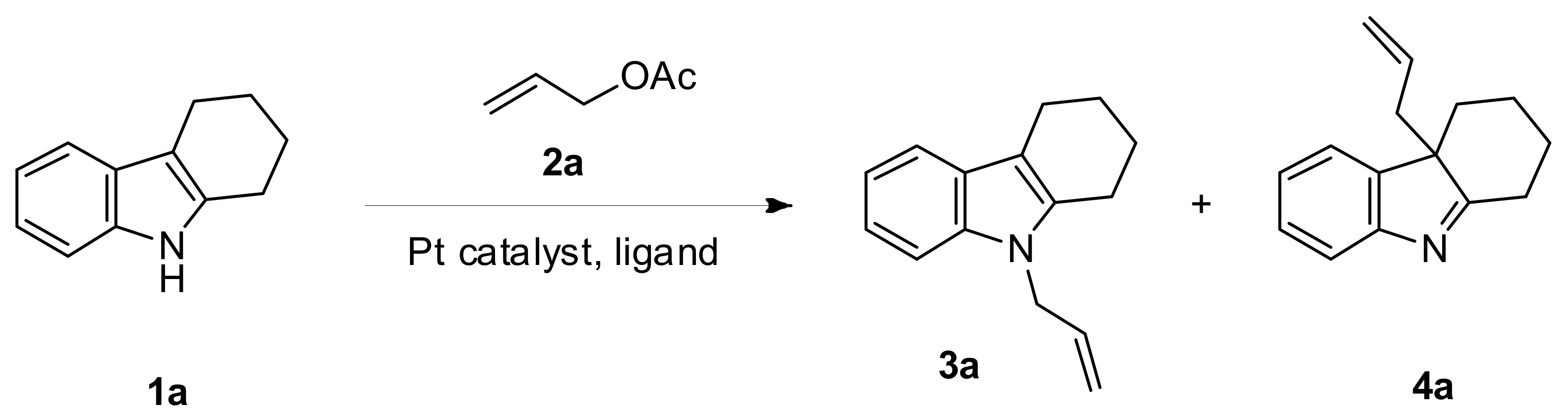
| Entry | Ligand | Platinum Catalyst | Solvent | Yield (%) (3a:4a) b |
|---|---|---|---|---|
| 1 | PPh3 | Pt(acac)2 | Benzene | 7 (7:0) |
| 2 | (2-CH3C6H4)3P | Pt(acac)2 | Benzene | 7 (0:7) |
| 3 | (3-CH3C6H4)3P | Pt(acac)2 | Benzene | 3 (0:3) |
| 4 | (4-CH3C6H4)3P | Pt(acac)2 | Benzene | 14 (8:6) |
| 5 | (4-FC6H4)3P | Pt(acac)2 | Benzene | 11 (0:11) |
| 6 | (4-ClC6H4)3P | Pt(acac)2 | Benzene | 99 (69:30) |
| 7 | (n-butyl)3P | Pt(acac)2 | Benzene | 30 (0:30) |
| 8 | (3-CH3OC6H4)3P | Pt(acac)2 | Benzene | 11 (3:8) |
| 9 | (4-CH3OC6H4)3P | Pt(acac)2 | Benzene | 18 (4:14) |
| 10 | (2-furyl)3P | Pt(acac)2 | Benzene | 8 (0:8) |
| 11 | (2,6-diCH3OC6H3)3P | Pt(acac)2 | Benzene | 19 (9:10) |
| 12 | (2,4,6-triCH3OC6H2)3P | Pt(acac)2 | Benzene | 6 (0:6) |
| 13 | Dppm c | Pt(acac)2 | Benzene | 9 (5:4) |
| 14 | Dppf d | Pt(acac)2 | Benzene | 13 (2:11) |
| 15 | Dppe e | Pt(acac)2 | Benzene | 14 (9:5) |
| 16 | Dppb f | Pt(acac)2 | Benzene | 12 (5:7) |
| 17 | - | Pt(acac)2 | Benzene | 0 (0:0) |
| 18 | (4-ClC6H4)3P | - | Benzene | 0 (0:0) |
| 19 g | (4-ClC6H4)3P | Pt(acac)2 | Benzene | 11 (5:6) |
| 20 h | (4-ClC6H4)3P | Pt(acac)2 | Benzene | 45 (32:13) |
| 21 | (4-ClC6H4)3P | cis-PtCl2(PhCN)2 | Benzene | 16 (6:10) |
| 22 | (4-ClC6H4)3P | cis-PtCl2(PPh3)2 | Benzene | 18 (5:13) |
| 23 | (4-ClC6H4)3P | Di(1,5-cyclooctadiene)Pt | Benzene | 22 (7:15) |
| 24 | (4-ClC6H4)3P | O[Si(CH3)2C=CH2]2Pt | Benzene | 12 (6:6) |
| 25 | (4-ClC6H4)3P | PtCl2 | Benzene | 36 (11:25) |
| 26 | (4-ClC6H4)3P | PtI2 | Benzene | 11 (0:11) |
| 27 | (4-ClC6H4)3P | Pt(CN)2 | Benzene | 20 (17:3) |
| 28 | - | Pt(CH2=CH2)(PPh3)2 | Benzene | 17 (2:15) |
| 29 | (4-ClC6H4)3P | Pt(CH2=CH2)(PPh3)2 | Benzene | 35 (5:30) |
| 30 | - | Pt(PPh3)4 | Benzene | 22 (2:20) |
| 31 | (4-ClC6H4)3P | Pt(PPh3)4 | Benzene | 64 (4:60) |
| 32 i | (4-ClC6H4)3P | Pt(acac)2 | Benzene | 0 (0:0) |
| 33 j | (4-ClC6H4)3P | Pt(acac)2 | Benzene | 11 (5:6) |
| 34 k | (4-ClC6H4)3P | Pt(acac)2 | Benzene | 75 (38:37) |
