Phenolic Content and Antioxidant Activity in Raw and Denatured Aqueous Extracts from Sprouts and Wheatgrass of Einkorn and Emmer Obtained under Salinity
Abstract
:1. Introduction
2. Results
2.1. Germination and Growth Performance
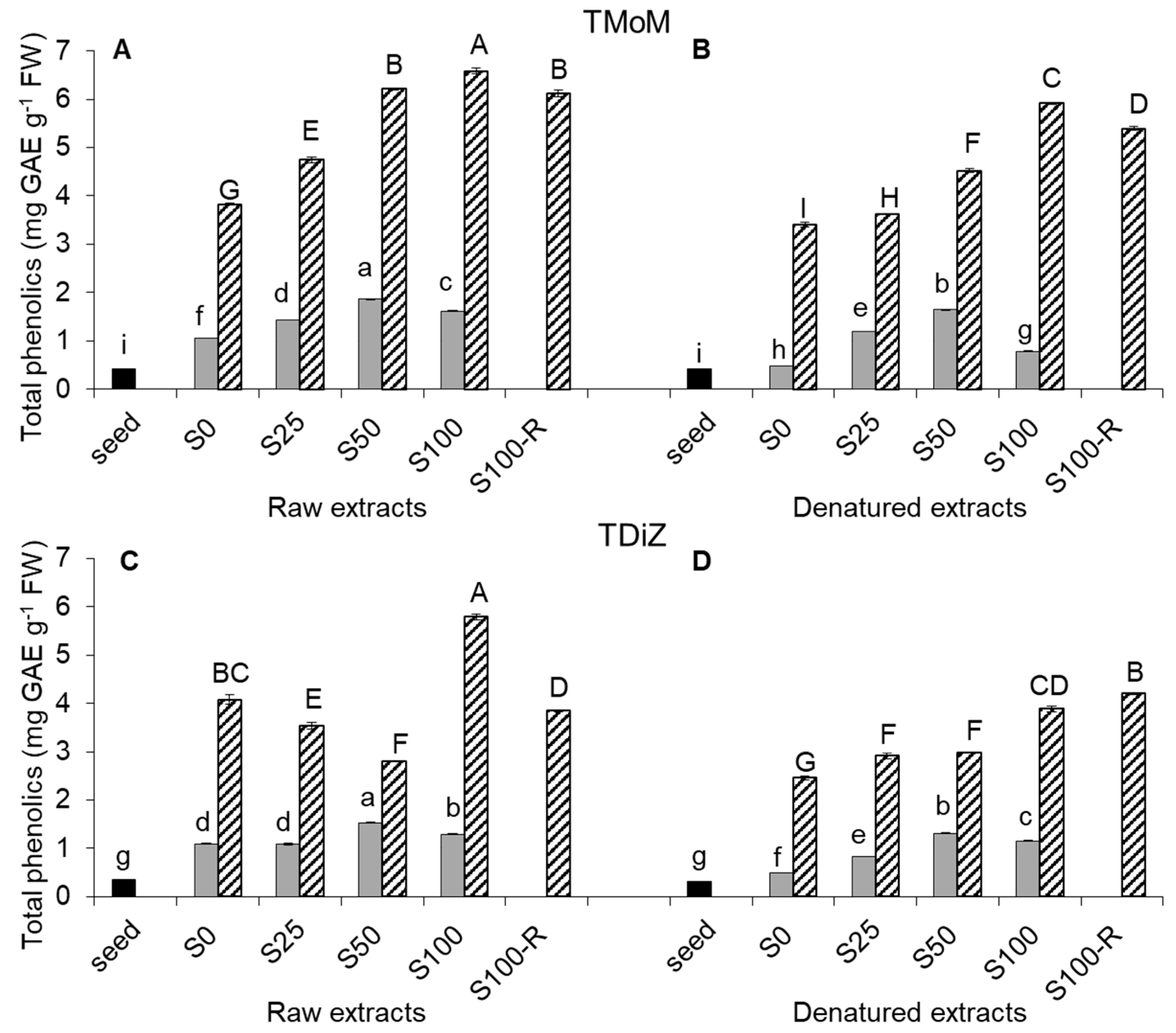
2.2. Total Phenolic Content
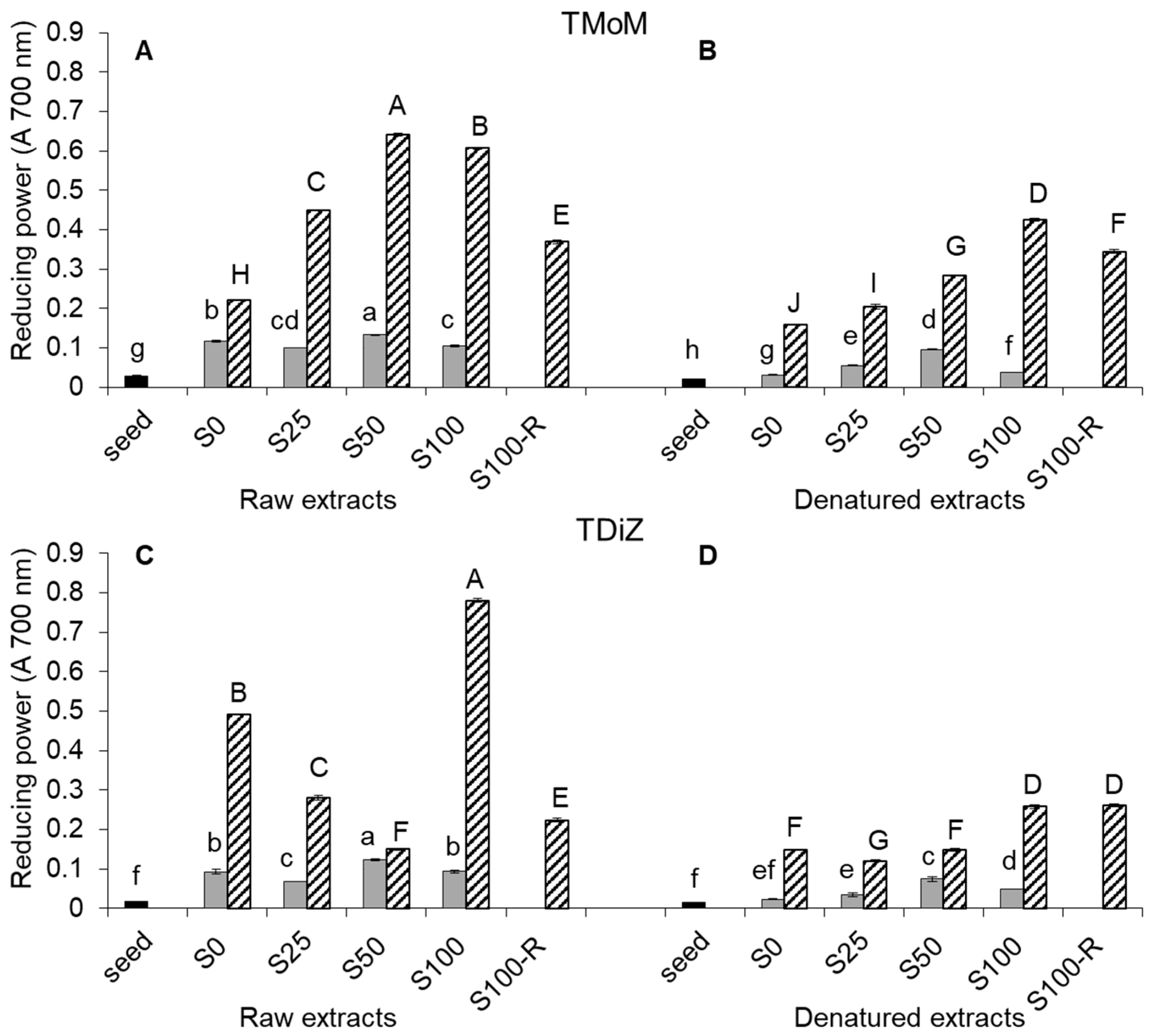
2.3. Reducing Power
2.4. Radical Scavenging
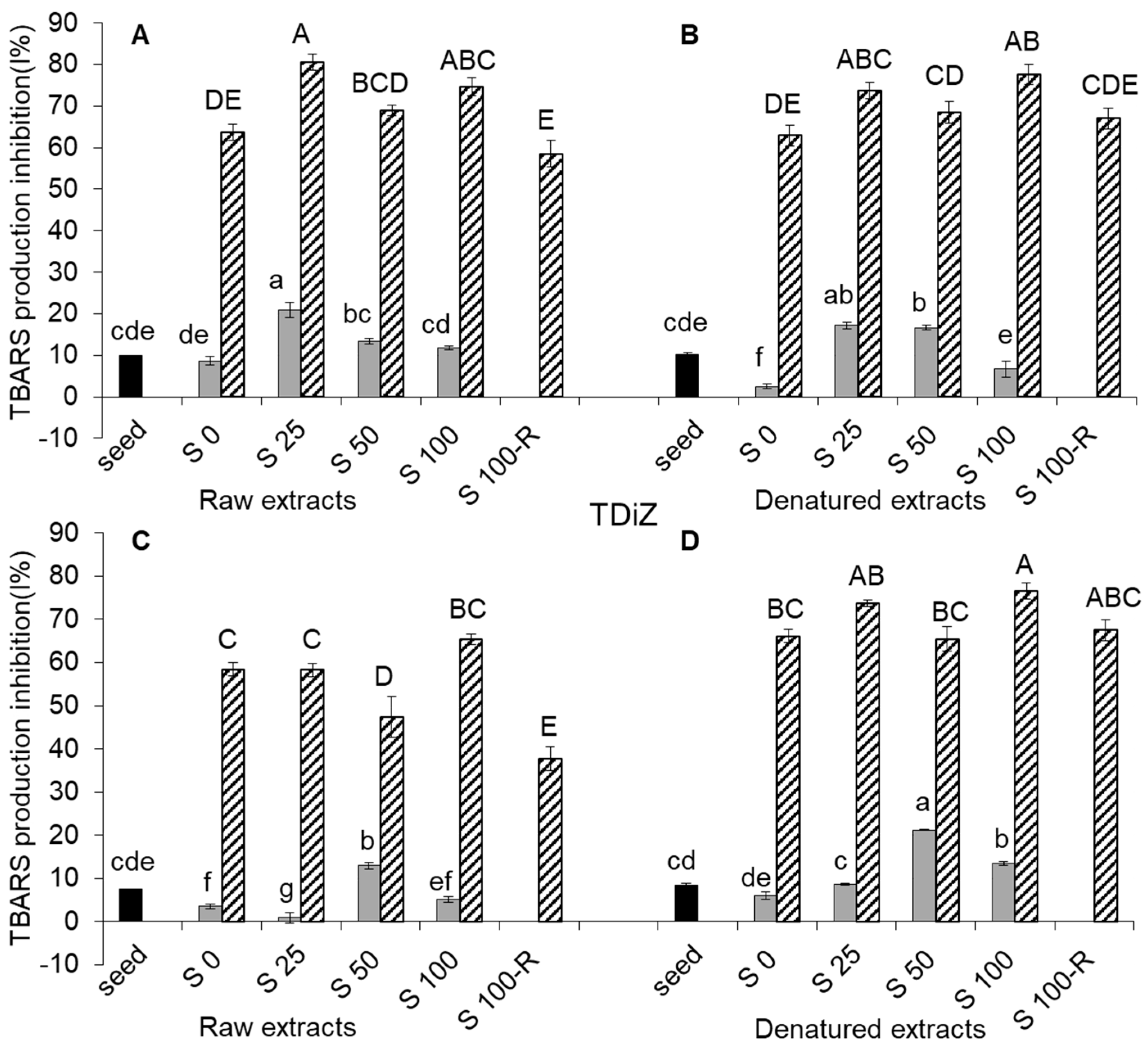
2.5. TBARS Production Inhibition
3. Discussion
4. Materials and Methods
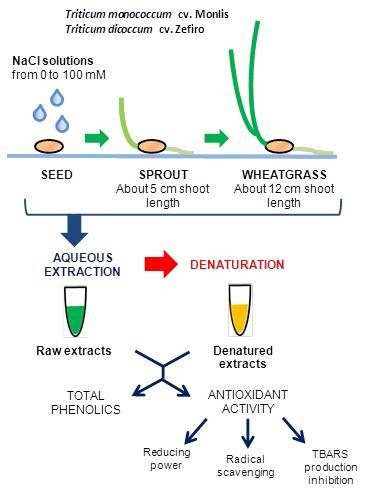
4.1. Plant Material and Experimental Design
4.2. Sample Preparation
4.3. Chemical Reagents
4.4. Total Phenolic Content
4.5. Reducing Power
4.6. Radical (Superoxide) Scavenging
4.7. TBARS Production (Lipid Peroxidation) Inhibition
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dykes, L.; Rooney, L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Food World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Lachman, L.; Hejtmankova, K.; Kotìkovà, Z. Tocols and carotenoids of einkorn, emmer and spring wheat varieties: Selection for breeding and production. J. Cereal Sci. 2013, 57, 207–214. [Google Scholar] [CrossRef]
- Calzuola, I.; Marsili, V.; Gianfranceschi, G.L. Synthesis of antioxidants in wheat sprouts. J. Agric. Food Chem. 2004, 52, 5201–5206. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Verma, P.; Pandey, B.R. Therapeutic potential of organic Triticum aestivum Linn. (Wheat Grass) in prevention and treatment of chronic disease: An overview. Int. J. Pharm. Sci. Drug Res. 2012, 4, 10–14. [Google Scholar]
- Falcioni, G.; Fedeli, D.; Tiano, L.; Calzuola, I.; Macinelli, L.; Marsili, V.; Gianfranceschi, G. Antioxidant activity of wheat sprouts extract in vitro: Inhibition of DNA oxidative damage. J. Food Sci. 2002, 67, 2918–2922. [Google Scholar] [CrossRef]
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated grains—Sources of bioactive compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Turkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 283–314. ISBN 978-1-4614-4747-4. [Google Scholar]
- Guo, L.; Yang, R.; Wang, Z.; Guo, Q.; Gu, Z. Effect of NaCl stress on health-promoting compounds and antioxidant activity in the sprouts of three broccoli cultivars. Int. J. Food Sci. Nutr. 2014, 65, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under moderate salinity increases phenolic content and antioxidant activity in rapeseed (Brassica napus var oleifera Del.) sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Park, K.J.; Kim, B.K.; Jeong, J.W.; Kim, H.J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.P.; Leong, L.P.; Koh, J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Drożdżyńska, A.; Gujska, E. Effect of boiling in water of barley and buckwheat groats on the antioxidant properties and dietary fiber composition. Plant Food Hum. Nutr. 2014, 69, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Bradford, K.J. Water relations in seed germination. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 351–396. [Google Scholar]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallangher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, X.; Choi, J.H. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish. J. Agric. Food Chem. 2006, 54, 7263–7269. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ouyang, C.; Wang, S.; Xu, Y.; Tang, L.; Chen, F. Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings. Plant Soil Environ. 2008, 54, 374–381. [Google Scholar]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Marjan, M.Z.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.J.; McDonald, M.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Sholz, G.; Lalijie, S.; Somoza, V.; Knorr, D.; Jasti, P.-R.; Eisenbrand, G. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Sari, F.; Velioglu, S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Ramesh, C.K.; Rehman, A.; Prabhakar, B.T.; Vijay Avin, B.R.; Aditya Rao, S.J. Antioxidant potentials in sprouts vs. seeds of Vigna radiata and Macrotyloma uniflorum. J. Appl. Pharm. Sci. 2011, 1, 99–103. [Google Scholar]
