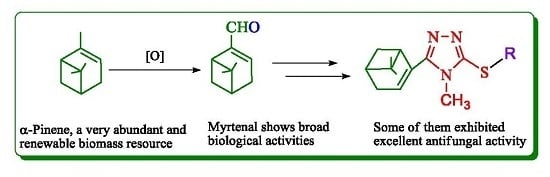
Synthesis and Antifungal Activity of Novel Myrtenal-Based 4-Methyl-1,2,4-triazole-thioethers
Abstract
:1. Introduction
2. Results and Discussion
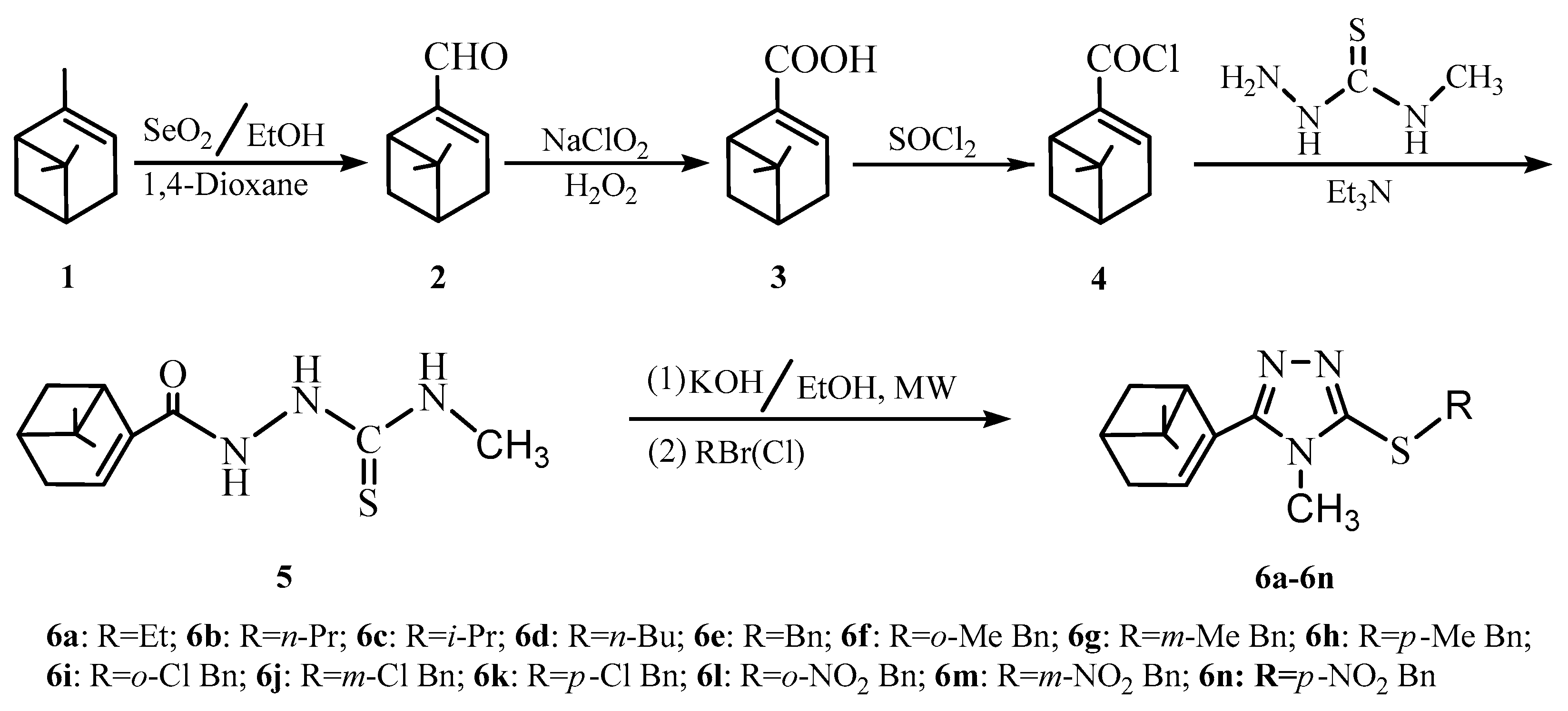
2.1. Synthesis and Characterization
2.2. Antifungal Activity
3. Experimental Section
3.1. General
3.2. Synthesis of Myrtenal from α-Pinene
3.3. Synthesis of Myrtenic Acid
3.4. Synthesis of Myrtenyl Chloride
3.5. Synthesis of Myrtenal-Based 4-Methylhydrazinecarbothioamide
3.6. General Procedure for the One-Pot Sequential Synthesis of Myrtenal-Based 4-Methyl-1,2,4-triazole-thioethers (6a–6n)
3.7. Antifungal Activity Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Setzer, W.N.; Vogler, B.; