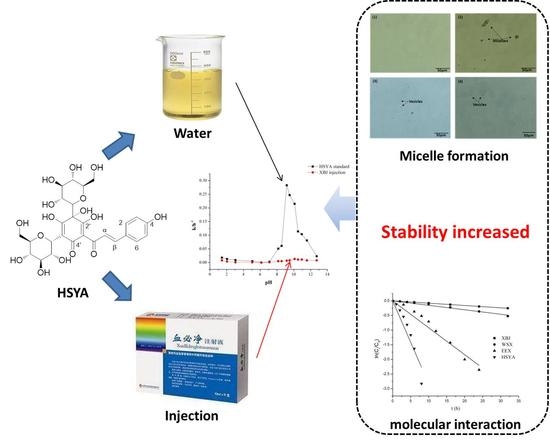
Superior Stability of Hydroxysafflor Yellow A in Xuebijing Injection and the Associated Mechanism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validation
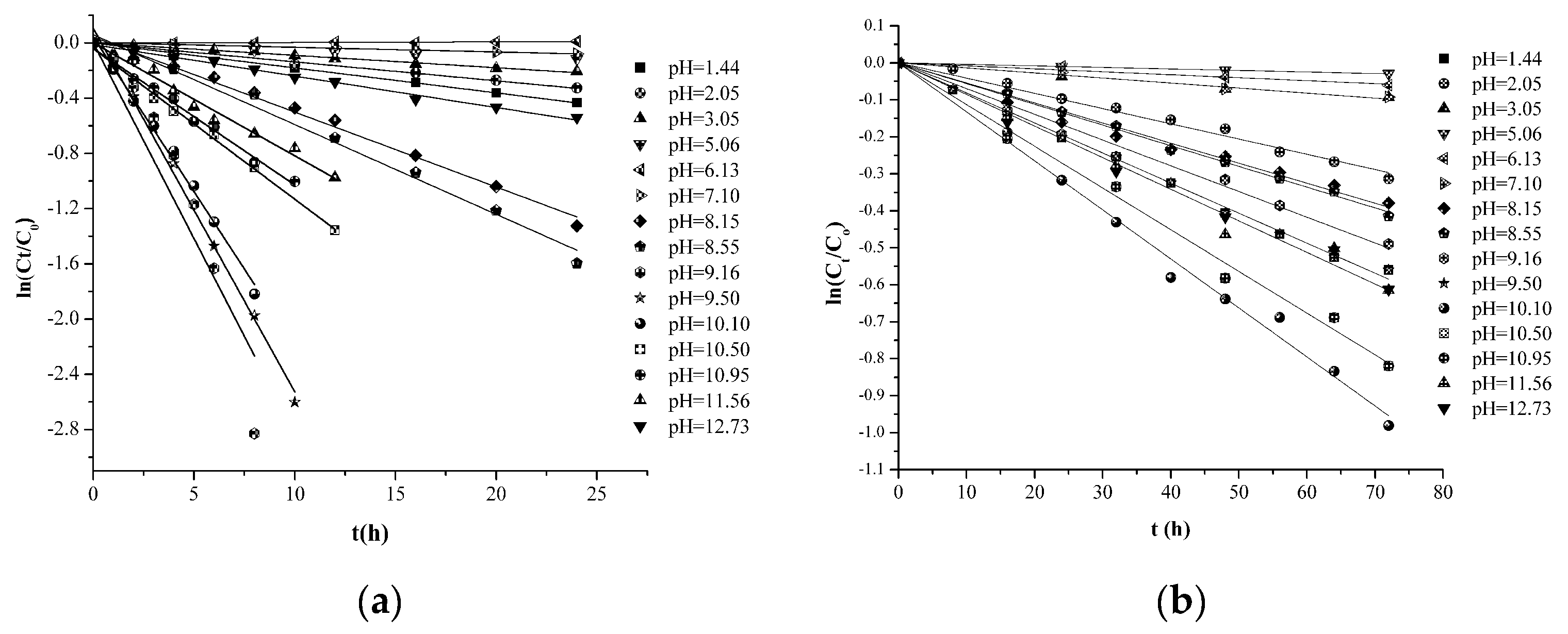
2.2. HSYA Degradation Follows First-Order Reaction Kinetics
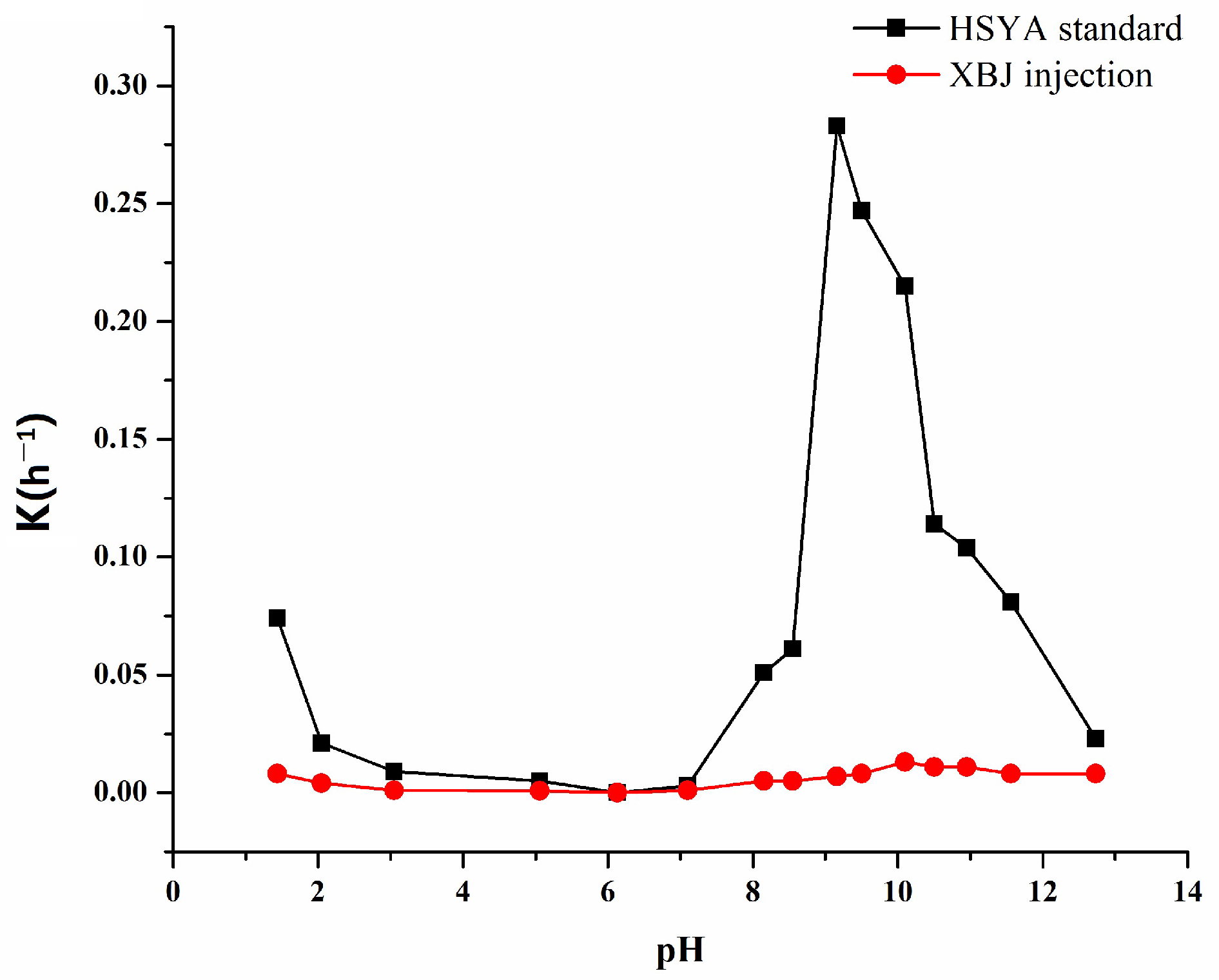
2.3. The pH Profile of HSYA Stability
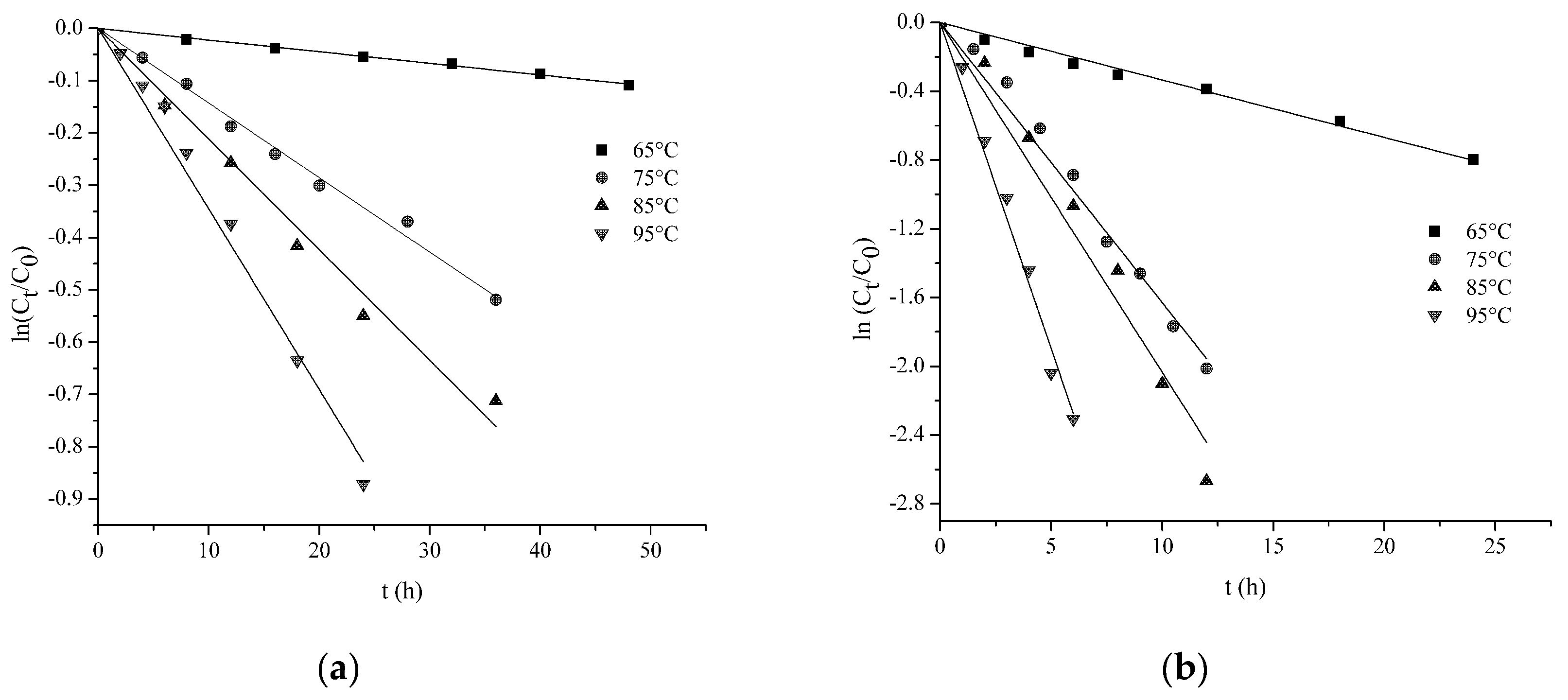
2.4. The Temperature Profile of HSYA Stability
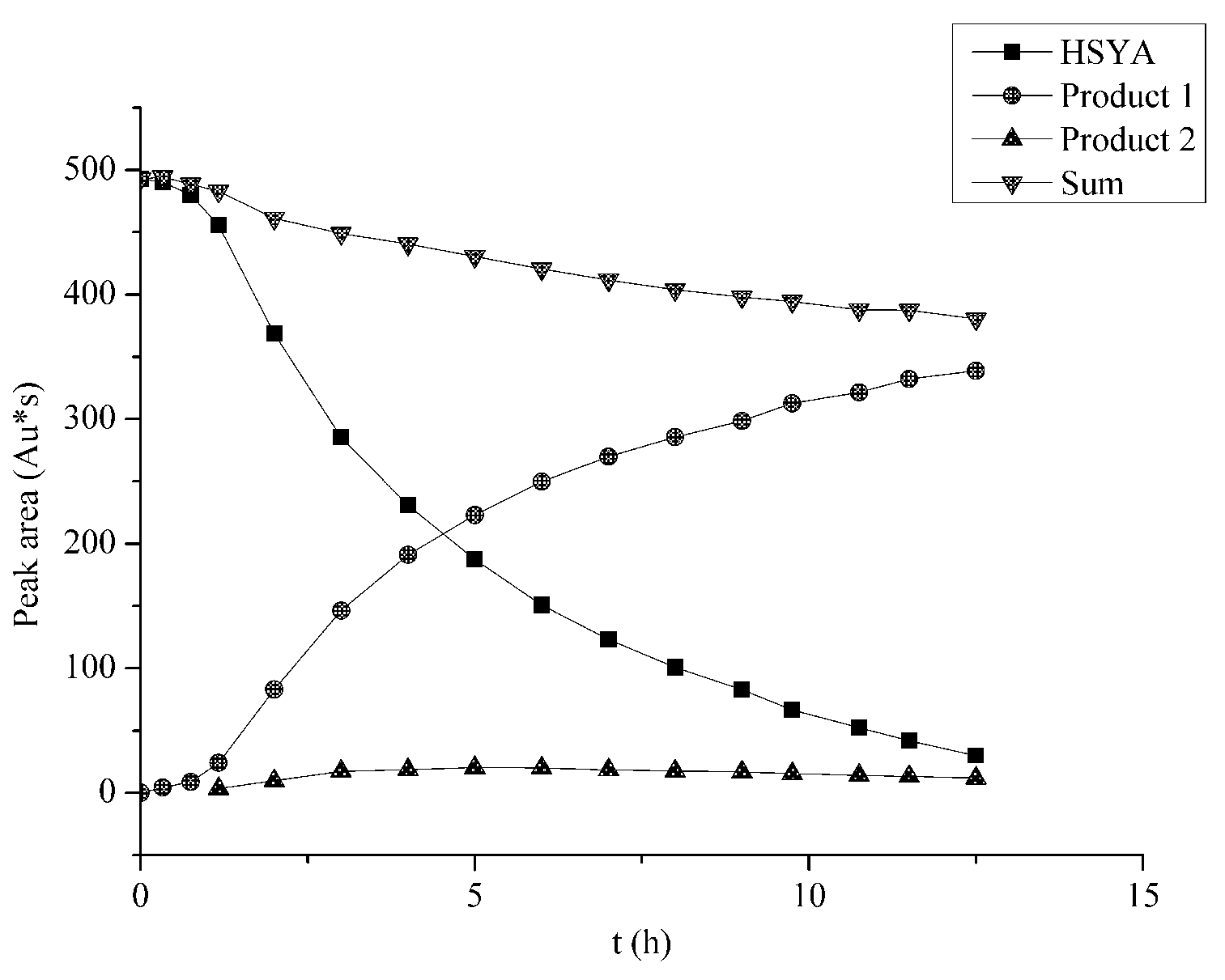
2.5. Mass Balance
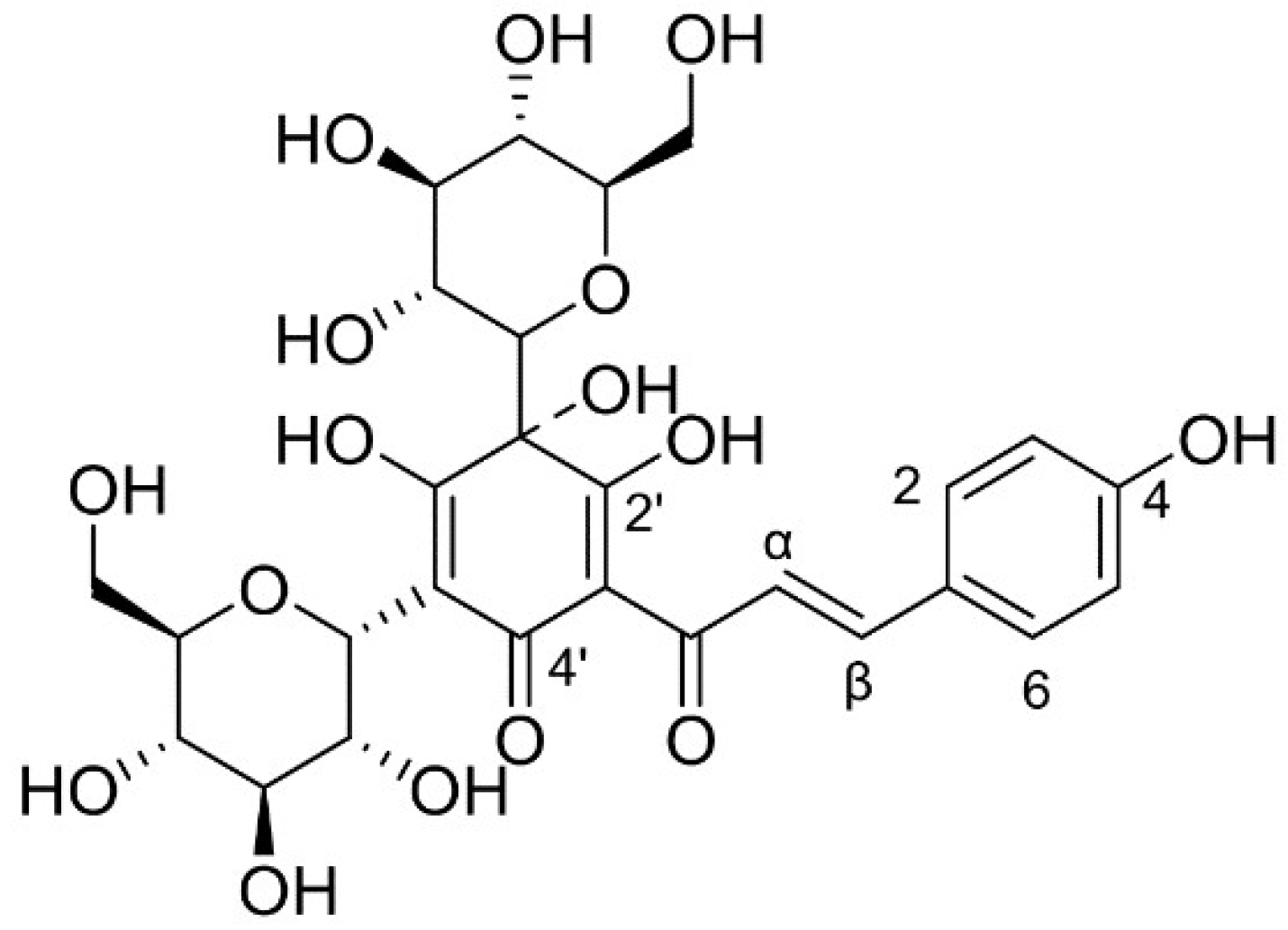
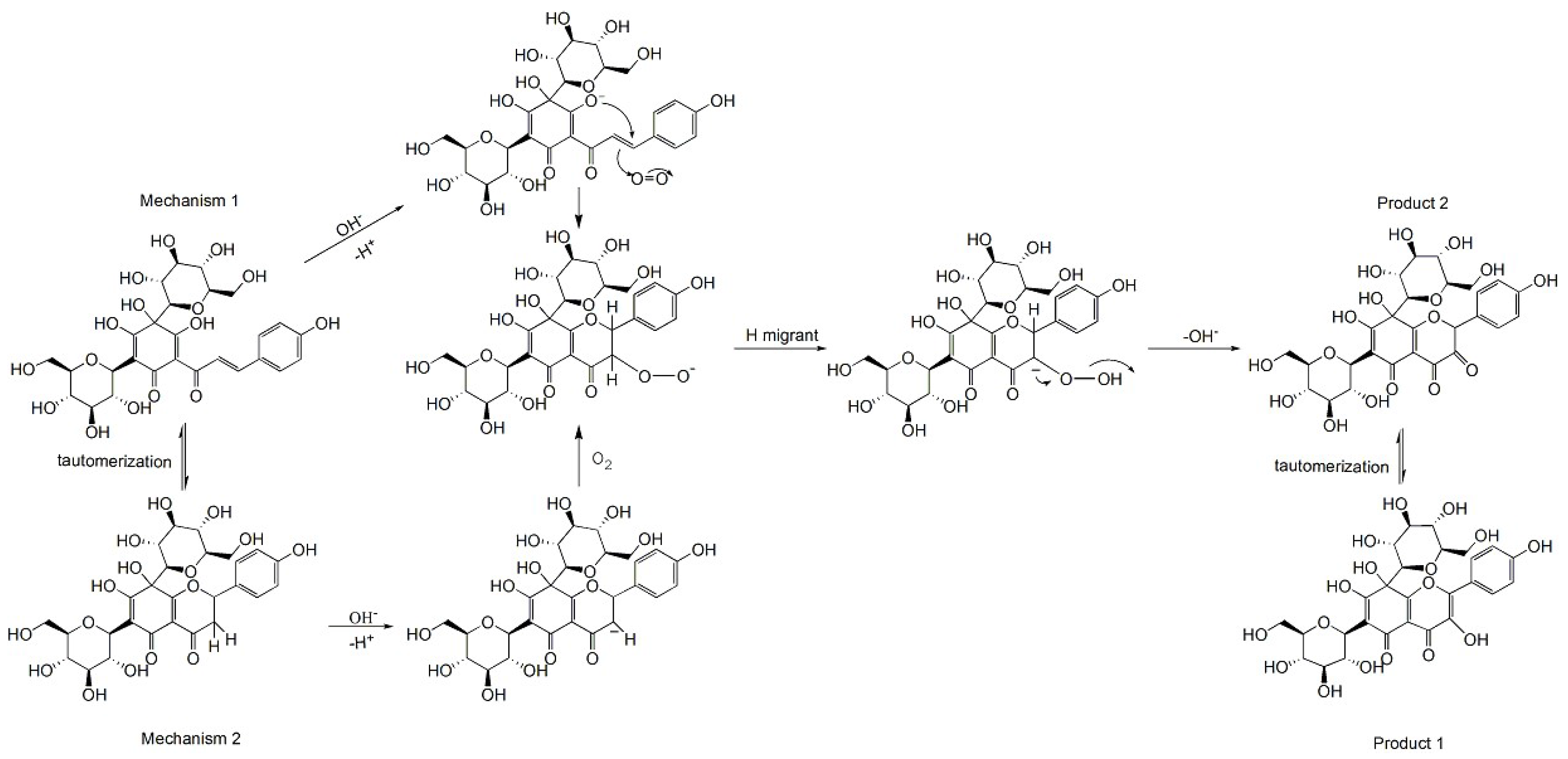
2.6. The HSYA Degradation Products and Proposed Degradation Pathway
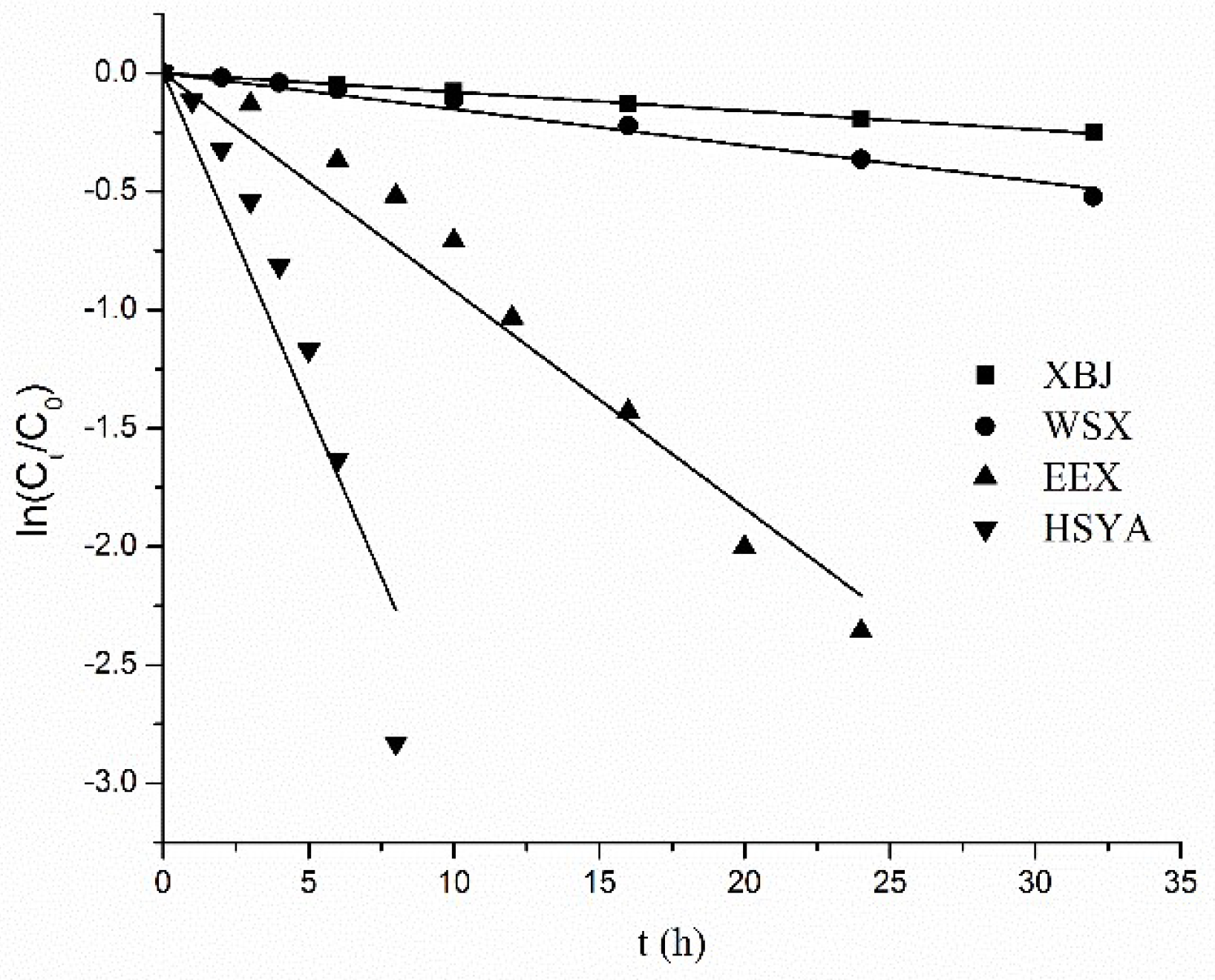
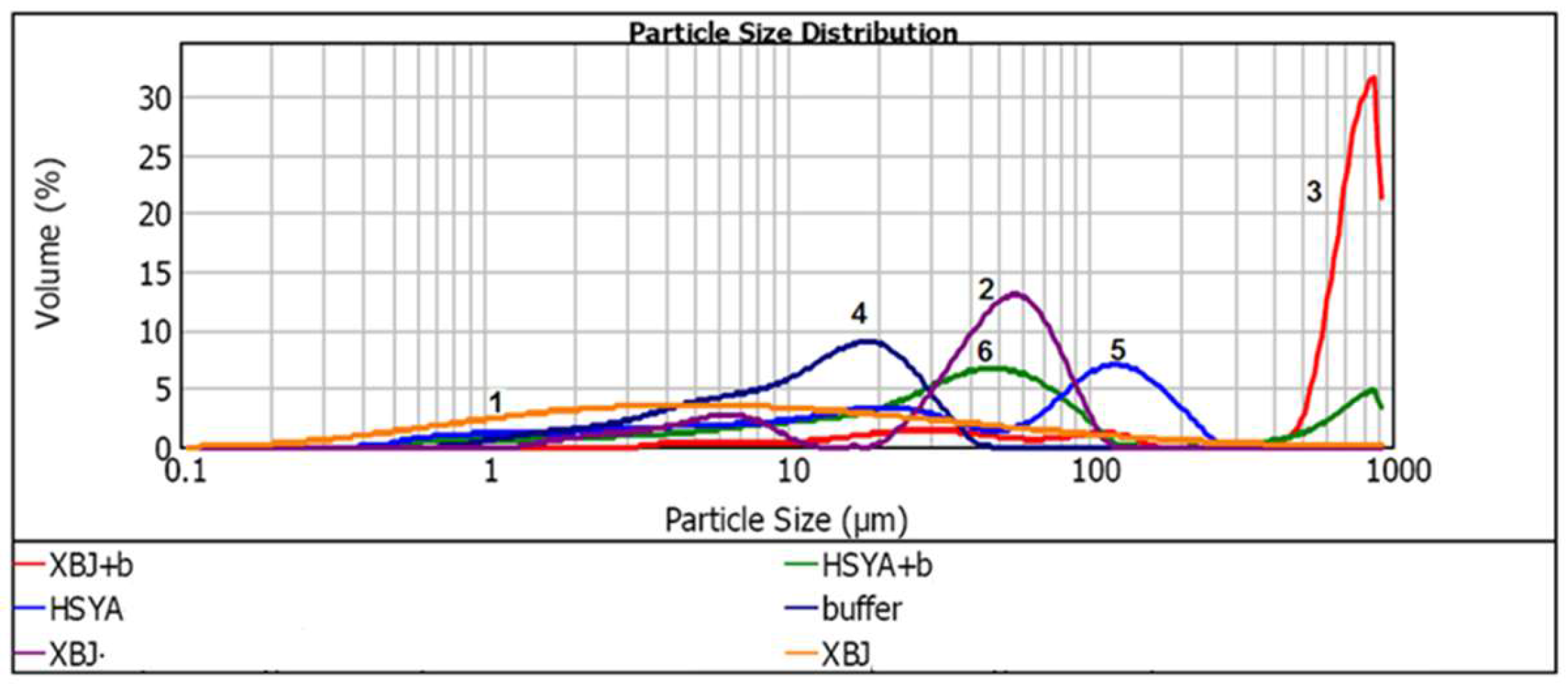
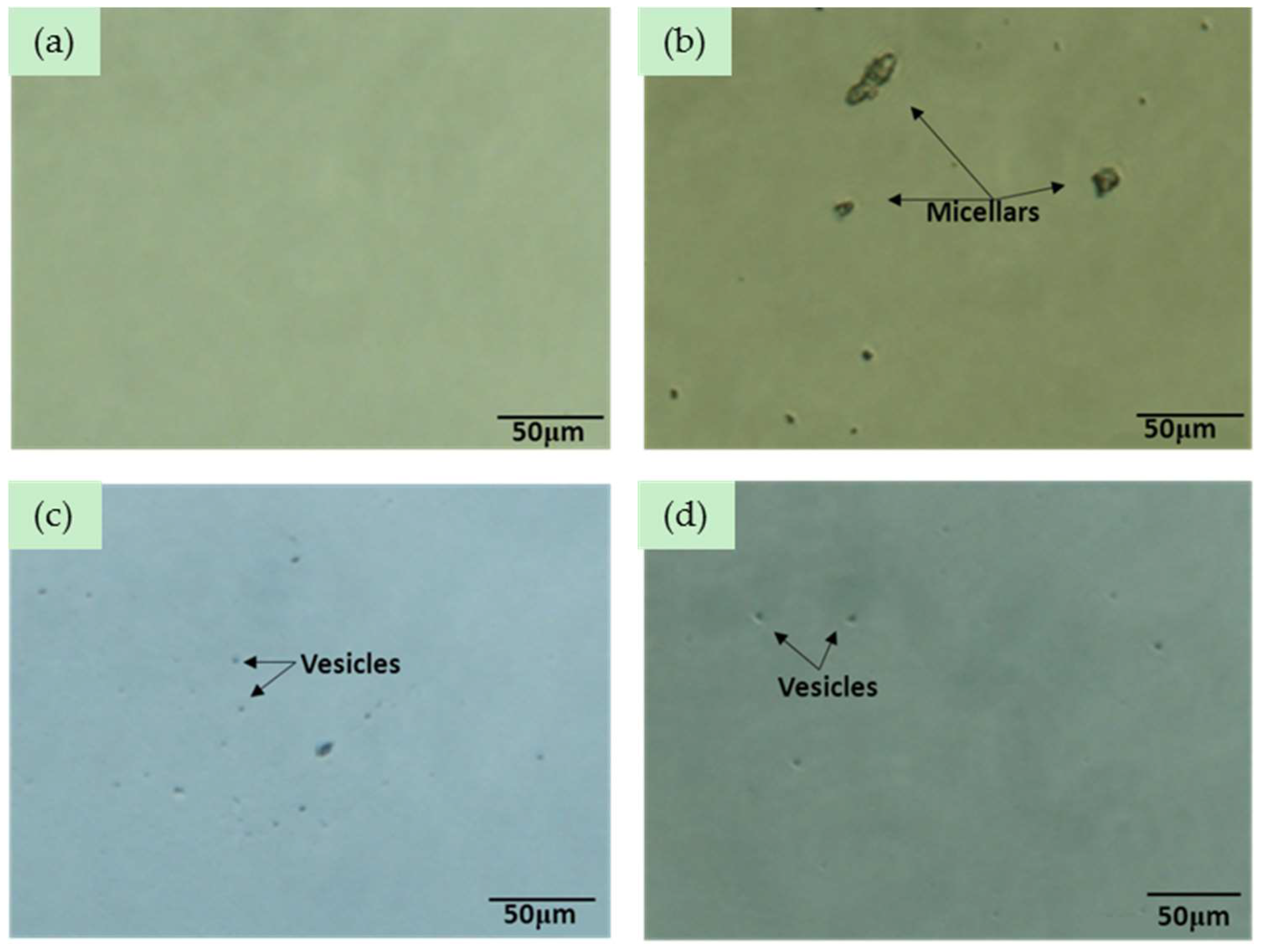
2.7. Factors that Stabilize HSYA in XBJ Injection
3. Materials and Methods
3.1. Reagents
3.2. HPLC Analysis
3.3. UPLC-Triple-Quattro/MS Method
3.4. Sample Preparation
3.5. Measurement of HSYA Stability at Different pHs
3.6. Measurement of HSYA Stability at Different Temperatures
3.7. Characterization of Degradation Products
3.8. The Effects of XBJ Components on HSYA Stability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ji, D.B.; Zhang, L.Y.; Li, C.L.; Ye, J.; Zhu, B.H. Effect of Hydroxysafflor yellow A on human umbilical vein endothelial cells under hypoxia. Vasc. Pharmacol. 