Evaluation of Poly(Lactic-co-glycolic) Acid Alone or in Combination with Hydroxyapatite on Human-Periosteal Cells Bone Differentiation and in Sinus Lift Treatment
Abstract
:1. Introduction
2. Results
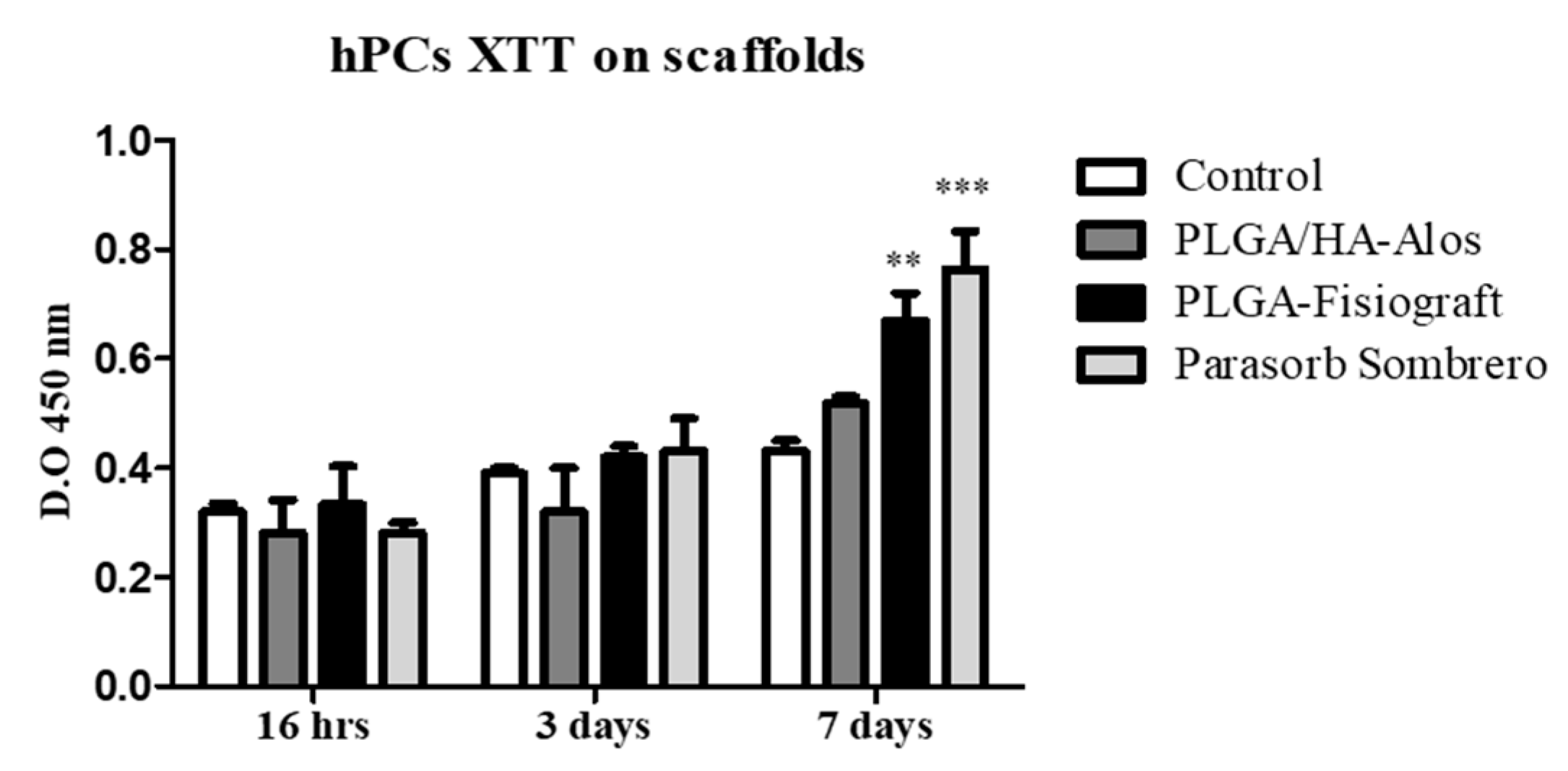
2.1. Effects of Scaffolds on the Proliferation of hPCs
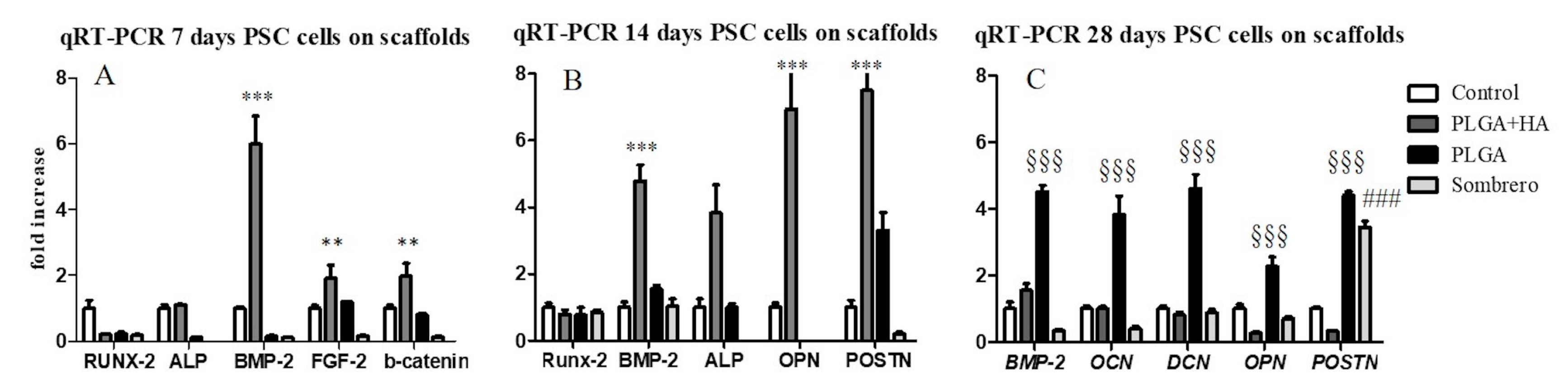
2.2. Gene Expression Analysis
2.3. Morphological Evaluation of Calcium Deposition
2.4. Bone Matrix Deposition: Quantification and Immunolocalization Analysis
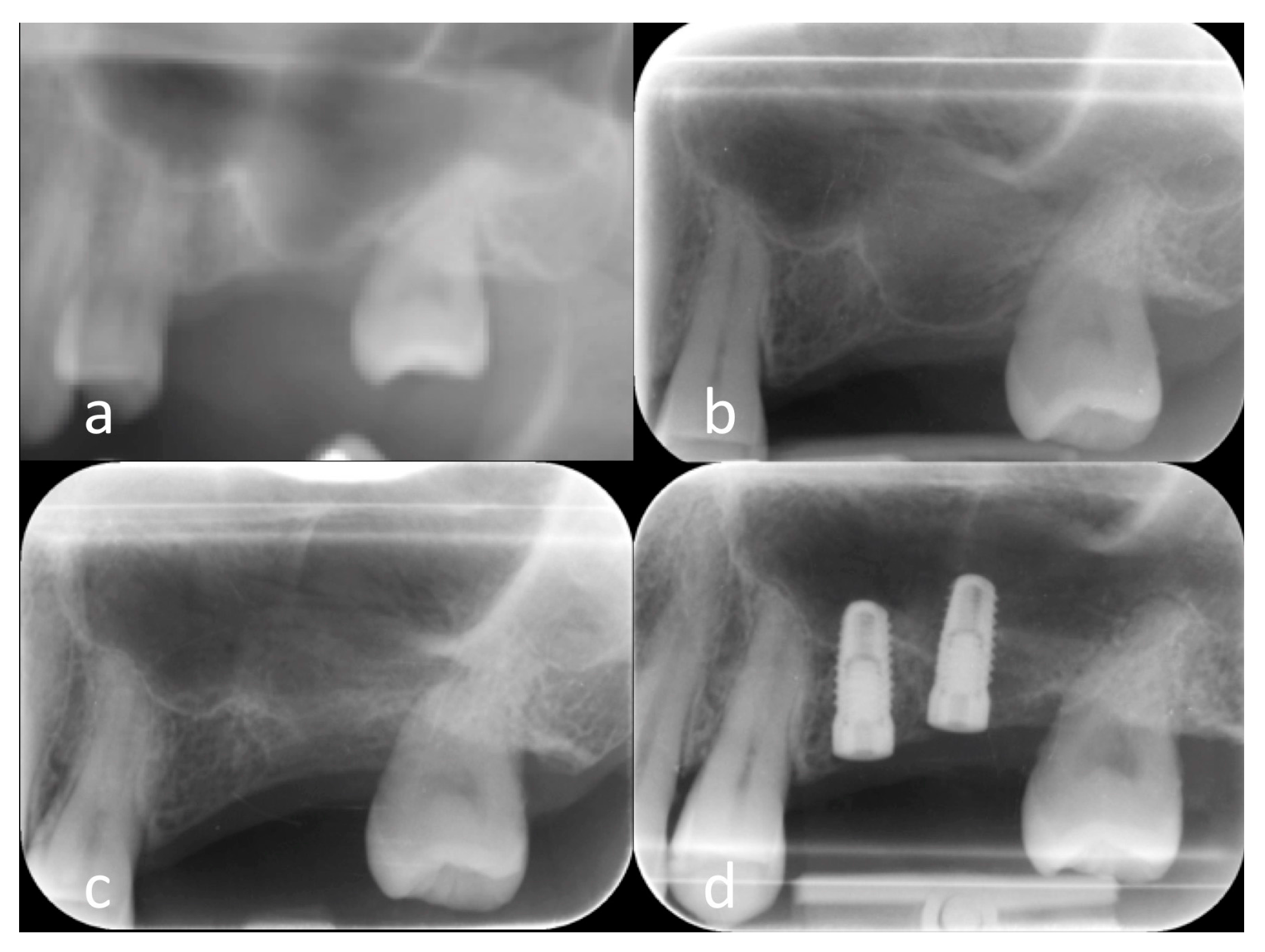
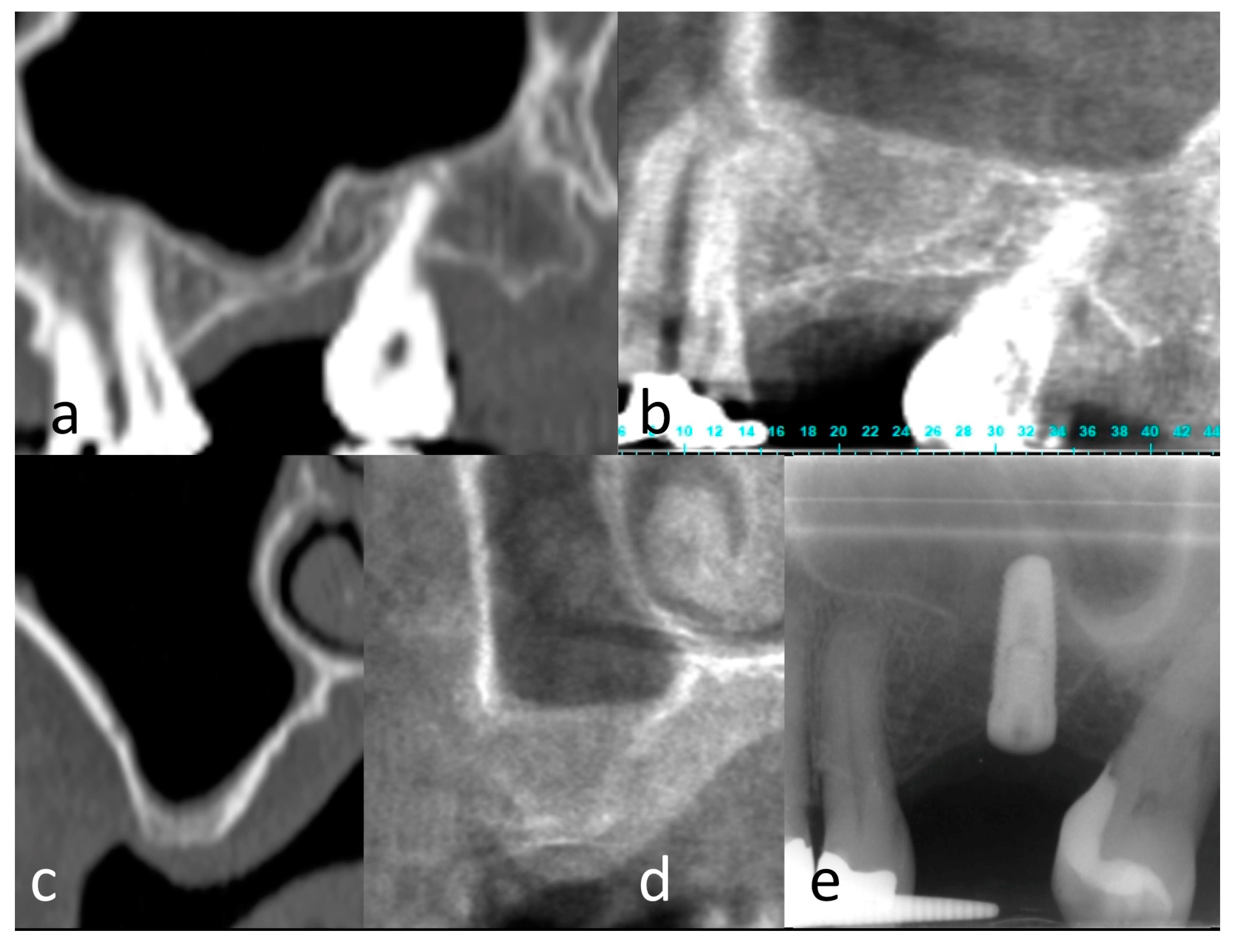
2.5. Clinical Results
3. Discussion
4. Materials and Methods
4.1. Scaffold Composition
4.2. Periosteum-Derived Mesenchymal Cell Isolation and Characterization
4.3. Attachment of hPCs to Scaffolds
4.4. Cell Viability Assay
4.5. Gene Expression Analysis
4.6. Bone ECM Protein Extraction and ELISAs
4.7. Alizarin Red S Test
4.8. Patients
4.9. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| α-MEM | Alpha-minimum essential medium |
| BCA | Bicinchoninic acid (assay) |
| β-cat | Βeta-catenin |
| BMP | Bone morphogenetic protein |
| FGF-2 | Fibroblast growth factor 2 |
| DCN | Decorin |
| DNA | Deoxyribonucleic acid |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| FACS | Fluorescence-activated cell sorting |
| GAPDH | Glyceraldehyde phosphate dehydrogenase |
| HA | Hydroxyapatite |
| hMSCs | Human mesenchymal stem cells |
| hPCs | Human periosteal cells |
| mRNA | Messenger RNA |
| OCN | Osteocalcin |
| OPN | Osteopontin |
| PLGA | Poly (lactic-co-glycolic) acid |
| PM | Proliferative medium |
| POSTN | Periostin |
| RNA | Ribonucleic acid |
| RUNX-2 | Runt-related transcription Factor |
| XTT | Cell proliferation kit II (XTT) |
References
