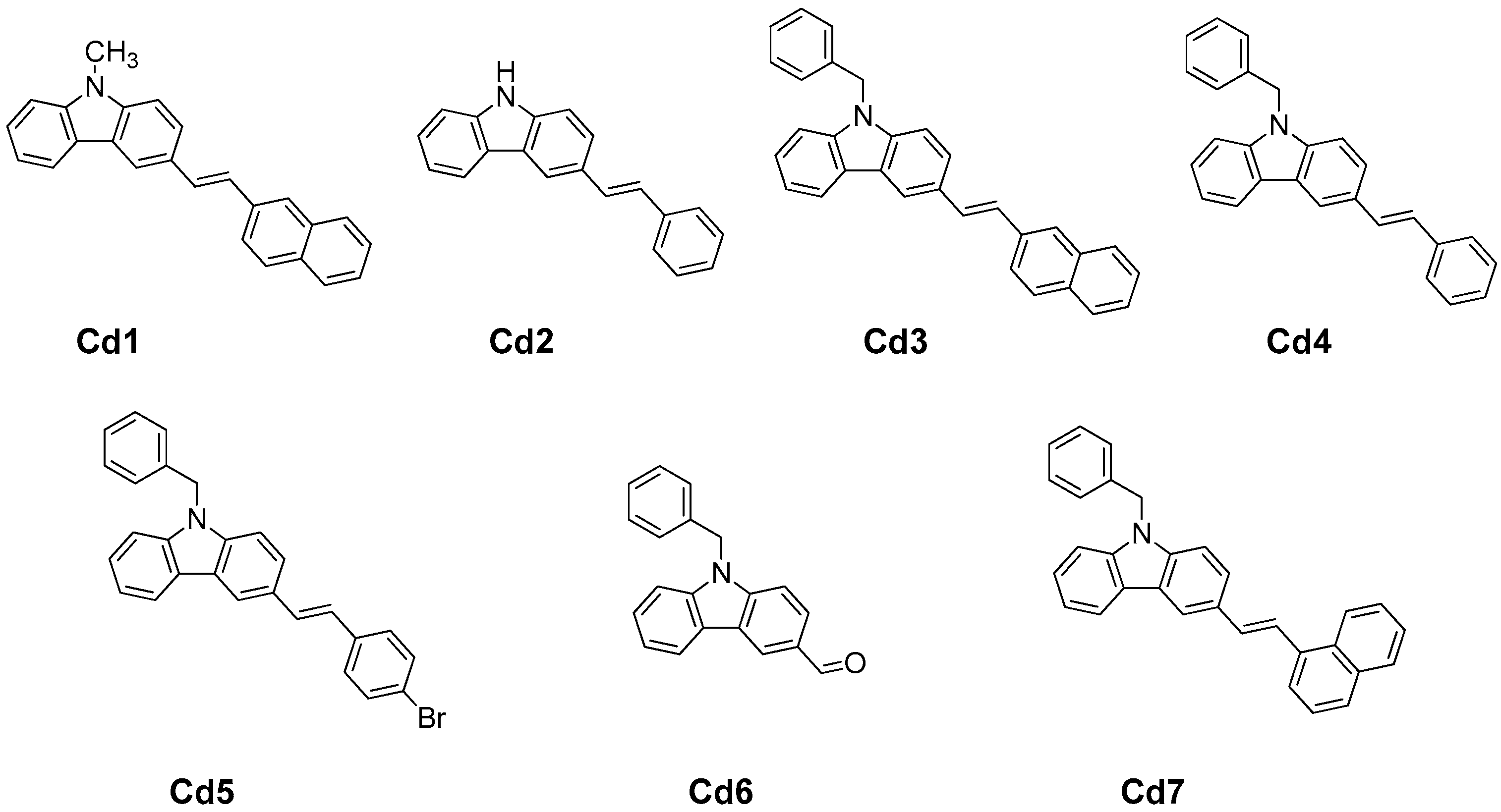
Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Results
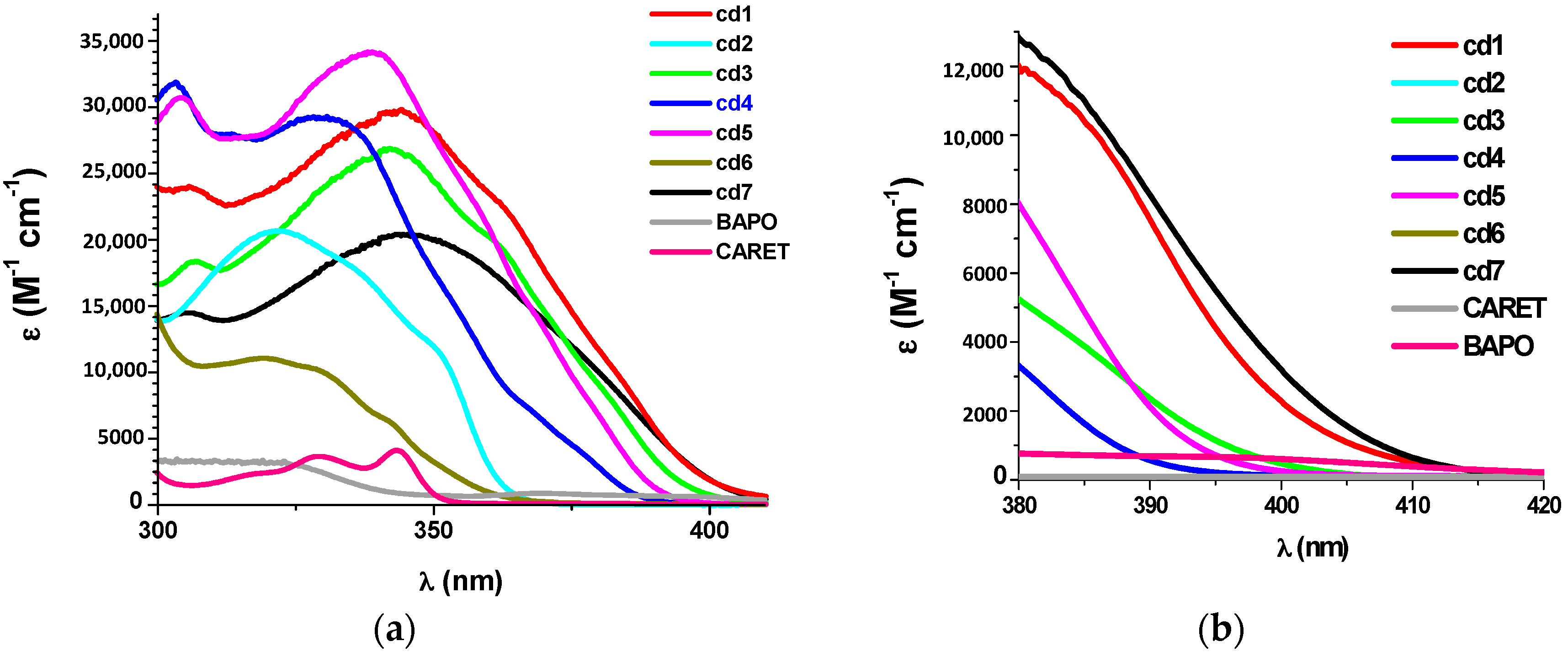
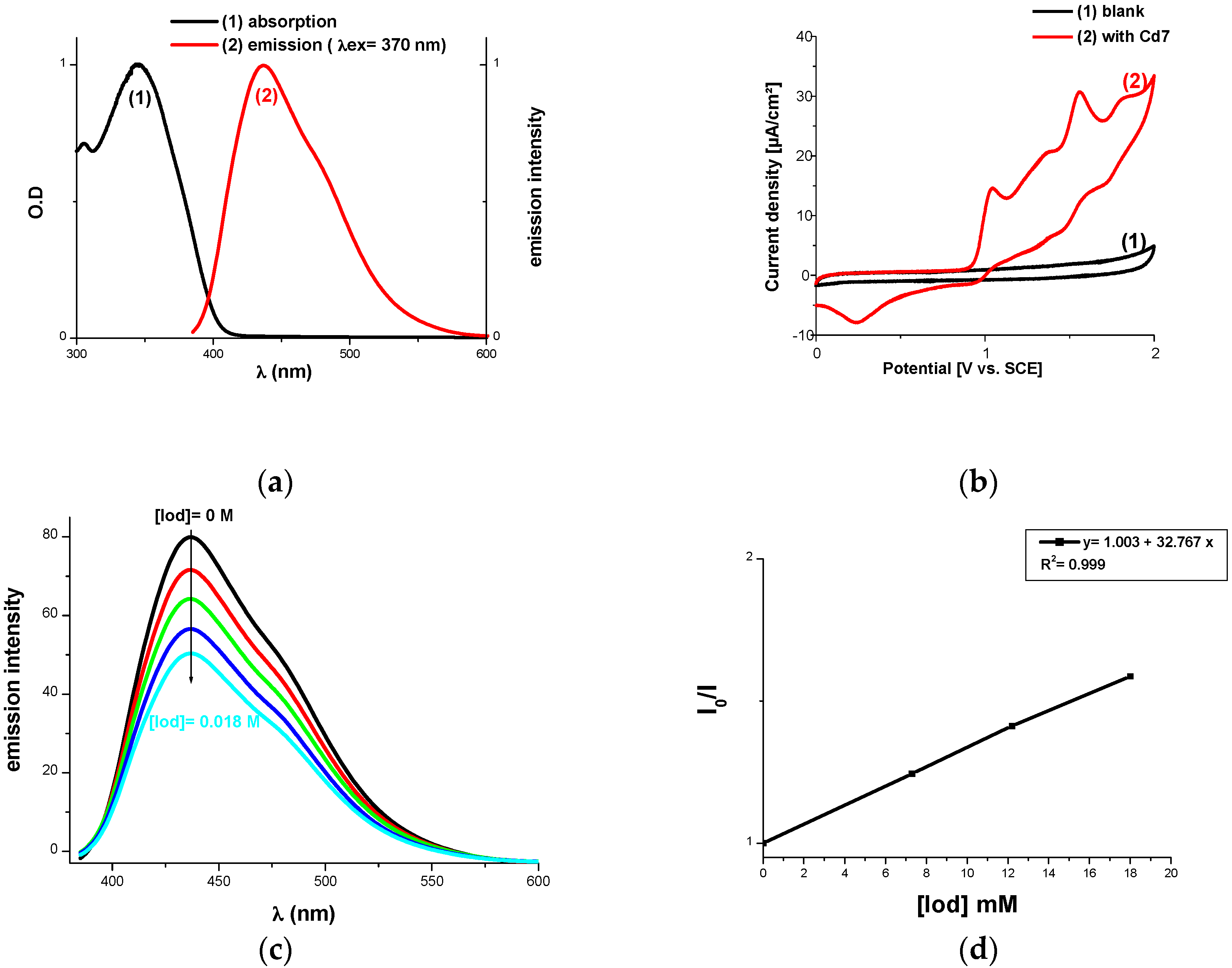
2.1.1. Light Absorption Properties of Cd1–Cd7
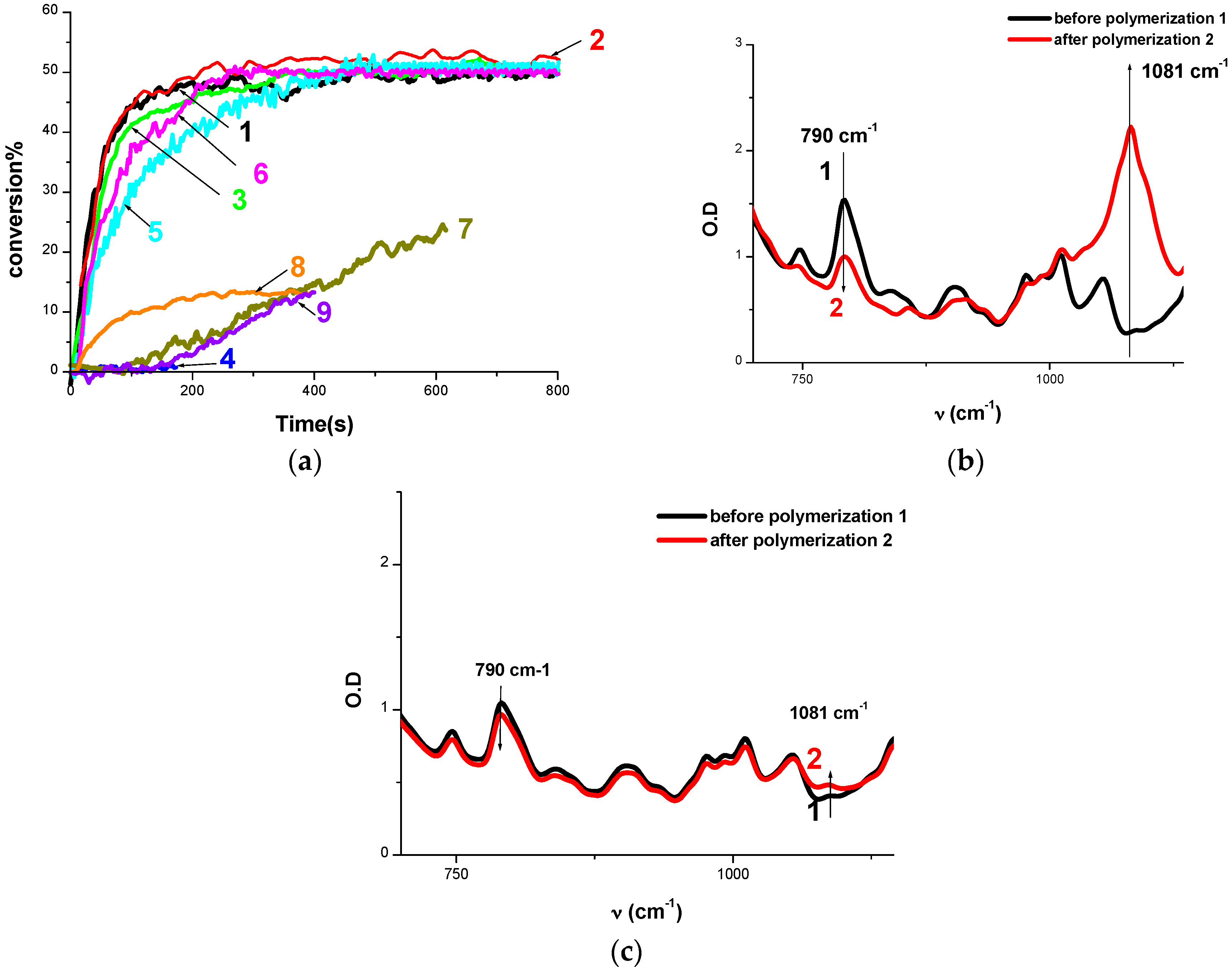
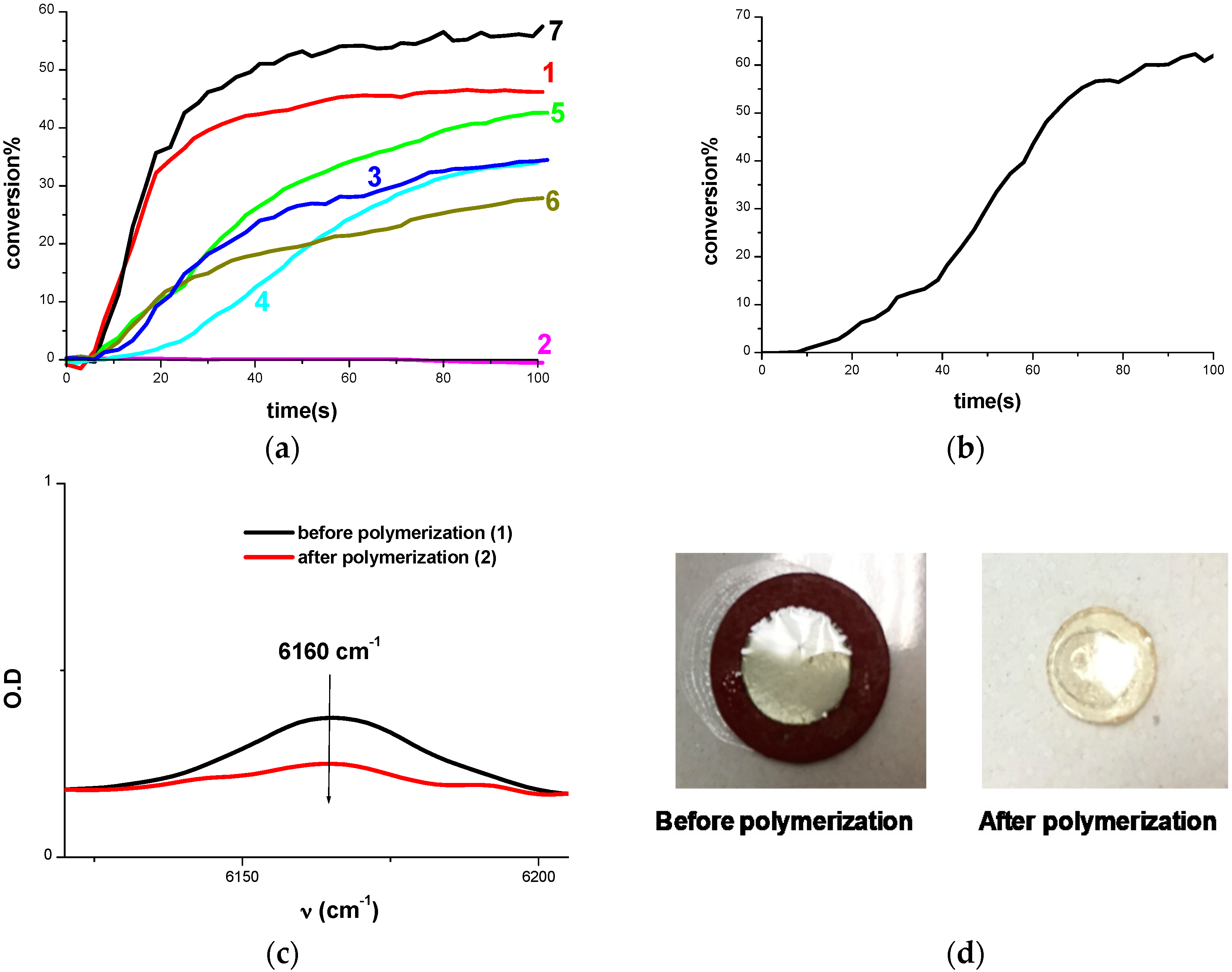
2.1.2. Cationic Photopolymerization (CP) of Epoxides
2.1.3. Free Radical Photopolymerization
2.1.4. Synthesis of Inter-Penetrated Networks (IPNs) through Photopolymerization
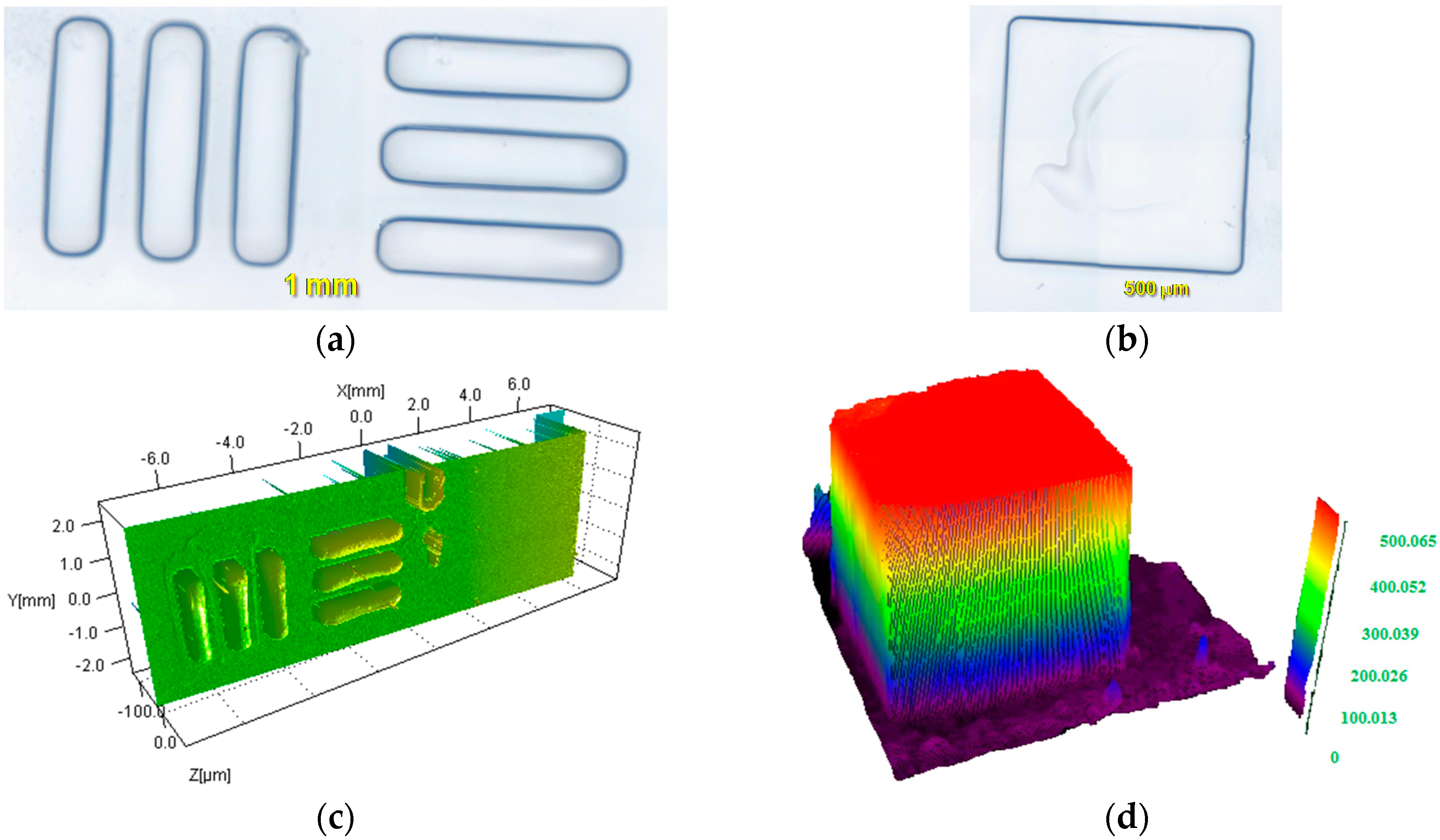
2.1.5. Surface Patterning or 3D Printing Using Cd1–Cd7 Based System upon LED@405 nm Projector
2.2. Photochemical Mechanisms
2.2.1. Steady State Photolysis
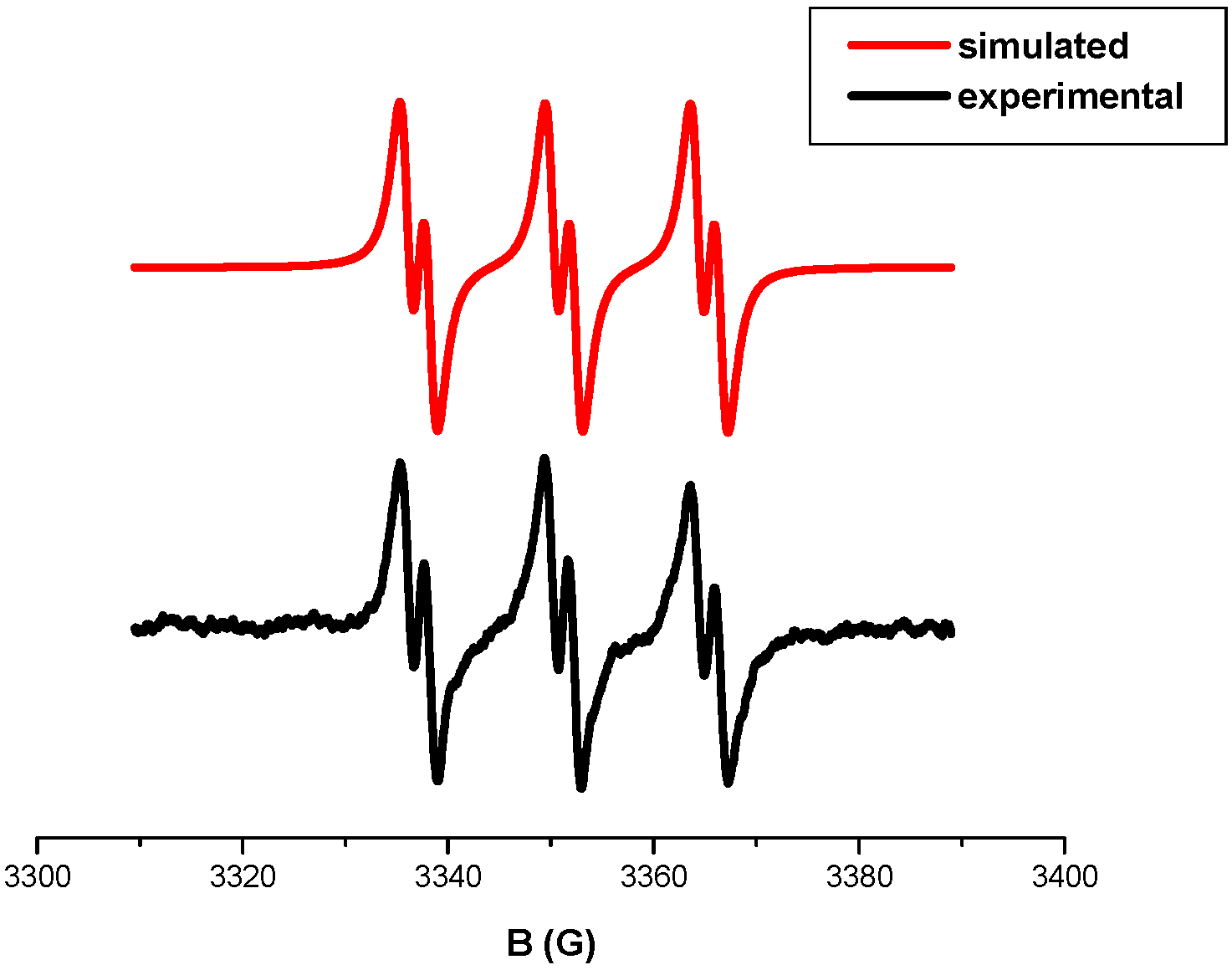
2.2.2. Fluorescence Quenching, Laser Flash Photolysis (LFP), Cyclic Voltammetry and ESR Experiments
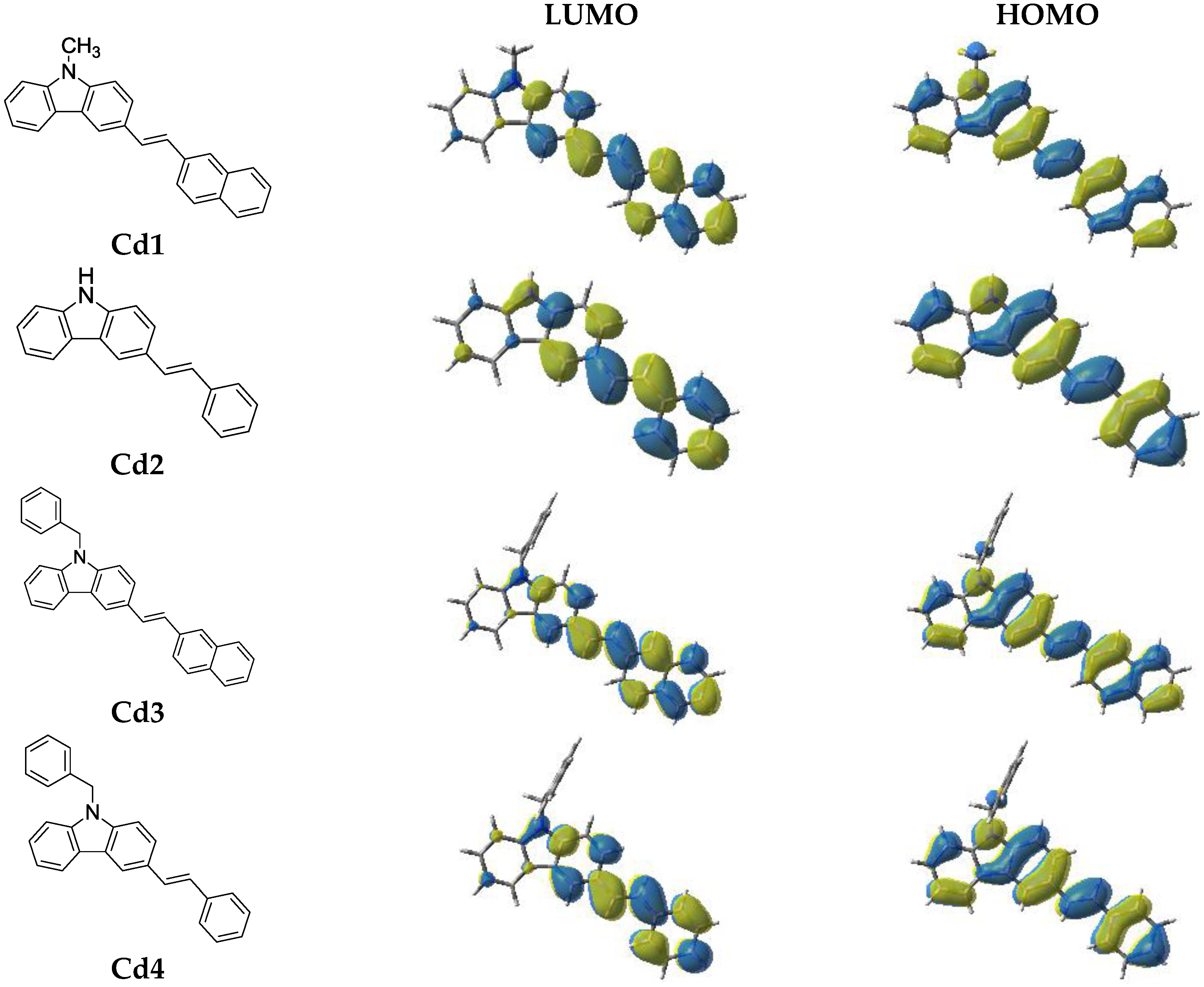
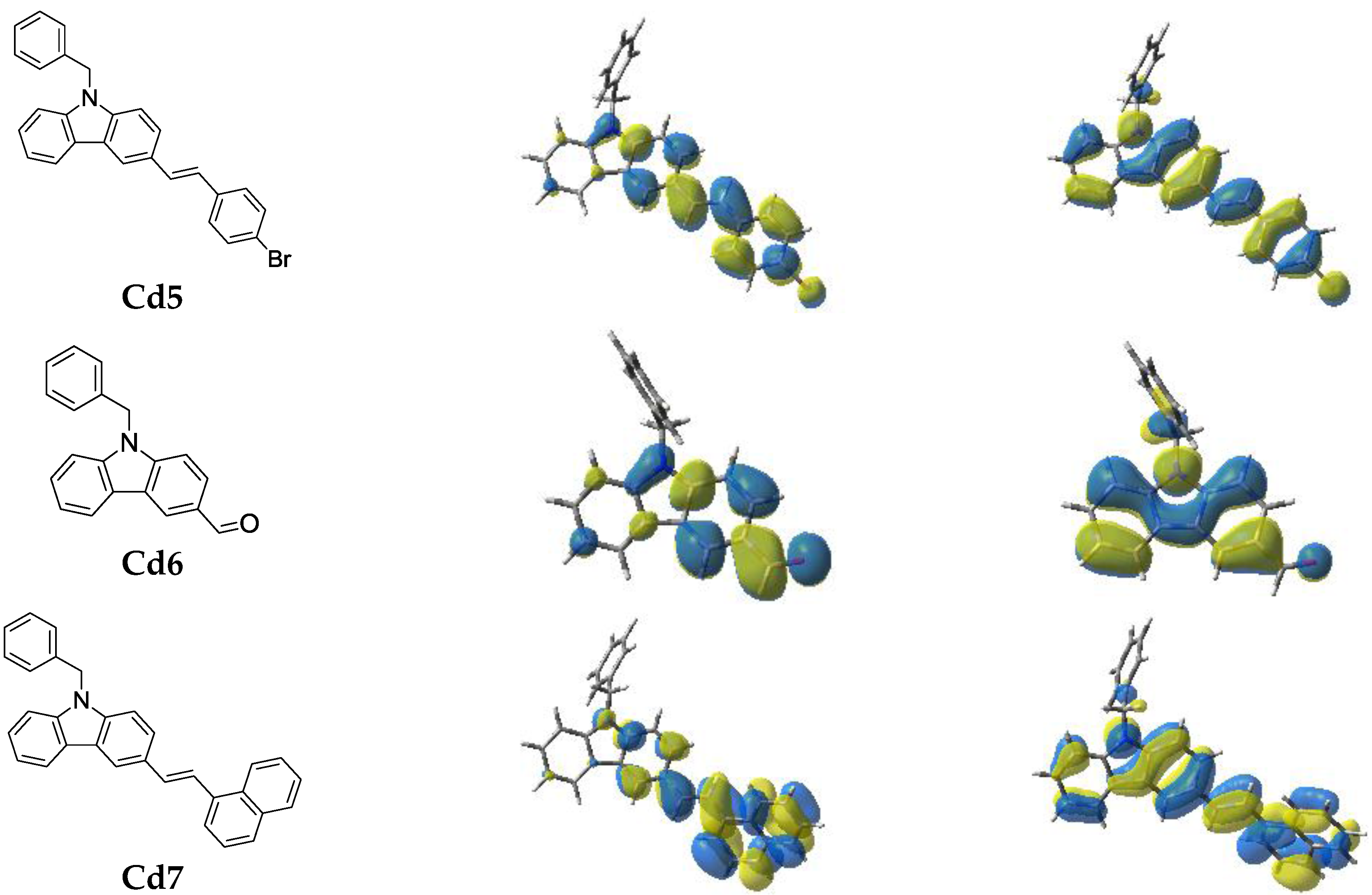
2.2.3. Structure/Reactivity/Efficiency Relationship
3. Materials and Methods
3.1. Chemical Compounds
3.2. Irradiation Sources
3.3. Free Radical (FRP) and Cationic (CP) Photopolymerization
3.4. Redox Potentials