2009, 50, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.H.; Zhang, L.; Zhang, W.H.; Rong, W.F.; Zhi, J.M. The effects of hydroxysafflor yellow A on blood pressure and cardiac function. J. Ethnopharmacol. 2012, 139, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.; Zhang, Q.; Liu, C.; Xie, H.; Yue, L.; Gao, X. Effects of hydroxy safflower Yellow-A on tumor capillary angiogenesis in transplanted human gastric adenocarcinoma BGC-823tumors in nude mice. J. Tradit. Chin. Med. 2012, 32, 243–248. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Lu, M.; Chen, Z.; Chen, C.; Han, L.; Xu, Y. Hydroxysafflor yellow A suppresses inflammatory responses of BV2 microglia after oxygen-glucose deprivation. Neurosci. Lett. 2013, 535, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Fan, L. Evidence-based application, adverse drug reactions and precaution of Xuebijing injection. Chin. J. New Drugs 2013, 22, 2449–2452. [Google Scholar]
- Lu, Y.Q.; Gu, L.H.; Huang, W.D. Effect of Xuebijing injection on peripheral T-lymphocyte subpopulations in patients with severe trauma. Chin. J. Traumatol. 2010, 13, 72–76. [Google Scholar] [PubMed]
- Shao, J.; Zhou, W.; Sheng, H.; Ni, T.; Lu, Y. Effect of Xuebijing injection on inflammatory factors in treatment of severe acute pancreatitis. Chin. J. New Drugs Clin. Remedies 2013, 32, 379–382. [Google Scholar]
- Qi, F.; Liang, Z.; She, D.; Yan, G.T.; Chen, L. A Clinical Study on the Effects and Mechanism of Xuebijing Injection in Severe Pneumonia Patients. J. Tradit. Chin. Med. 2011, 31, 46–49. [Google Scholar] [CrossRef]
- Huang, H.; Ji, L.; Song, S.; Wang, J.; Wei, N.; Jiang, M.; Luo, G. Identification of the major constituents in Xuebijing injection by HPLC-ESI-MS. Phytochem. Anal. 2011, 22, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Huang, H.; Jiang, M.; Bai, G.; Luo, G. Simultaneous HPLC determination of 11 essential compounds in Xuebijing injection. Chin. J. Chin. Mater. Med. 2010, 35, 2395–2398. [Google Scholar]
- Jiang, M.; Zhou, M.; Han, Y.; Xing, L.; Zhao, H.; Dong, L.; Luo, G. Identification of NF-kappaB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J. Ethnopharmacol. 2013, 147, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Bansal, G.; Suthar, N.; Kaur, J.; Jain, A. Stability testing of herbal drugs: Challenges, regulatory compliance and perspectives. Phytother. Res. 2016, 30, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.A.; Yoshida, M.I.; Belinelo, V.J.; Valotto, R.S. Degradation kinetics of atorvastatin under stress conditions and chemical analysis by HPLC. Molecules 2013, 18, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, B.; David, L.; Chişbora, C.; Cimpoiu, C. Degradation