- Hughes, D.; Song, B. Dental and Nondental Stem Cell Based Regeneration of the Craniofacial Region: A Tissue Based Approach. Stem Cells Int. 2016, 2016, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Raval, R.; Khandeparker, R.V.; Chidrawar, S.K.; Khan, A.A.; Ganpat, M.S. Tissue Engineering: Step Ahead in Maxillofacial Reconstruction. J. Int. Oral Health 2015, 7, 138–142. [Google Scholar] [PubMed]
- Fishero, B.A.; Kohli, N.; Das, A.; Christophel, J.J.; Cui, Q. Current concepts of bone tissue engineering for craniofacial bone defect repair. Craniomaxillofac Trauma Reconstr. 2015, 8, 23–30. [Google Scholar] [PubMed]
- Gong, T.; Heng, B.C.; Lo, E.C.; Zhang, C. Current Advance and Future Prospects of Tissue Engineering Approach to Dentin/Pulp Regenerative Therapy. Stem Cells Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Fujii, S.; Tomokiyo, A.; Wada, N.; Akamine, A. Periodontal tissue engineering: Defining the triad. Int. J. Oral Maxillofac. Implants 2013, 28, e461–e471. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Presta, R.; Benedetti, L.; Cusella De Angelis, M.G.; Lupi, S.M.; Rodriguez, Y.; Baena, R. Emerging Perspectives in Scaffold for Tissue Engineering in Oral Surgery. Stem Cells Int. 2017, 2017, 4585401. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Espro, C.; Galvagno, S.; Tampieri, A.; Montesi, M.; Panseri, S.; Sandri, M. Tethering of Gly-Arg-Gly-Asp-Ser-Pro-Lys Peptides on Mg-Doped Hydroxyapatite. Engineering 2017, 3, 55–59. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, J.Y.; Hao, Y.M.; Cao, C.H.; Zou, D.R. Maxillary sinus floor elevation with a tissue-engineered bone composite of deciduous tooth stem cells and calcium phosphate cement in goats. J. Tissue Eng. Regen. Med. 2017, 11, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Guo, L.; Lu, J.; Zhang, X.; Wei, J.; Liu, C.; Zhang, Z.; Jiang, X. Engineering