3.5. ESR Spin Trapping (ESR-ST) Experiments
3.6. Absorption Experiments
3.7. Fluorescence Experiments
3.8. Computational Procedure
3.9. 3D Printing Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fouassier, J.-P.; Lalevée, J. Photoinitiators for Polymer Synthesis, Scope, Reactivity, and Efficiency; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; ISBN 978-3-527-33210-6. [Google Scholar]
- Fouassier, J.P. Photoinitiation, Photopolymerization and Photocuring: Fundamentals and Applications; Gardner Publications: New York, NY, USA, 1995; ISBN-10 1569901465. [Google Scholar]
- Dietliker, K.A. Compilation of Photoinitiators Commercially Available for UV Today; Sita Technology Ltd.: London, UK, 2002; ISBN 0947798676 9780947798673. [Google Scholar]
- Davidson, S. Exploring the Science, Technology and Application of UV and EB Curing; Sita Technology Ltd.: London, UK, 1999; ISBN-10 0947798412. [Google Scholar]
- Jenkins, A. Photoinitiators for Free Radical Cationic and Anionic Photopolymerisation, 2nd ed.; Crivello, J.V., Dietliker, K., Bradley, G., Eds.; John Wiley & Sons: Chichester, UK, 1998; ISBN 0-471-97892-2. [Google Scholar]