- Liu, B.; Guo, X.; Zhu, K.; Liu, Y. Nutritional evaluation and antioxidant activity of sesame sprouts. Food Chem. 2011, 129, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovic, J.; Radotic, K.; Mitrovic, A. Changes in activities of antioxidant enzymes during Chenopodium murale seed germination. Biol. Plant. 2008, 52, 396–400. [Google Scholar] [CrossRef]
- Esfandiari, E.; Shekari, F.; Shekari, F.; Esfandiari, M. The effect of salt stress on antioxidant enzymes’ activity and lipid peroxidation on the wheat seedling. Not. Bot. Horti Agrobot. 2007, 35, 48–56. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C.; Agarwal, S.; Meena, R.C. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant. 2005, 49, 85–91. [Google Scholar] [CrossRef]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. India 2002, 82, 1227–1238. [Google Scholar]
- Koracevic, D.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Jimenèz-Monreal, A.M.; Garcìa-Diz, L.; Martìnez-Tomè, M.; Mariscal, M.; Murcia, M.A. Influence of cooking methods on antioxidant activity of vegetables. J. Food Sci. 2009, 74, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Pasdar, N.; Li-Chan, E.C.Y. Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Kirby, A.J.; Schmidt, R.J. The antioxidant activity of Chinese herbs for eczema and of placebo herbs. J. Ethnopharmacol. 1997, 56, 103–108. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Genotype | Treatment (mM) | G (%) | T50 (d) | Wheatgrass Individuals (%) |
|---|---|---|---|---|
| TMoM | S0 | 82 (1.5) | 3.4 (0.1) | 96 (1.3) |
