Improved Performance of Magnetic Cross-Linked Lipase Aggregates by Interfacial Activation: A Robust and Magnetically Recyclable Biocatalyst for Transesterification of Jatropha Oil
Abstract
:1. Introduction
2. Results and Discussion
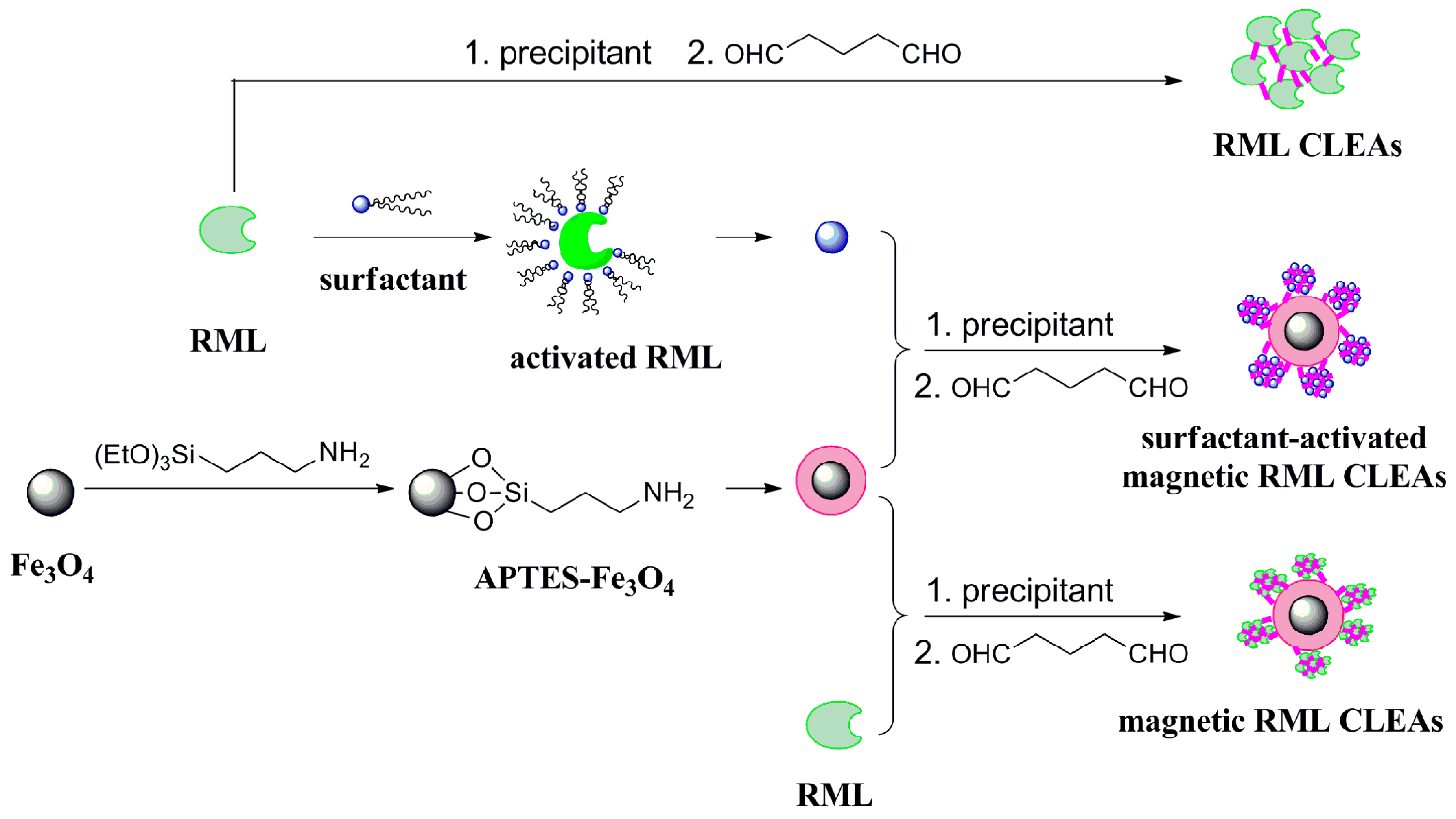
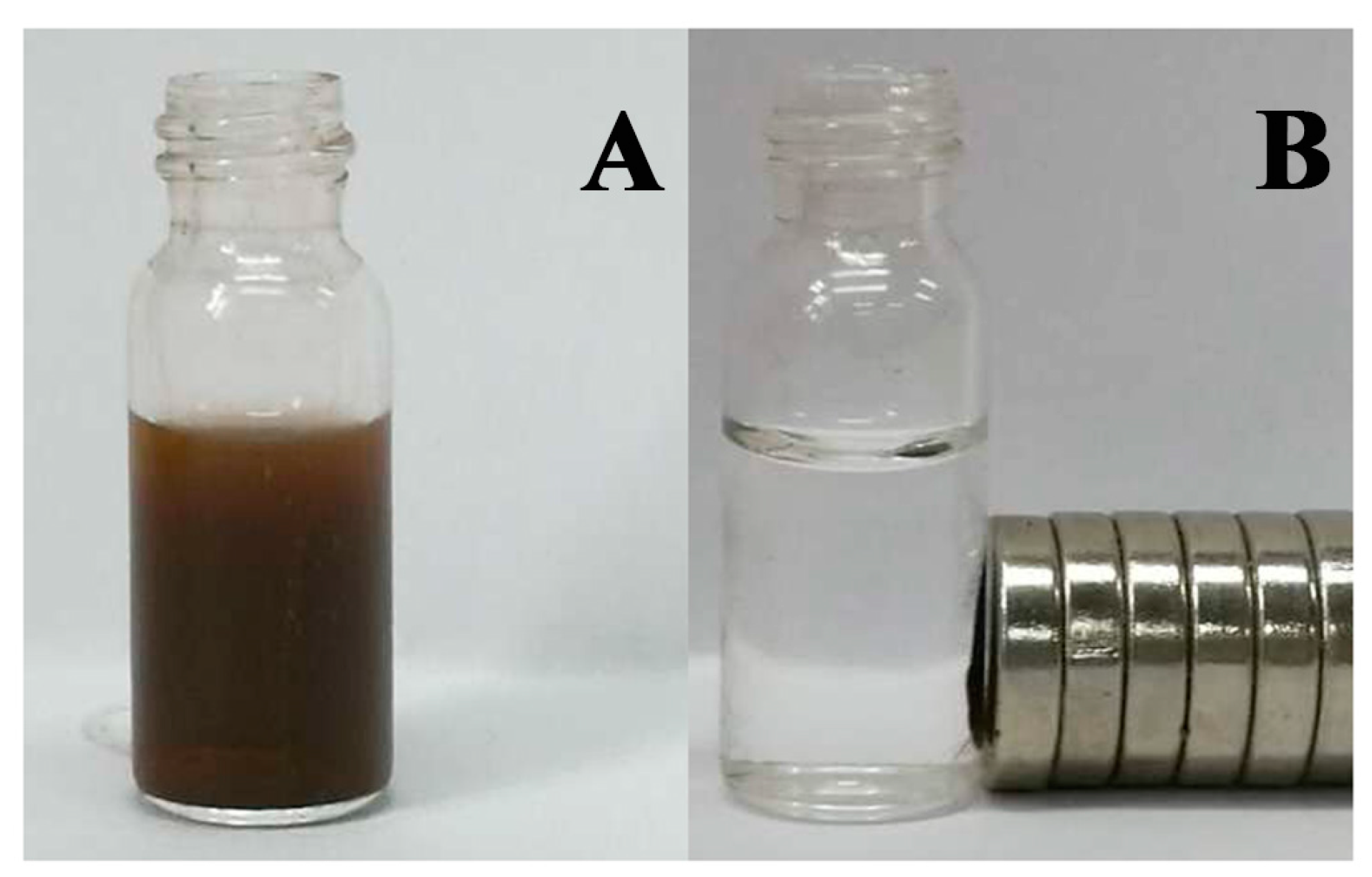
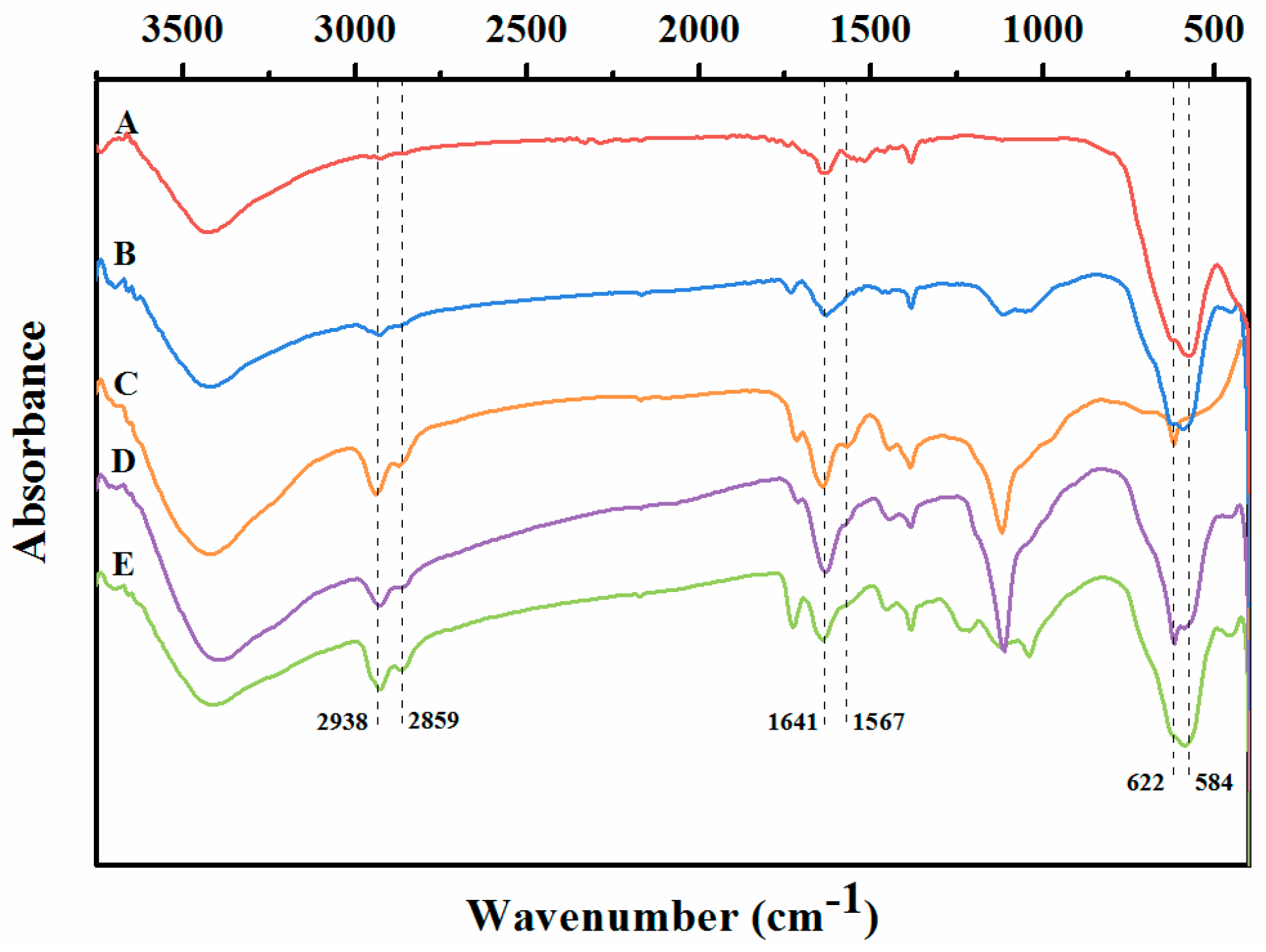
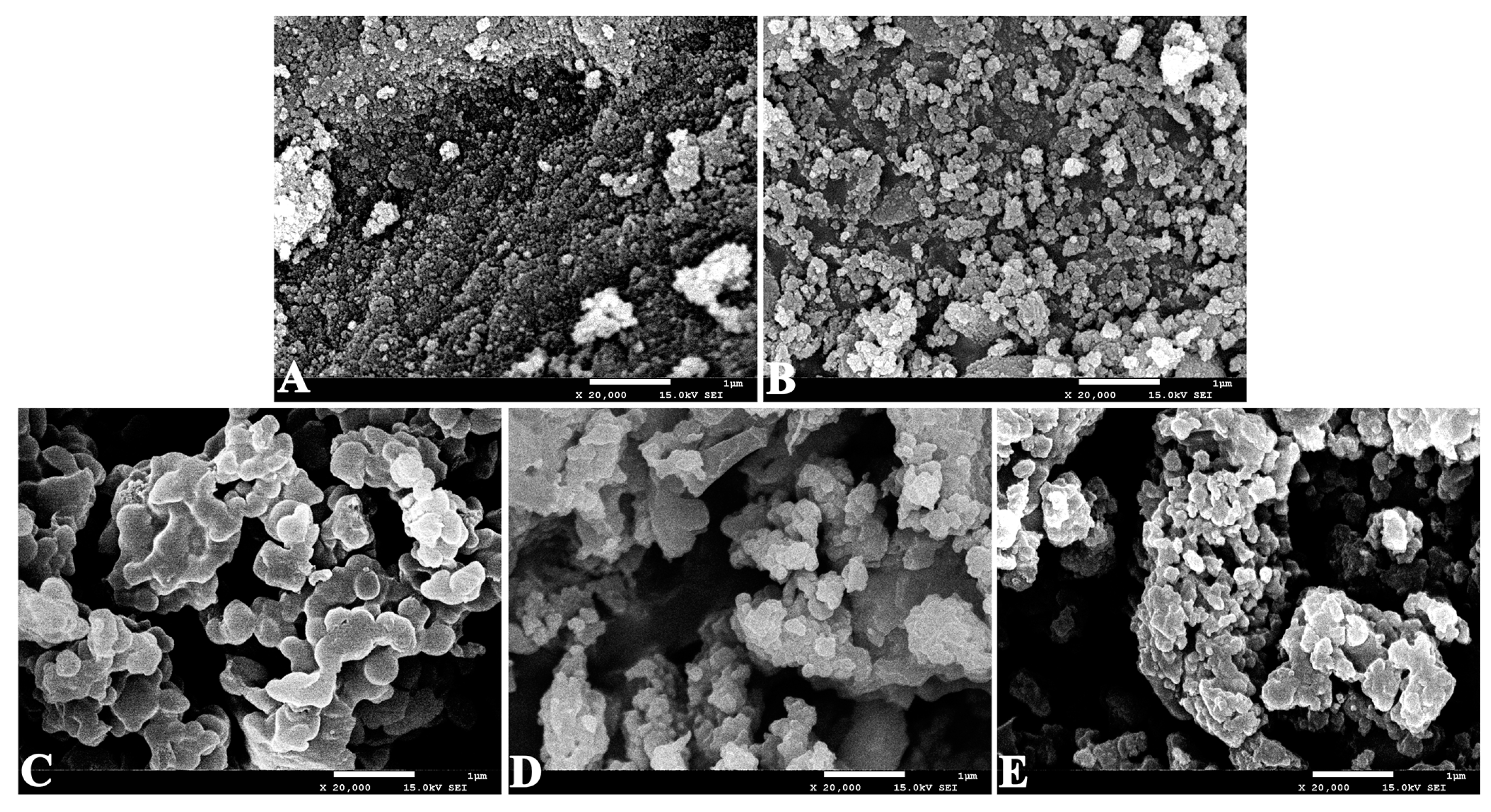
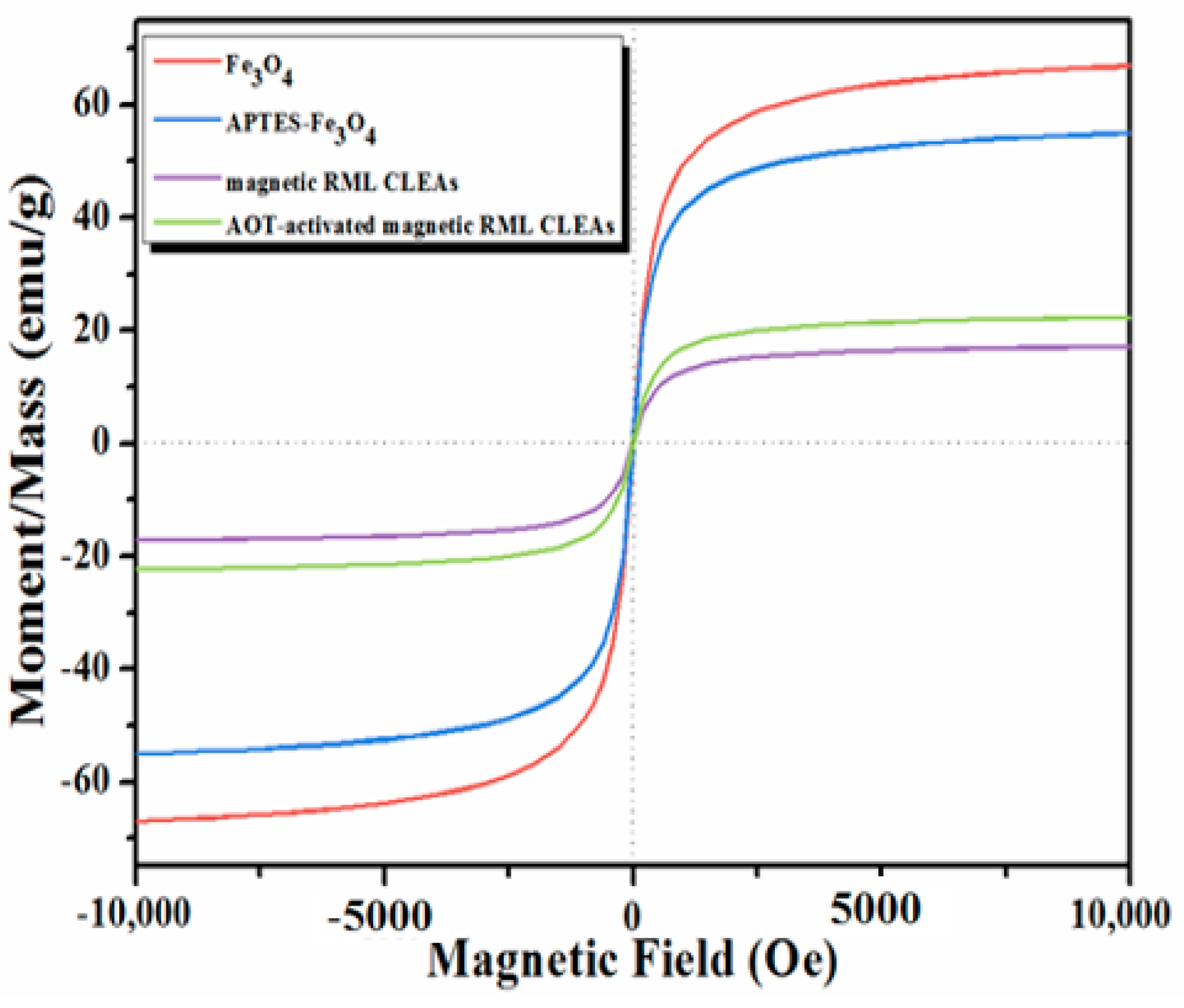
2.1. Characterization of the Immobilized Lipase
2.2. Optimization of Magnetic RML CLEAs Preparation Parameters
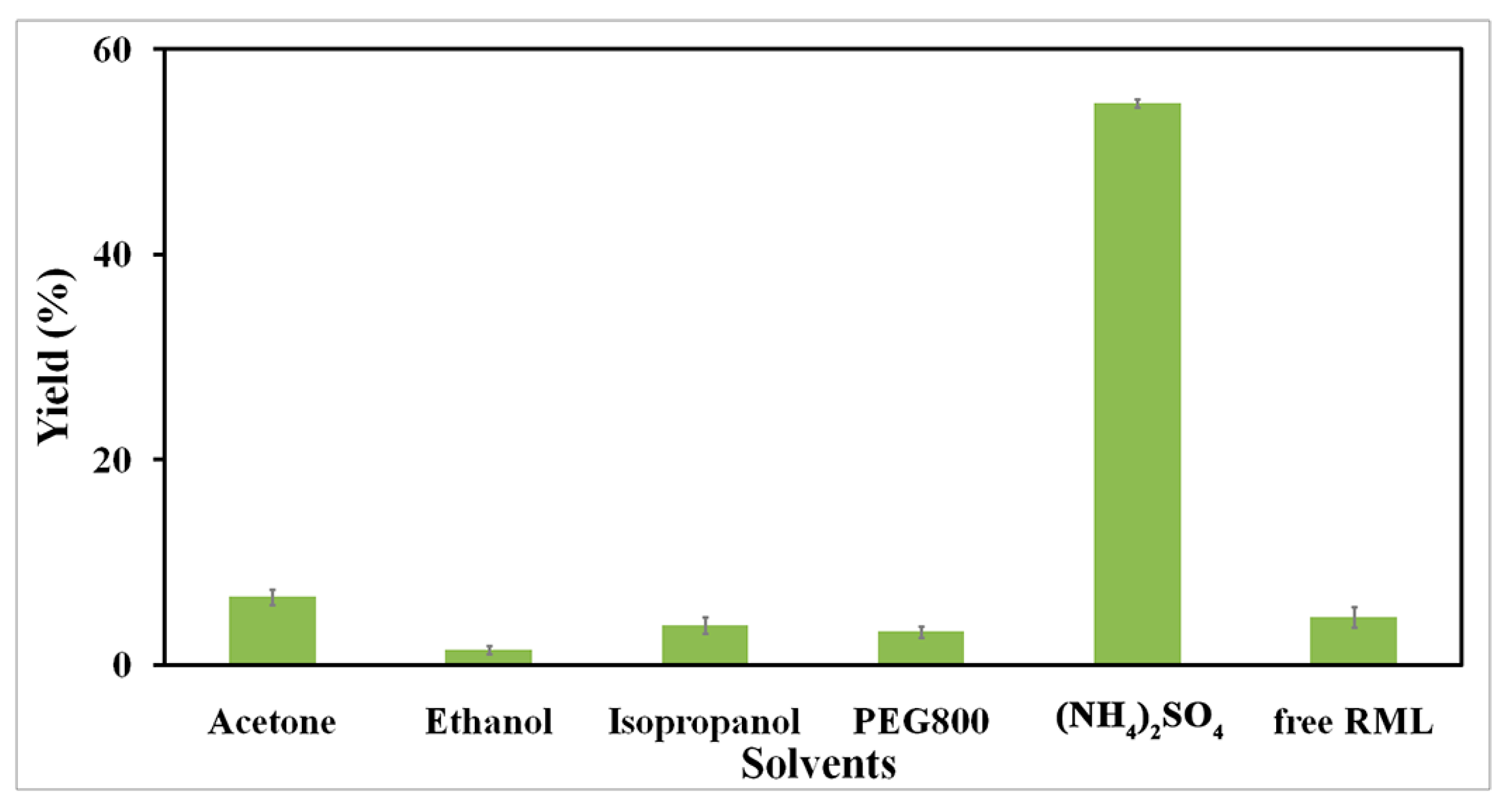
2.2.1. Effect of Precipitant
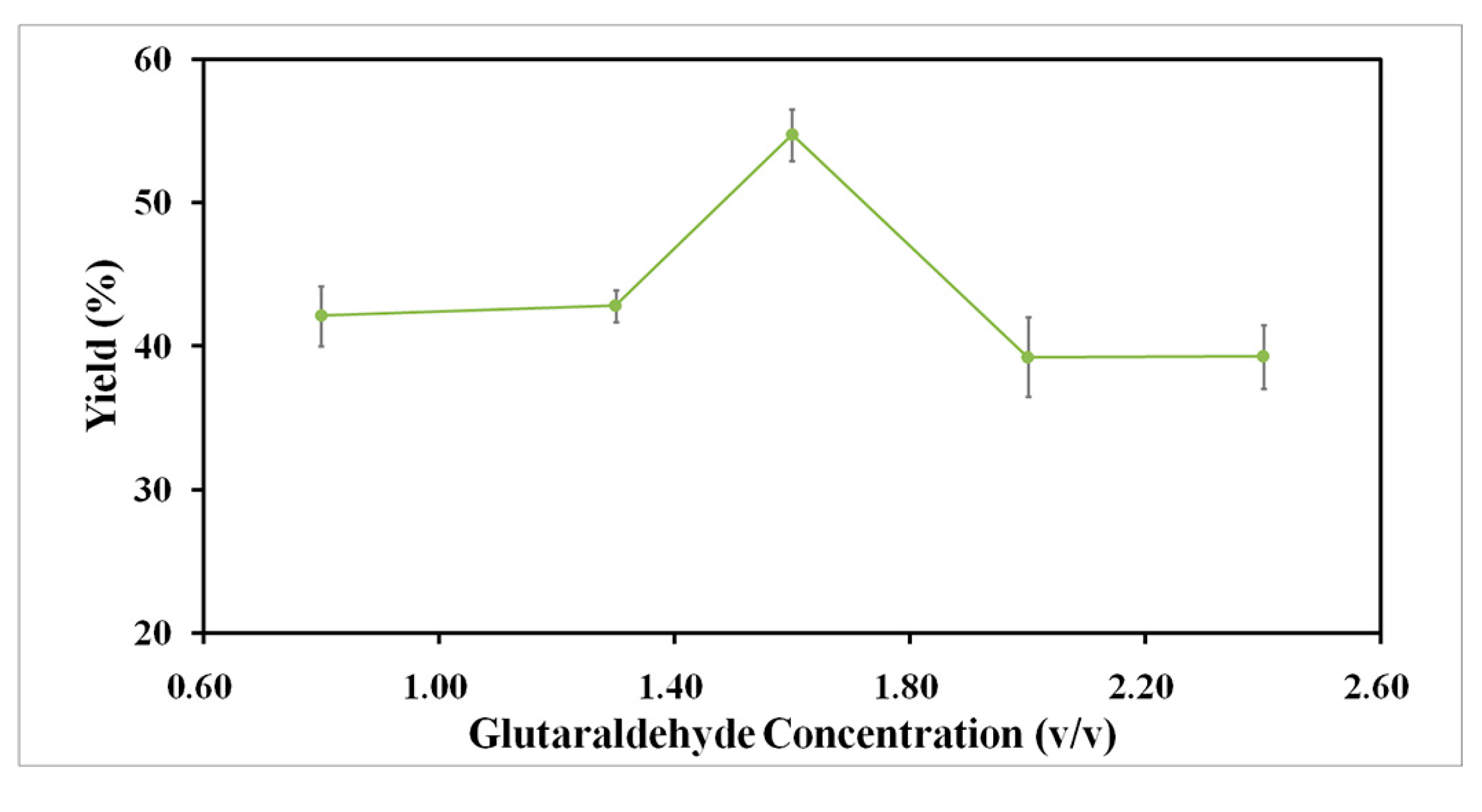
2.2.2. Effect of Glutaraldehyde Concentration
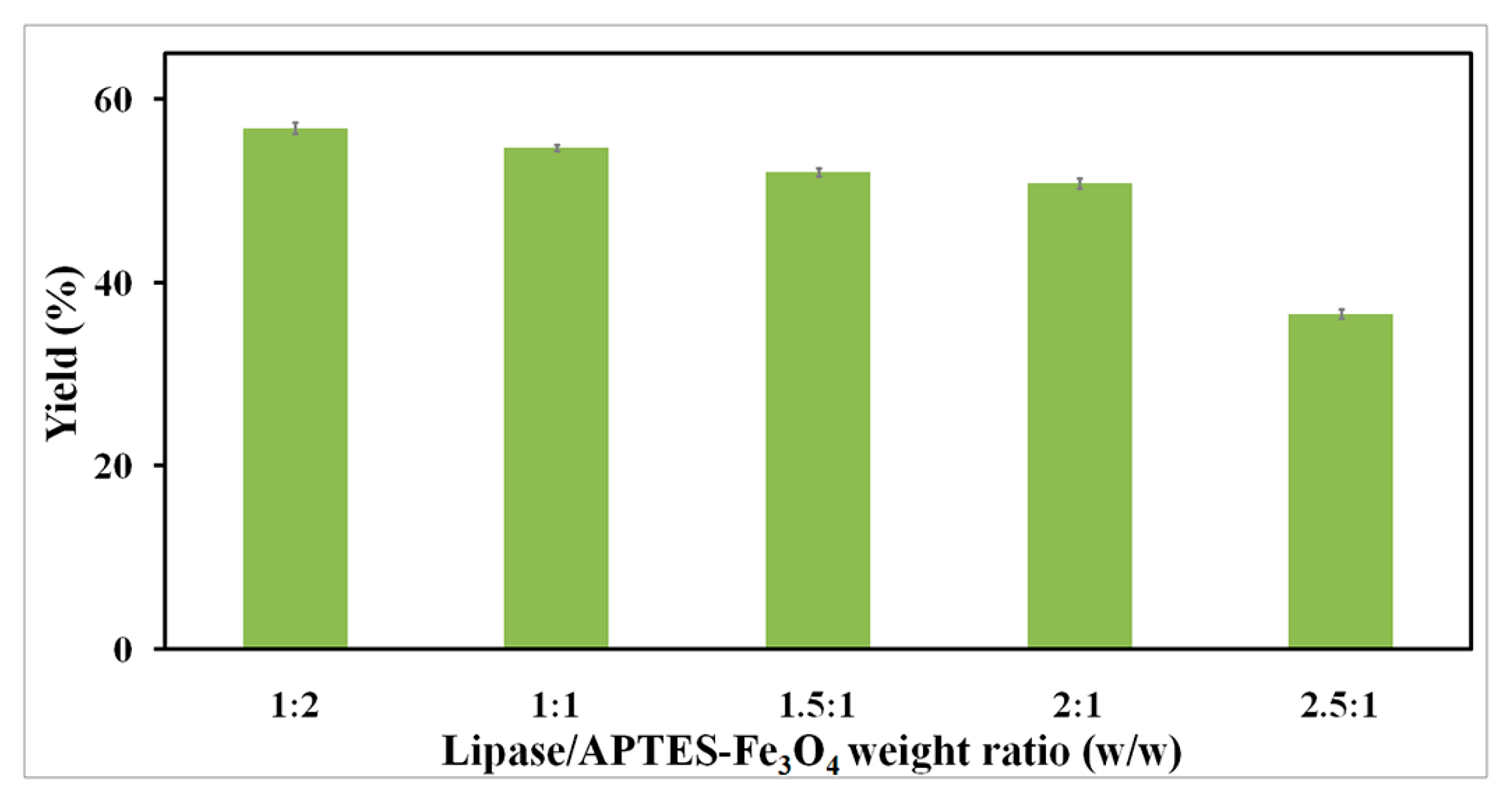
2.2.3. Effect of Lipase-to-Nanoparticle Ratio
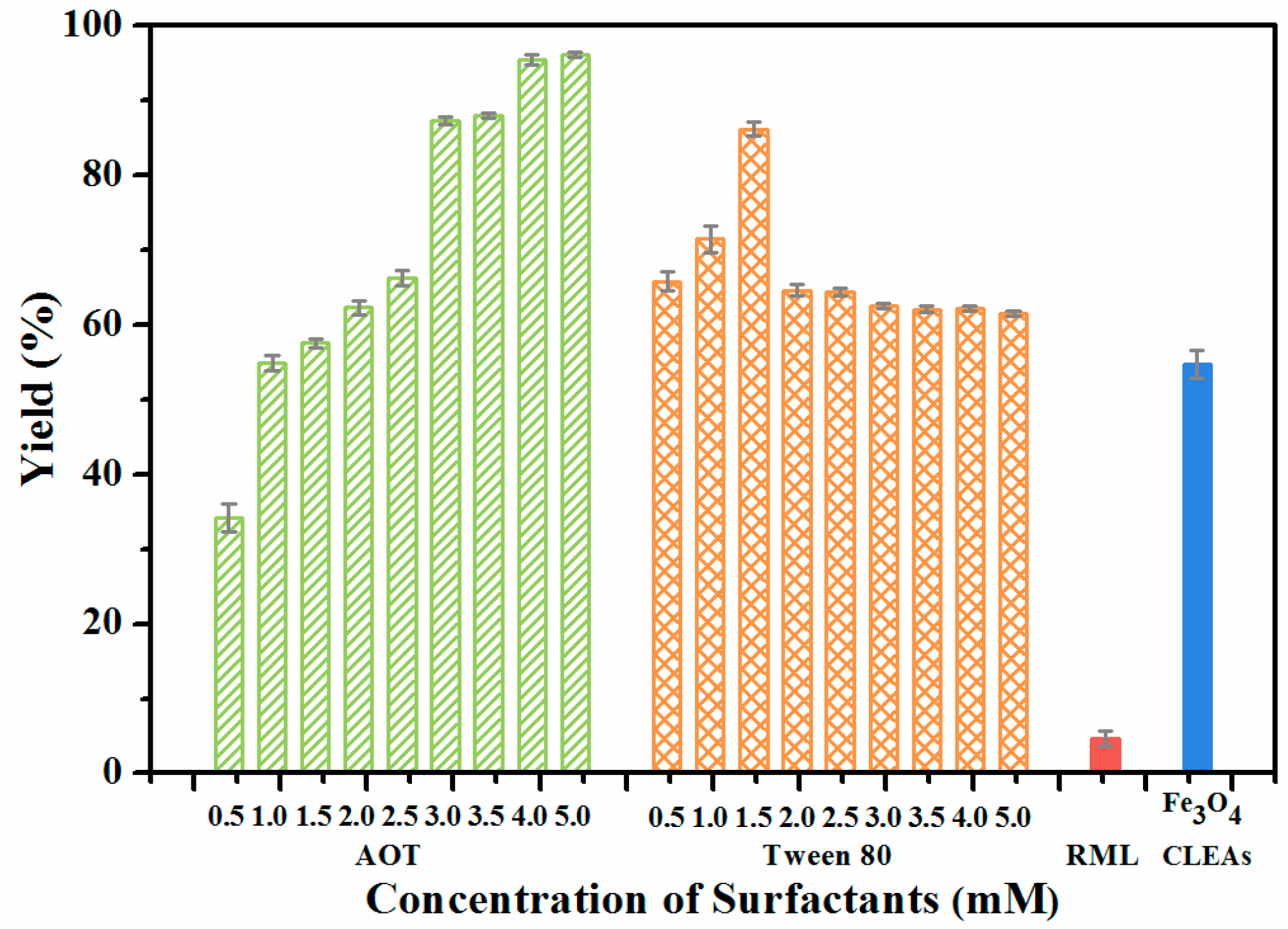
2.3. Surfactants Activated Lipase Magnetic CLEAs
2.4. Application in Biodiesel Production
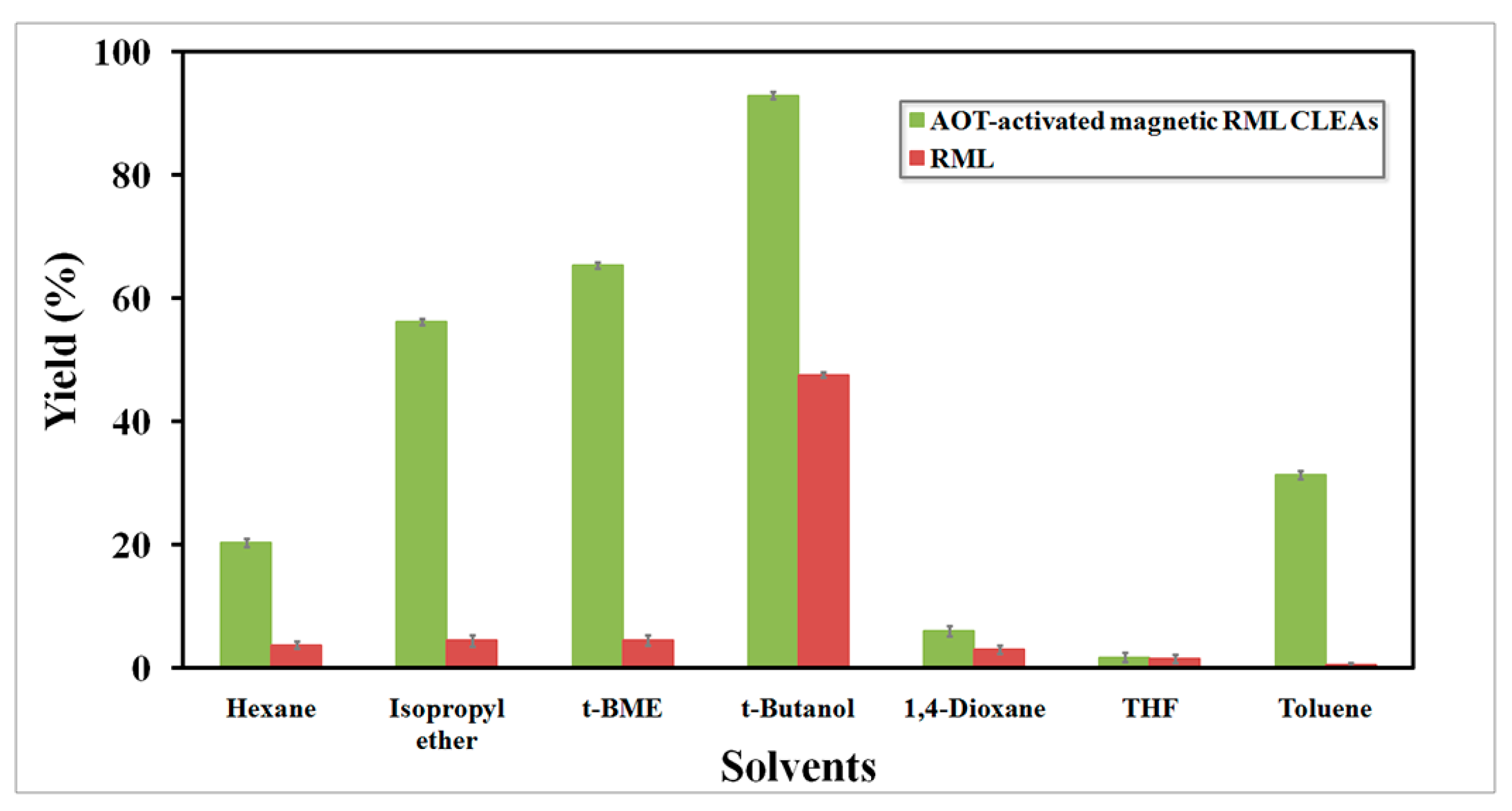
2.4.1. Effect of Organic Solvents on Biodiesel Production
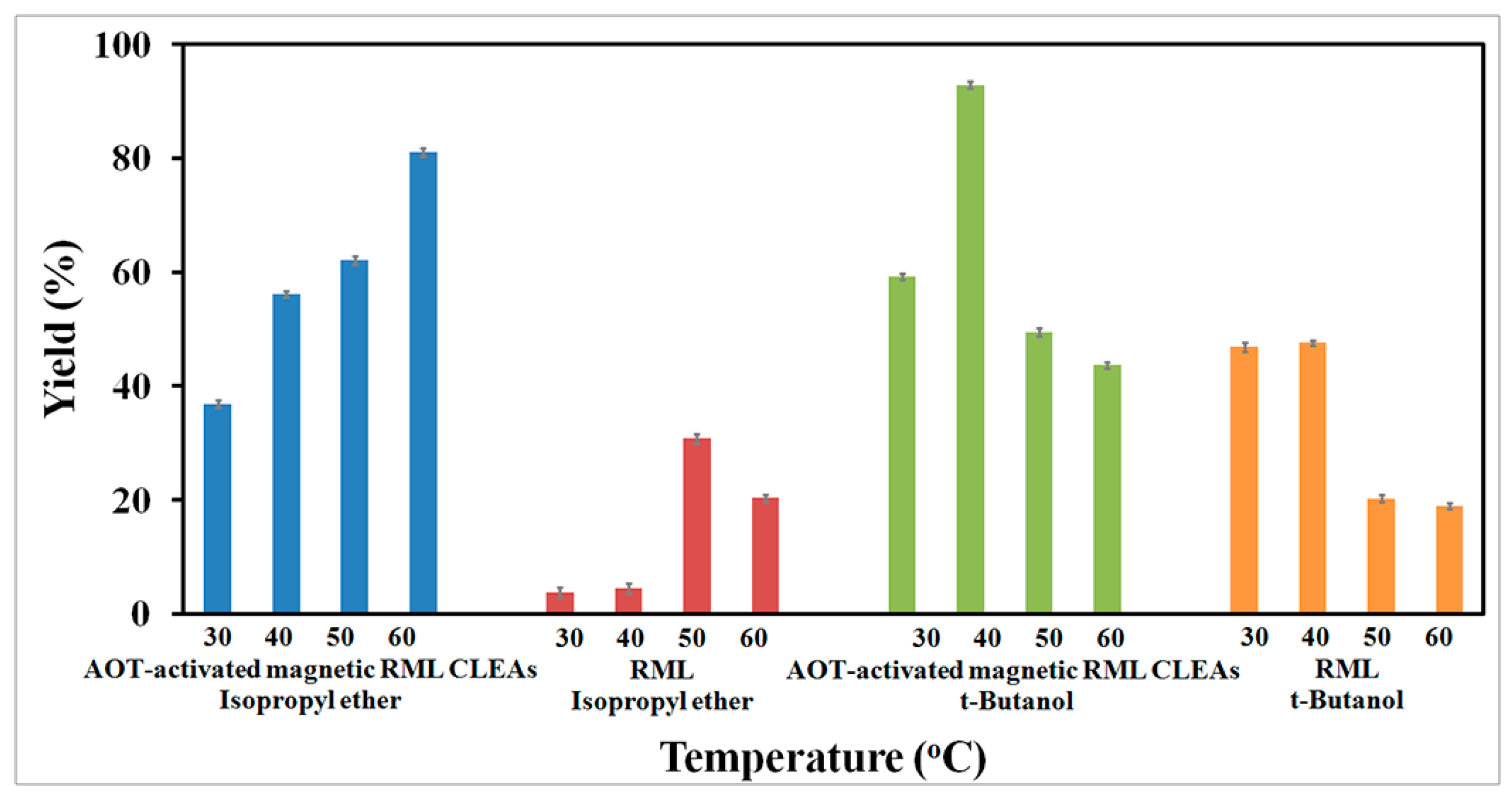
2.4.2. Effect of Temperatures on Biodiesel Production
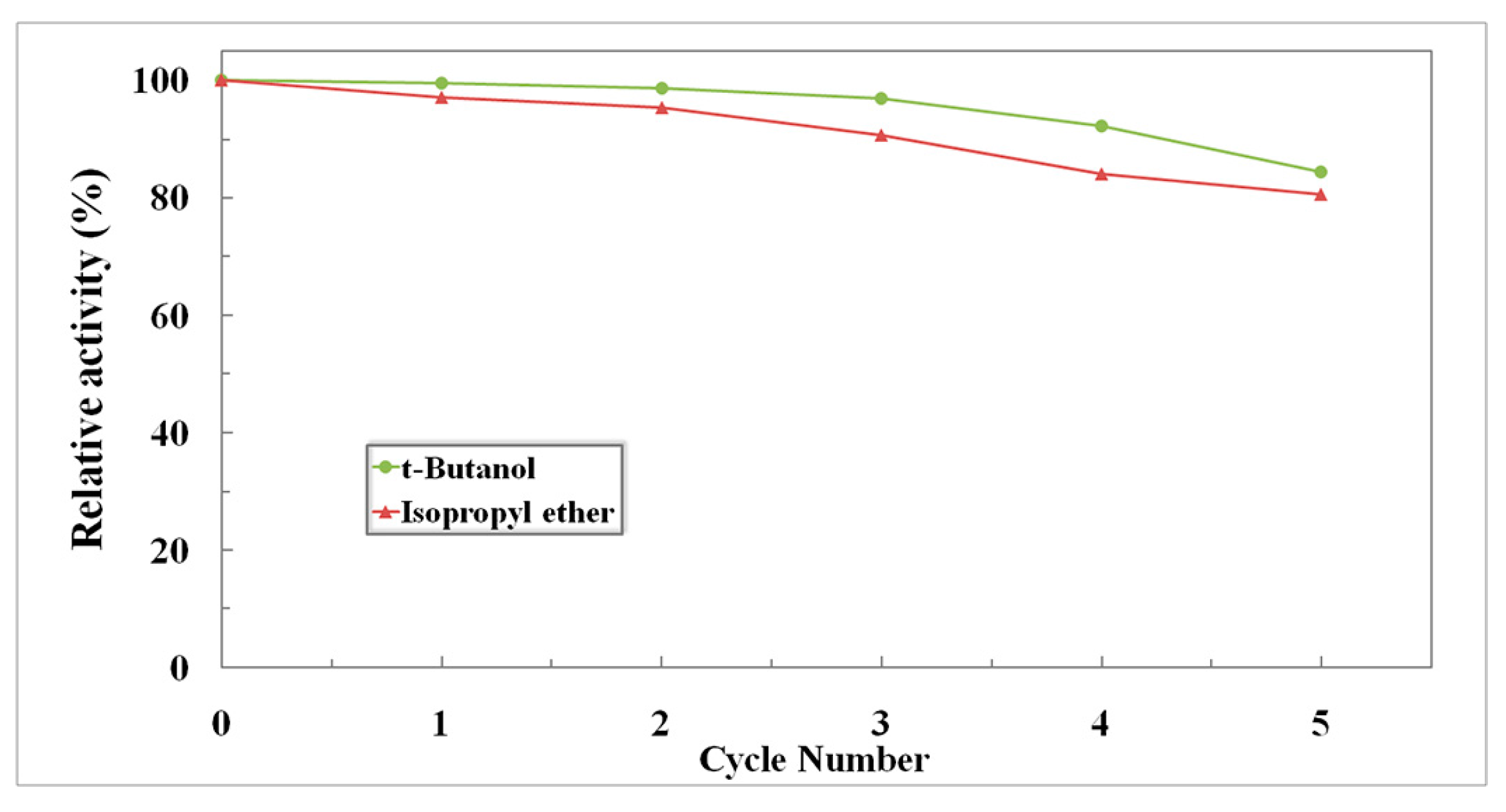
2.4.3. Reusability
3. Materials and Methods
3.1. Materials
3.2. Functionalization of Magnetic Nanoparticles
3.3. Immobilization of Lipase
3.3.1. Preparation of RML CLEAs
3.3.2. Preparation of Magnetic RML CLEAs
3.3.3. Preparation of Surfactants-Activated Magnetic RML CLEAs
3.4. Assay of Immobilized RML
3.4.1. Activity Assay
3.4.2. Biodiesel Production
3.4.3. Reusability
3.4.4. HPLC Analysis
3.4.5. GC Analysis
3.5. Characterization
3.5.1. FT-IR
3.5.2. SEM
3.5.3. Magnetization Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adrio, J.L.; Demain, A.L. Microbial enzymes: Tools for biotechnological processes. Biomolecules 2014, 4, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzyme Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Secundo, F.; Carrea, G.; Tarabiono, C.; Gatti-Lafranconi, P.; Brocca, S.; Lotti, M.; Jaeger, K.E.; Puls, M.; Eggert, T. The lid is a structural and functional determinant of lipase activity and selectivity. J. Mol. Catal. B Enzym. 2006, 39, 166–170. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.M.; Liu, L.L.; Yang, R.C. Improved performance of immobilized lipase by interfacial activation on Fe3O4 @PVBC nanoparticles. RSC Adv. 2017, 7, 35169–35174. [Google Scholar] [CrossRef]