kinetics of anthocyanins from European cranberrybush (Viburnum opulus L.) fruit extracts. Effects of temperature, pH and storage solvent. Molecules 2012, 17, 11655–11666. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Pu, R.; Zhao, H.; Liu, X.; Ma, C.; Wang, B.; Guo, D. Stability and degradation of hydroxysafflor yellow A and anhydrosafflor yellow B in the Safflower injection studied by HPLC-DAD-ESI-MSn. J. Chin. Pharm. Sci. 2011, 20, 47–56. [Google Scholar] [CrossRef]
- Li, H.; Huang, L.; Ping, Q.; Zhao, H. Study on the stability of Safflower Yellow. Strait Pharm. J. 2009, 21, 12–14. [Google Scholar]
- Li, X.; Huang, L.; Fu, Z. The influence of light on stability of Safflowler Yellow. Strait Pharm. J. 2011, 23, 64–66. [Google Scholar]
- Ren, A.; Gao, R.; Zhu, L.; He, S. Thermolability in the process of safflower infusion solution by HPLC fingerprint. Chin. Tradit. Pat. Med. 2009, 31, 165–168. [Google Scholar]
- ICH. Stability Testing of New Drug Substances and Products (Q1AR) International Conference on Harmonization; IFPMA: Gevena, Switzerland, 2000. [Google Scholar]
- Guo, Y.X.; Xiu, Z.L.; Zhang, D.J.; Wang, H.; Wang, L.X.; Xiao, H.B. Kinetics and mechanism of degradation of lithospermic acid B in aqueous solution. J. Pharm. Biomed. Anal. 2007, 43, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Salmani, J.M. M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; Probing the effects of solvents, pH and light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.P.; Wang, C.R.; Lai, L.S. Thermal degradation kinetics analysis of monacolin K in Monascus-fermented products. Lwt-Food Sci. Technol. 2009, 42, 292–296. [Google Scholar] [CrossRef]
- Paula, H.; Aaron, D.P.; Michael, D.J. Study of relative response factors and mass balance in forced degradation studies with liquid chromatography/photo-diode array detector/evaporative light scattering detector/mass spectrometry system. J. Chromatogr. A 2017, 1512, 61–70. [Google Scholar]
- Liu, Y. Pharmacodynamic Substances Studies of Active Fractions from Panax Notoginseng (Burk.) F.H. Chen & Fragmentation Behaviors of Quinochalone Compounds from Carthamus tinctorius L. and HSYA’s Internal Distribution Studies. Ph.D. Thesis, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China, 2009. [Google Scholar]
- Chawla, H.M.; Chibber, S.S. Biologically patterned sensitized photooxygenation of chalcones. Tetrahedron Lett. 1976, 17, 2171–2172. [Google Scholar] [CrossRef]
- Montenegro, M.A.; Nazareno, M.A.; Borsarelli, C.D. Kinetic study of the photosensitized oxygenation of the flavanone naringin and its chalcone. J. Photoch. Photobiol. A 2007, 186, 47–56. [Google Scholar] [CrossRef]
- Cisak, A.; Mielczarek, C. Practical and Theoretical Aspects of Flavanone-Chalcone Isomerisations. J. Chem. Soc. Perkin Trans. 1992, 9, 1603–1607. [Google Scholar] [CrossRef]
- Maswal, M.; Dar, A.A. Mixed micelles of sodium cholate and Brij30: Their rheological behaviour and capability towards solubilization and stabilization of rifampicin. Colloids Surf. A 2013, 436, 704–713. [Google Scholar] [CrossRef]
- Dai, X.X.; Shi, X.Y.; Yin, Q.Q.; Ding, H.O.; Qiao, Y.J. Multiscale study on the interaction mechanism between ginsenoside biosurfactant and saikosaponin. J. Colloid Interface Sci. 2013, 396, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.A.; Muller, B.W. Solubility and stability of clonazepam in mixed micelles. Int. J. Pharm. 1998, 169, 55–64. [Google Scholar] [CrossRef]
- Liu, X.; Zhi, H.; Du, F.; Ye, Z.; Wang, N.; Qin, W.; Li, J. A HPLC-UV method for the determination of puerarin in rat plasma after intravenous administration of PEGylated puerarin conjugate. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| pH | HSYA in Aqueous Solution | HSYA in XBJ Injection | ||||
|---|---|---|---|---|---|---|
| k (h−1) | r2 | t1/2 (h) | k (h−1) | r2 | t1/2 (h) | |
| 1.44 | 0.018 | 0.996 | 38.5 | 0.008 | 0.996 | 86.6 |
| 2.05 | 0.013 | 0.995 | 53.3 | 0.004 | 0.986 | 173.3 |
| 3.05 | 0.009 | 0.992 | 77.0 | 0.001 | 0.986 | 693.0 |