of bone using porous calcium phosphate cement and bone marrow stromal cells for maxillary sinus augmentation with simultaneous implant placement in goats. Tissue Eng. Part A 2012, 18, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, M.; Pecci-Lloret, M.P.; García-Bernal, D.; Aznar-Cervantes, S.; Oñate-Sánchez, R.E.; Moraleda, J.M.; Cenis, J.L.; Rodríguez-Lozano, F.J. Biological effects of silk fibroin 3D scaffolds on stem cells from human exfoliated deciduous teeth (SHEDs). Odontology 2017. [Google Scholar] [CrossRef] [PubMed]
- Y Baena, R.R.; Lupi, S.M.; Pastorino, R.; Maiorana, C.; Lucchese, A.; Rizzo, S. Radiographic evaluation of regenerated bone following poly(lactic-co-glycolic) acid/hydroxyapatite and deproteinized bovine bone graft in sinus lifting. J. Craniofac. Surg. 2013, 24, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Graziano, A.; Benedetti, L.; Imbriani, M.; Romano, F.; Ferrarotti, F.; Aimetti, M.; Cusella de Angelis, G.M. Osteogenic Potential of Human Oral-Periosteal Cells (PCs) Isolated From Different Oral Origin: An In Vitro Study. J. Cell. Physiol. 2016, 231, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Bikle, D.D. Osteogenic Differentiation of Periosteal Cells during Fracture Healing. J. Cell. Physiol. 2017, 232, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Zaffe, D.; Leghissa, G.C.; Pradelli, J.; Botticelli, A.R. Histological study on sinus lift grafting by Fisiograft and Bio-Oss. J. Mater. Sci. Mater. Med. 2005, 16, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Fini, M.; Giavaresi, G.; Rimondini, L.; Ambrosio, L.; Giardino, R. In vivo preclinical efficacy of a PDLLA/PGA porous copolymer for dental application. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Omata, K.; Hashimoto, Y.; Tabata, Y.; Satoh, T. Alveolar bone tissue engineering using composite scaffolds for drug delivery. Jpn. Dent. Sci. Rev. 2010, 46, 188–192. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; D’Aquino, R.; Cusella-De Angelis, M.G.; De Francesco, F.; Giordano, A.; Laino, G.; Piattelli, A.; Traini, T.; De Rosa, A.; Papaccio, G. Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J. Cell. Physiol. 2008, 214, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.J.; Weiss-Bilka, H.E.; Meagher, M.J.; Liu, Y.; Gargac, J.A.; Niebur, G.L.; Wagner, D.R.; Roeder, R.K. Hydroxyapatite reinforced collagen scaffolds with improved architecture and mechanical properties. Acta Biomater. 2015, 17, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.L.; Peters, M.C.; Kohn, D.H.; Mooney, D.J. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2000, 21, 2521–2527. [Google Scholar] [CrossRef]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kunert-Keil, C.; Gredes, T.; Heinemann, F.; Dominiak, M.; Botzenhart, U.U.; Gedrange, T. Socket augmentation using a commercial collagen-based product-an animal study in pigs. Mater. Sci. Eng. C 2015, 46, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Lin, C.Y.; Saito, E.; Schek, R.D.; Taboas, J.M.; Williams, J.M.; Partee, B.; Flanagan, C.L.; Diggs, A.; Wilke, E.N. Engineering craniofacial scaffolds. Orthod. Craniofacial Res. 2005, 8, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.G.; Colombo, M.; Veronesi, G.; Caprioglio, A.; Mangano, C. Mesenchymal stem cells in maxillary sinus augmentation: A systematic review with meta-analysis. World J. Stem Cells 2015, 7, 976–991. [Google Scholar] [CrossRef] [PubMed]