| S25 | 84 (0.3) | 3.5 (0.2) | 99 (1.2) | |
| S50 | 85 (1.3) | 3.8 (0.1) | 96 (1.1) | |
| S100 | 82 (0.7) | 4.1 (0.2) | 94 (1.2) | |
| S100-R | - | - | 97 (1.0) | |
| TDiZ | S0 | 79 (3.3) | 3.6 (0.0) | 99 (1.3) |
| S25 | 81 (1.1) | 3.5 (0.3) | 92 (0.1) | |
| S50 | 85 (2.7) | 4.2 (0.1) | 77 (8.4) | |
| S100 | 76 (4.3) | 5.5 (0.6) | 11 (1.0) | |
| S100-R | - | - | 90 (2.5) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcinelli, B.; Benincasa, P.; Calzuola, I.; Gigliarelli, L.; Lutts, S.; Marsili, V. Phenolic Content and Antioxidant Activity in Raw and Denatured Aqueous Extracts from Sprouts and Wheatgrass of Einkorn and Emmer Obtained under Salinity. Molecules 2017, 22, 2132. https://doi.org/10.3390/molecules22122132
Falcinelli B, Benincasa P, Calzuola I, Gigliarelli L, Lutts S, Marsili V. Phenolic Content and Antioxidant Activity in Raw and Denatured Aqueous Extracts from Sprouts and Wheatgrass of Einkorn and Emmer Obtained under Salinity. Molecules. 2017; 22(12):2132. https://doi.org/10.3390/molecules22122132
Chicago/Turabian StyleFalcinelli, Beatrice, Paolo Benincasa, Isabella Calzuola, Lilia Gigliarelli, Stanley Lutts, and Valeria Marsili. 2017. "Phenolic Content and Antioxidant Activity in Raw and Denatured Aqueous Extracts from Sprouts and Wheatgrass of Einkorn and Emmer Obtained under Salinity" Molecules 22, no. 12: 2132. https://doi.org/10.3390/molecules22122132