- Lalevée, J.; Fouassier, J.-P. (Eds.) Dyes and Chromophores in Polymer Science; ISTE Wiley: London, UK, 2015; ISBN 9781119006671. [Google Scholar]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New Photoinitiating Systems and Strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerelli, M.; Kermagoret, A.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photocatalysts in Polymerization Reactions. ChemCatChem 2016, 8, 1617–1631. [Google Scholar] [CrossRef]
- Treat, N.J.; Fors, B.P.; Kramer, J.W.; Christianson, M.; Chiu, C.-Y.; Read de Alaniz, J.; Hawker, C.J. Controlled Radical Polymerization of Acrylates Regulated by Visible Light. ACS Macro Lett. 2014, 3, 580–584. [Google Scholar] [CrossRef]
- Pan, X.; Lamson, M.; Yan, J.; Matyjaszewski, K. Photoinduced Metal-Free Atom Transfer Radical Polymerization of Acrylonitrile. ACS Macro Lett. 2015, 4, 192–196. [Google Scholar] [CrossRef]
- Boyer, C.; Corrigan, N.A.; Jung, K.; Nguyen, D.; Nguyen, T.-K.; Adnan, N.N.M.; Oliver, S.; Shanmugam, S.; Yeow, J. Copper-Mediated Living Radical Polymerization (Atom Transfer Radical Polymerization and Copper(0) Mediated Polymerization): From Fundamentals to Bioapplications. Chem. Rev. 2016, 116, 1803–1949. [Google Scholar] [CrossRef] [PubMed]