- Ki, K.; Dean, D.; Lu, A.; Mikos, A.G.; Fisher, J.P. Early osteogenic signal expression of rat bone marrow stromal cells is influenced by both hydroxyapatite nanoparticle content and initial cell seeding density in biodegradable nanocomposite scaffolds. Acta Biomater. 2011, 7, 1249–1264. [Google Scholar]
- Baena, R.R.Y.; D’Aquino, R.; Graziano, A.; Trovato, L.; Aloise, A.C.; Ceccarelli, G.; Cusella, G.; Pelegrine, A.A.; Lupi, S.M. Autologous Periosteum-Derived Micrografts and PLGA/HA Enhance the Bone Formation in Sinus Lift Augmentation. Front. Cell Dev. Biol. 2017, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Genetos, D.C.; Leach, J.K. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng. Part A 2010, 16, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Biancu, S.; Iezzi, G.; Piattelli, A. Ridge preservation following tooth extraction using a polylactide and polyglycolide sponge as space filler: A clinical and histological study in humans. Clin. Oral Implants Res. 2003, 14, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez y Baena, R.; Pastorino, R.; Gherlone, E.F.; Perillo, L.; Lupi, S.M.; Lucchese, A. Histomorphometric Evaluation of Two Different Bone Substitutes in Sinus Augmentation Procedures: A Randomized Controlled Trial in Humans. Int. J. Oral Maxillofac. Implants 2017, 32, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Madhumala, R.; Saranyan, R.; Sreekanth, P. Use of fisiograft in intrabony defects- a clinical and radiological study. J. Clin. Diagn. Res. 2013, 7, 2364–2367. [Google Scholar] [CrossRef] [PubMed]
- Ohgushi, H.; Okumura, M.; Yoshikawa, T.; Inoue, K.; Senpuku, N.; Tamai, S.; Shors, E.C. Bone formation process in porous calcium carbonate and hydroxyapatite. J. Biomed. Mater. Res. 1992, 26, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Kaito, T.; Myoui, A.; Takaoka, K.; Saito, N.; Nishikawa, M.; Tamai, N.; Ohgushi, H.; Yoshikawa, H. Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite. Biomaterials 2005, 26, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Aboudzadeh, N.; Imani, M.; Shokrgozar, M.A.; Khavandi, A.; Javadpour, J.; Shafieyan, Y.; Farokhi, M. Fabrication and characterization of poly(d,l-lactide-co-glycolide)/hydroxyapatite nanocomposite scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. A 2010, 94, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Caves, J.M.; Haller, C.A.; Dai, E.; Liu, L.; Grainger, S.; Chaikof, E.L. Acellular vascular grafts generated from collagen and elastin analogs. Acta Biomater. 2013, 9, 8067–8074. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Zaffaroni, N.; Novara, F.; Cometa, A.M.; Avanzini, M.A.; Moretta, A.; Montagna, D.; Maccario, R.; Villa, R.; Daidone, M.G.; et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007, 67, 9142–9149. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.J.; Goodwin, C.J.; Holt, S.J. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul. 1995, 5, 69–84. [Google Scholar] [PubMed]
- Prè, D.; Ceccarelli, G.; Gastaldi, G.; Asti, A.; Saino, E.; Visai, L.; Benazzo, F.; Cusella De Angelis, M.G.; Magenes, G. The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. Bone 2011, 49, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Saino, E.; Grandi, S.; Quartarone, E.; Maliardi, V.; Galli, D.; Bloise, N.; Fassina, L.; De Angelis, M.G.; Mustarelli, P.; Imbriani, M.; et al. In vitro calcified matrix deposition by human osteoblasts onto a zinc-containing bioactive glass. Eur. Cell Mater. 2011, 21, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Majors, A.K.; Boehm, C.A.; Nitto, H.; Midura, R.J.; Muschler, G.F. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J. Orthop. Res. 1997, 15, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar] [PubMed]