| 35 | (4-ClC6H4)3P | Pt(acac)2 | Toluene | 82 (62:20) |
| 36 | (4-ClC6H4)3P | Pt(acac)2 | CH2Cl2 | 43 (40:3) |
| 37 | (4-ClC6H4)3P | Pt(acac)2 | THF | 52 (34:18) |
| 38 | (4-ClC6H4)3P | Pt(acac)2 | Dioxane | 78 (63:15) |
| 39 | (4-ClC6H4)3P | Pt(acac)2 | DMF | 67 (53:14) |
| Entry | 2 | Yield b (%) | |
|---|---|---|---|
| 1 | 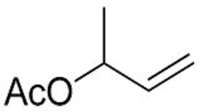 2b | 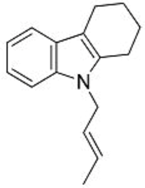 3b 54 (E/Z = 90/10) d | 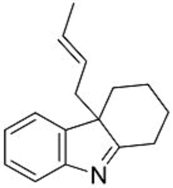 4b 23 (E/Z = 83/17) d |
| 2 | 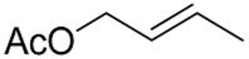 2c | 3b 62 (E/Z = 89/11) d | 4b 25 (E/Z = 95/5) d |
| 3 | 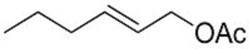 2d | 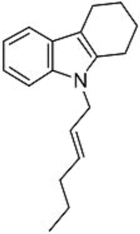 3c 45 (E/Z = 85/15) d |  4c 27 (E/Z = 90/10) d |
| 4 | 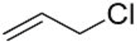 2e | 3a 34 | 4a 12 |
| 5 c | 2e | 3a 55 | 4a 20 |
| 6 | 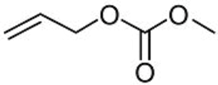 2f | 3a 87 | 4a 5 |
| Entry | 1 | Yield b (%) | |
|---|---|---|---|
| 1 | 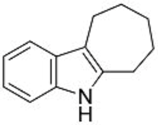 1b | 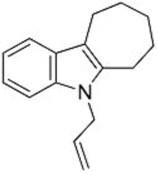 3d 67 | 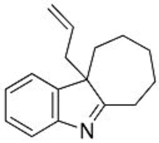 4d 30 |
| 2 | 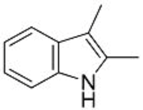 1c | 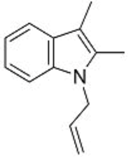 3e 68 | 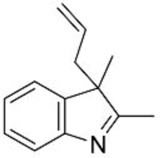 4e 30 |
| 3 |  1d | 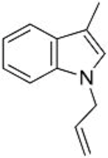 3f 47 | 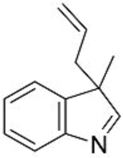 4f 24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.-J.; Wu, W.-T.; Yang, S.-C. Platinum-Catalyzed Allylation of 2,3-Disubstituted Indoles with Allylic Acetates. Molecules 2017, 22, 2097. https://doi.org/10.3390/molecules22122097
Peng B-J, Wu W-T, Yang S-C. Platinum-Catalyzed Allylation of 2,3-Disubstituted Indoles with Allylic Acetates. Molecules. 2017; 22(12):2097. https://doi.org/10.3390/molecules22122097
Chicago/Turabian StylePeng, Bai-Jing, Wen-Ting Wu, and Shyh-Chyun Yang. 2017. "Platinum-Catalyzed Allylation of 2,3-Disubstituted Indoles with Allylic Acetates" Molecules 22, no. 12: 2097. https://doi.org/10.3390/molecules22122097