- Tasdelen, M.A.; Yilmaz, G.; Iskin, B.; Yagci, Y. Photoinduced Free Radical Promoted Copper(I)-Catalyzed Click Chemistry for Macromolecular Syntheses. Macromolecules 2012, 45, 56–61. [Google Scholar] [CrossRef]
- Corrigan, N.; Xu, J.; Boyer, C. A Photoinitiation System for Conventional and Controlled Radical Polymerization at Visible and NIR Wavelengths. Macromolecules 2016, 49, 3274–3285. [Google Scholar] [CrossRef]
- Yeow, J.; Shanmugam, S.; Corrigan, N.; Kuchel, R.P.; Xu, J.; Boyer, C. A Polymerization-Induced Self-Assembly Approach to Nanoparticles Loaded with Singlet Oxygen Generators. Macromolecules 2016, 49, 7277–7285. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Carbazole Scaffold Based Photoinitiator/Photoredox Catalysts: Toward New High Performance Photoinitiating Systems and Application in LED Projector 3D Printing Resins. Macromolecules 2017, 50, 2747–2758. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Chachaj-Brekiesz, A.; Ortyl, J.; Lalevée, J. Meta-Terphenyl Derivative/Iodonium Salt/9H-Carbazole-9-Ethanol Photoinitiating Systems for Free Radical Promoted Cationic Polymerization upon Visible Lights. Macromol. Chem. Phys. 2016, 217, 1955–1965. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Poriel, C.; Dumur, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Lalevée, J. Zinc Tetraphenylporphyrin as High Performance Visible Light Photoinitiator of Cationic Photosensitive Resins for LED Projector 3D Printing Applications. Macromolecules 2017, 50, 746–753. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Kermagoret, A.; Versace, D.-L.; Toufaily, J.; Hamieh, T.; Graff, B.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper Photoredox Catalysts for Polymerization upon near UV or Visible Light: Structure/Reactivity/Efficiency Relationships and Use in LED Projector 3D Printing Resins. Polym. Chem. 2017, 8, 568–580. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Photochemical Production of Interpenetrating Polymer Networks; Simultaneous Initiation of Radical and Cationic Polymerization Reactions. Polymers 2014, 6, 2588–2610. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.-A.; Peter, M.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Efficient Dual Radical/Cationic Photoinitiator under Visible Light: A New Concept. Polym. Chem. 2011, 2, 1986–1991. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.-A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. A Novel Photopolymerization Initiating System Based on an Iridium Complex Photocatalyst. Macromol. Rapid Commun. 2011, 32, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Rehm, D.; Weller, A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.-A.; Morlet-Savary, F.; Fouassier, J.P. Green Bulb Light Source Induced Epoxy Cationic Polymerization under Air Using Tris(2,2′-bipyridine)ruthenium(II) and Silyl Radicals. Macromolecules 2010, 43, 10191–10195. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian, Inc.: Pittsburgh, PA, USA, 1996; ISBN 0-9636769-3-8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, J.R.E.; Burant, J.C.; et al. Gaussian 03; Revision B.03; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structure Design of Naphthalimide Derivatives: Toward Versatile Photoinitiators for Near-UV/Visible LEDs, 3D Printing, and Water-Soluble Photoinitiating Systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Cd1–Cd7 are available from the authors. |


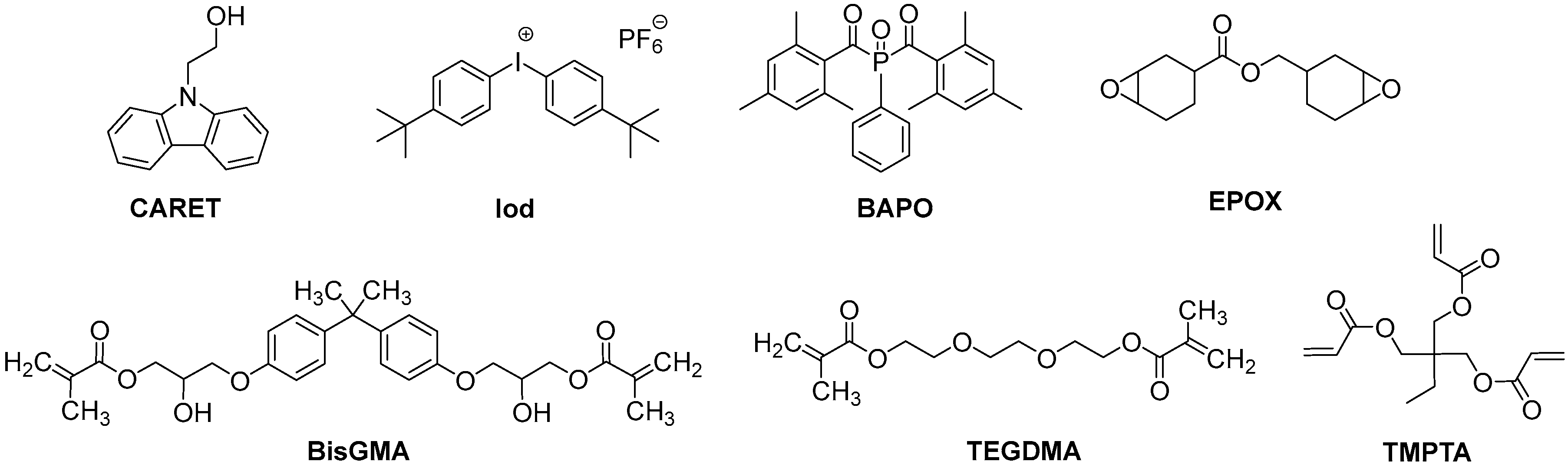
| Cd1 | Cd2 | Cd3 | Cd4 | Cd5 | Cd6 | Cd7 | BAPO | CARET | |
|---|---|---|---|---|---|---|---|---|---|
| ε 1 | 1000 | n.a 2 | 180 | 90 | 140 | n.a 2 | 1500 | 500 | n.a 2 |
| Cd1 | Cd2 | Cd3 | Cd4 | Cd5 | Cd6 | Cd7 | BAPO | CARET | |
|---|---|---|---|---|---|---|---|---|---|
| EPOX 1 | 44% | 0% | 41% | 27% | 30% | 37% | 44% | 10% | 1% |
| TMPTA 2 | 46% | 0% | 34% | 34% | 43% | 28% | 57% | 60% | |
| BisGMA/TEGDMA 3 | 57% | 0% | 60% |
| Eox (eV) 1 | ES1 (eV) | ΔGetS1 (eV) | ET1 (eV) 2 | ΔGetT1 2 (eV) | Φet (S1) | |
|---|---|---|---|---|---|---|
| Cd1 | 0.92 | 3.17 | −2.05 | 1.97 | −0.85 | 0.34 |
| Cd2 | 1.06 | 3.5 | −2.22 | 1.99 | −0.73 | 0.5 |
| Cd3 | 0.97 | 3.2 | −2.02 | 1.97 | −0.81 | 0.41 |
| Cd4 | 0.96 | 3.35 | −2.19 | 1.99 | −0.83 | 0.5 |
| Cd5 | 0.99 | 3.25 | −2.06 | 1.97 | −0.79 | |
| Cd6 | 1.49 | 3.5 | −1.81 | 3.04 | −1.36 | |