- Rodriguez, A.; Anastassov, G.E.; Lee, H.; Buchbinder, D.; Wettan, H. Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J. Oral Maxillofac. Surg. 2003, 61, 157–163. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, R.; Trovato, L.; Graziano, A.; Ceccarelli, G.; Cusella De Angelis, M.G.; Marangini, A.; Nisio, A.; Galli, M.; Pasi, M.; Finotti, M.; et al. Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. J. Transl. Sci. 2016. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Genes | Accession Number | FW | RW | T° Annealing |
|---|---|---|---|---|
| ALP | NM_000478.5 | 5′-CTATCCTGGCTCCGTGTCC-3′ | 5′-AGCCCAGAGATGCAATCG-3′ | 60° |
| FGF-2 | NM_002006.4 | 5′-CGGCTGTACTGCAAAAACGG-3′ | 5′-TTGTAGCTTGATGTGGAGGGTCG-3′ | 60° |
| RUNX-2 | NM_001278478.1 | 5′-ACAGTAGATGGACCTCGGGA-3′ | 5′-ATACTGGGATGAGGAATGCG-3′ | 60° |
| OPN | NM_001040058.1 | 5′-GTGATTTGCTTTTGCCTCCT-3′ | 5′-GCCACAGCATCTGGGTATTT-3′ | 60° |
| OCN | NM_199173.5 | 5′-AAGAGACCCAGGCGCTACCT-3′ | 5′-AACTCGTCACAGTCCGGATTG-3′ | 60° |
| BMP-2 | NM_001200.3 | 5′-CCTCCGTGGGGATAGAACTT-3′ | 5′-CACTGTGCGCAGCTTCC-3′ | 60° |
| POSTN | NM_006475.2 | 5′-GAGGTCACCAAGGTCACCAAA-3′ | 5′-GGGTGTGTCTCCCTGAAGC-3′ | 60° |
| DCN | NM_001920.4 | 5′-ACCCCCTCCTCCTTTCCACACC-3′ | 5′-ACCAGGGAACCTTTTAATCCGGGAA-3′ | 60° |
| β-Catenin | NM_001098209.1 | 5′-GTCTGAGGAGCAGCTTCAGT-3′ | 5′-CCATTGTCCACGCTGGATTT-3′ | 60° |
| * GAPDH | NM_002046.5 | 5′-AGCCTCAAGATCATCAGCAATGCC-3′ | 5′-TGTGGTCATGAGTCCTTCCACGAT-3′ | 60° |
| Control | PLGA (Fisiograft®) | PLGA + HA (Alos®) | Parasorb Sombrer® | ||||
|---|---|---|---|---|---|---|---|
| pg | pg | Retio/Related to Control | pg | Retio/Related to Control | pg | Retio/Related to Control | |