| 5.06 | 0.005 | 0.971 | 138.6 | 0.0008 | 0.992 | 866.3 |
| 6.13 | 0.001 | 0.985 | 693.0 | 0.001 | 0.980 | 693.0 |
| 7.10 | 0.003 | 0.987 | 231.0 | 0.005 | 0.975 | 693.0 |
| 8.15 | 0.051 | 0.984 | 13.6 | 0.005 | 0.996 | 138.6 |
| 8.55 | 0.061 | 0.989 | 11.4 | 0.007 | 0.984 | 138.6 |
| 9.16 | 0.283 | 0.910 | 2.4 | 0.008 | 0.990 | 99.0 |
| 9.50 | 0.247 | 0.992 | 2.8 | 0.013 | 0.991 | 77.0 |
| 10.15 | 0.215 | 0.991 | 3.2 | 0.011 | 0.990 | 53.3 |
| 10.50 | 0.114 | 0.985 | 6.1 | 0.011 | 0.991 | 63.0 |
| 10.95 | 0.104 | 0.983 | 6.7 | 0.008 | 0.981 | 63.0 |
| 11.56 | 0.081 | 0.99 | 8.6 | 0.008 | 0.989 | 86.6 |
| 12.73 | 0.023 | 0.985 | 30.1 | 0.008 | 0.996 | 86.6 |
| T/°C | HSYA in XBJ Injection | HSYA in Aqueous Solution | ||||
|---|---|---|---|---|---|---|
| k (h−1) | R2 | Ea (kJ∙mol−1) | k (h−1) | R2 | Ea (kJ∙mol−1) | |
| 65 | 0.002 | 0.985 | 92.90 | 0.033 | 0.994 | 78.53 |
| 75 | 0.014 | 0.985 | 0.163 | 0.992 | ||
| 85 | 0.021 | 0.971 | 0.203 | 0.983 | ||
| 95 | 0.034 | 0.987 | 0.379 | 0.985 | ||
| Compounds | t (min) | UV (λ, nm) | [M − H]+ | [M − H]− | Fragmentations |
|---|---|---|---|---|---|
| HSYA | 4.72 | 228,404 | 613 | 611 | 593, 491; [M − H2O]−, [M − C4H8O4]− |
| Product 1 | 3.08 | 290, 376 | 627 | 625 | 505; [M − C4H8O4]− |
| Product 2 | 2.49 | 280, 383 | 627 | 625 | 505; [M − C4H8O4]− |
| k (h−1) | r2 | t1/2 (h) | t0.9 (h) | |