| Cd7 | 0.98 | 3.13 | −1.95 | 1.89 | −0.72 | 0.43 |
| Trend of bathochromic shift | Cd7~Cd1 > Cd3 > Cd5 > Cd4 > Cd2 > Cd6 |
| Trend of CP efficiency | Cd7~Cd1 > Cd3 > Cd5 > Cd4 > Cd6~Cd2 |
| Trend of FRP efficiency | Cd7 > Cd1 > Cd5 > Cd3 > Cd4 > Cd6~Cd2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mousawi, A.; Garra, P.; Dumur, F.; Bui, T.-T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; et al. Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing. Molecules 2017, 22, 2143. https://doi.org/10.3390/molecules22122143
Al Mousawi A, Garra P, Dumur F, Bui T-T, Goubard F, Toufaily J, Hamieh T, Graff B, Gigmes D, Fouassier JP, et al. Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing. Molecules. 2017; 22(12):2143. https://doi.org/10.3390/molecules22122143
Chicago/Turabian StyleAl Mousawi, Assi, Patxi Garra, Frédéric Dumur, Thanh-Tuan Bui, Fabrice Goubard, Joumana Toufaily, Tayssir Hamieh, Bernadette Graff, Didier Gigmes, Jean Pierre Fouassier, and et al. 2017. "Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing" Molecules 22, no. 12: 2143. https://doi.org/10.3390/molecules22122143