| ALP | 6.59 ± 2.05 | 10.09 ± 1.60 | 1.53 * | 11.29 ± 2.2 | 1.71 *** | 10.20 ± 3.1 | 1.5 |
| OSN | 1.20 ± 0.27 | 1.81 ± 1.29 | 1.5 * | 2.16 ± 0.23 | 1.8 *** | 1.75 ± 0.4 | 1.45 |
| OPN | 5.73 ± 1.13 | 8.76 ± 0.69 | 1.52 * | 15.80 ± 3.6 | 2.75 *** | 9.6 ± 1.12 | 1.67 * |
| BMP-2 | 0 | 0 | 123 | 123 | 0 | ||
| OSC | 419 ± 7.81 | 586 ± 30.12 | 1.39 * | 643 ± 24.2 | 1.53 *** | 352.50 ± 40.25 | 0.84 |
| DCN | 36.24 ± 5.20 | 38.56 ± 2.5 | 1.06 * | 55.44 ± 3.4 | 1.52 *** | 37.50 ± 10.63 | 1.03 |
| Type-I-collagen | 65.9 ± 3.24 | 27.6 ± 3.6 | 0.4 * | 73.8 ± 8.7 | 1.11 *** | 22.2 ± 5.21 | 0.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, G.; Presta, R.; Lupi, S.M.; Giarratana, N.; Bloise, N.; Benedetti, L.; Cusella De Angelis, M.G.; Rodriguez y Baena, R. Evaluation of Poly(Lactic-co-glycolic) Acid Alone or in Combination with Hydroxyapatite on Human-Periosteal Cells Bone Differentiation and in Sinus Lift Treatment. Molecules 2017, 22, 2109. https://doi.org/10.3390/molecules22122109
Ceccarelli G, Presta R, Lupi SM, Giarratana N, Bloise N, Benedetti L, Cusella De Angelis MG, Rodriguez y Baena R. Evaluation of Poly(Lactic-co-glycolic) Acid Alone or in Combination with Hydroxyapatite on Human-Periosteal Cells Bone Differentiation and in Sinus Lift Treatment. Molecules. 2017; 22(12):2109. https://doi.org/10.3390/molecules22122109
Chicago/Turabian StyleCeccarelli, Gabriele, Rossella Presta, Saturnino Marco Lupi, Nefele Giarratana, Nora Bloise, Laura Benedetti, Maria Gabriella Cusella De Angelis, and Ruggero Rodriguez y Baena. 2017. "Evaluation of Poly(Lactic-co-glycolic) Acid Alone or in Combination with Hydroxyapatite on Human-Periosteal Cells Bone Differentiation and in Sinus Lift Treatment" Molecules 22, no. 12: 2109. https://doi.org/10.3390/molecules22122109