- Adkins, S.S.; Hobbs, H.R.; Benaissi, K.; Johnston, K.P.; Poliakoff, M.; Thomas, N.R. Stable colloidal dispersions of a lipase-perfluoropolyether complex in liquid and supercritical carbon dioxide. J. Phys. Chem. B 2008, 112, 4760–4769. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, Y.K.; Lee, J.; Lee, E.; Park, J.; Kim, M.J. Ionic-surfactant-coated Burkholderia cepacia lipase as a highly active and enantioselective catalyst for the dynamic kinetic resolution of secondary alcohols. Angew. Chem. Int. Ed. 2011, 50, 10944–10948. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Ghosh, M.; Das, P.K. Gold nanorod in reverse micelles: A fitting fusion to catapult lipase activity. Chem. Commun. 2011, 47, 9864–9866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Wang, N.; Zhang, L.; Wu, W.X.; Hu, C.L.; Yu, X.Q. Effects of additives on lipase immobilization in microemulsion-based organogels. Appl. Biochem. Biotechnol. 2014, 172, 3128–3140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Wang, N.; Zhou, Y.J.; He, T.; Yu, X.Q. Enhancement of activity and stability of lipase by microemulsion-based organogels (MBGs) immobilization and application for synthesis of arylethyl acetate. J. Mol. Catal. B Enzym. 2012, 78, 65–71. [Google Scholar] [CrossRef]
- Wu, C.; Song, B.D.; Xing, A.H.; Hayashi, Y.; Talikder, M.M.R.; Wang, S.C. Esterification reactions catalysed by surfactant-coated Candida rugosa lipase in organic solvents. Process Biochem. 2002, 37, 1229–1233. [Google Scholar] [CrossRef]
- Mingarro, I.; Abad, C.; Braco, L. Interfacial activation-based molecular bioim-printing of lipolytic enzymes. Proc. Natl. Acad. Sci. USA 1995, 92, 3308–3312. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E. Inorganic materials as supports for covalent enzyme immobilization: Methods and mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.M.; Wang, J.W.; Tan, R.X. Immobilization of glucose oxidase on chitosan-SiO2 gel. Enzym. Microb. Technol. 2004, 34, 126–131. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Q.; Hu, Z.L.; Sheldon, R.A.; Yang, Z. Catalytic performance of cross-linked enzyme aggregates of Penicillium expansum lipase and their use as catalyst for biodiesel production. Process Biochem. 2012, 47, 2058–2063. [Google Scholar] [CrossRef]
- Cui, J.D.; Lin, T.; Feng, Y.X.; Tan, Z.L.; Jia, S.R. Preparation of spherical cross-linked lipase aggregates with improved activity, stability and reusability characteristic in water-in-ionic liquid microemulsion. J. Chem. Technol. Biotechnol. 2017, 92, 1785–1793. [Google Scholar] [CrossRef]
- Xu, D.Y.; Yang, Z. Cross-linked tyrosinase aggregates for elimination of phenolic compounds from wastewater. Chemosphere 2013, 92, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.Y.; Chen, J.Y.; Yang, Z. Use of cross-linked tyrosinase aggregates as catalyst for synthesis of l-DOPA. Biochem. Eng. J. 2012, 63, 88–94. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Seow, N.; Yang, K.L. Hollow cross-linked enzyme aggregates (h-CLEA) of laccase with high uniformity and activity. Colloids Surf. B Biointerfaces 2017, 151, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Devi, B.L.A.P.; Guo, Z.; Xu, X. Characterization of cross-linked lipase aggregates. J. Am. Oil Chem. Soc. 2009, 86, 637–642. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Cui, J.D.; Cui, L.L.; Zhang, S.P.; Zhang, Y.F.; Su, Z.G.; Ma, G.H. Hybrid magnetic cross-linked enzyme aggregates of phenylalanine ammonia lyase from Rhodotorula glutinis. PLoS ONE 2014, 9, e97221. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Taboada-Puig, R.; Junghanns, C.; Demarche, P.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Combined cross-linked enzyme aggregates from versatile peroxidase and glucose oxidase: Production, partial characterization and application for the elimination of endocrine disruptors. Bioresour. Technol. 2011, 102, 6593–6599. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, Y.; Guo, C.; Huang, W.; Liu, C. Enhanced phenol degradation in coking wastewater by immobilized laccase on magnetic mesoporous silica nanoparticles in a magnetically stabilized fluidized bed. Bioresour. Technol. 2012, 110, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.Q.; Zhang, W.W.; Yang, Z.J.; Yang, X.L.; Wang, N.; Yu, X.Q. Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules 2017, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Huang, Z.L.; Yang, T.X.; Huang, J.Z.; Yang, Z. Enzymatic production of biodiesel from millettia pinnata seed oil in ionic liquids. Bioenergy Res. 2014, 7, 1519–1528. [Google Scholar] [CrossRef]
- Liu, X.; Guan, Y.; Shen, R.; Liu, H. Immobilization of lipase onto micron-size magnetic beads. J. Chromatogr. B. 2005, 822, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Joshi, G.; Chougle, R.; Nainegali, B.; Desai, S.; Joshi, A.; Kambale, S.; Kamat, P.; Haripurkar, R.; Jadhav, S.; et al. Preparation of stable cross-linked enzyme aggregates (CLEAs) of NADH-dependent nitrate reductase and its use for silver nanoparticle synthesis from silver nitrate. Catal. Commun. 2014, 53, 62–66. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.; van Rantwijk, F.; van der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as an industrial biocatalyst in chemical process. J. Mol. Catal. B Enzym. 2010, 64, 1–22. [Google Scholar] [CrossRef]
- Holmquist, M.; Norin, M.; Hult, K. The role of arginines in stabilizing the active open-lid conformation of Rhizomucor miehei lipase. Lipids 1993, 28, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, S.; Verma, C.S.; Hubbard, R.E.; Caves, L.S.D. Identifying key electrostatic interactions in Rhizomucor miehei lipase: The influence of solvent dielectric. Theor. Chem. Acc. 1999, 101, 175–179. [Google Scholar]