|---|---|---|---|---|
| HSYA | 0.283 | 0.910 | 2.45 | 0.37 |
| WSX | 0.091 | 0.963 | 7.62 | 1.15 |
| EEX | 0.015 | 0.981 | 46.2 | 7.03 |
| XBJ | 0.008 | 0.990 | 86.6 | 13.1 |
| HSYA+mixture | 0.136 | 0.942 | 5.10 | 0.78 |
| Particle Size (μm) | |||
|---|---|---|---|
| Samples | d(0.1)/μm | d(0.5)/μm | d(0.9)/μm |
| XBJ injection | 0.851 | 6.116 | 58.531 |
| XBJ + Water | 5.201 | 47.210 | 78.927 |
| XBJ + Buffer | 35.759 | 729.872 | 894.720 |
| Buffer | 3.030 | 12.472 | 26.260 |
| HSYA + Water | 2.258 | 32.988 | 159.783 |
| HSYA + Buffer | 4.528 | 36.681 | 647.348 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, W.; Zhang, H.; Wang, M.; Liu, Y.; Sun, L.; Ren, X. Superior Stability of Hydroxysafflor Yellow A in Xuebijing Injection and the Associated Mechanism. Molecules 2017, 22, 2129. https://doi.org/10.3390/molecules22122129
Pu W, Zhang H, Wang M, Liu Y, Sun L, Ren X. Superior Stability of Hydroxysafflor Yellow A in Xuebijing Injection and the Associated Mechanism. Molecules. 2017; 22(12):2129. https://doi.org/10.3390/molecules22122129
Chicago/Turabian StylePu, Weiling, Huijie Zhang, Meng Wang, Yanan Liu, Lili Sun, and Xiaoliang Ren. 2017. "Superior Stability of Hydroxysafflor Yellow A in Xuebijing Injection and the Associated Mechanism" Molecules 22, no. 12: 2129. https://doi.org/10.3390/molecules22122129