- Du, W.; Liu, D.; Li, L.; Dai, L. Mechanism exploration during lipase-mediated methanolysis of renewable oils for biodiesel production in a tert-butanol system. Biotechnol. Prog. 2007, 23, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yang, H.; Liu, W.; Wang, N.; Yu, X. Improved Performance of Magnetic Cross-Linked Lipase Aggregates by Interfacial Activation: A Robust and Magnetically Recyclable Biocatalyst for Transesterification of Jatropha Oil. Molecules 2017, 22, 2157. https://doi.org/10.3390/molecules22122157
Zhang W, Yang H, Liu W, Wang N, Yu X. Improved Performance of Magnetic Cross-Linked Lipase Aggregates by Interfacial Activation: A Robust and Magnetically Recyclable Biocatalyst for Transesterification of Jatropha Oil. Molecules. 2017; 22(12):2157. https://doi.org/10.3390/molecules22122157
Chicago/Turabian StyleZhang, Weiwei, Huixia Yang, Wanyi Liu, Na Wang, and Xiaoqi Yu. 2017. "Improved Performance of Magnetic Cross-Linked Lipase Aggregates by Interfacial Activation: A Robust and Magnetically Recyclable Biocatalyst for Transesterification of Jatropha Oil" Molecules 22, no. 12: 2157. https://doi.org/10.3390/molecules22122157



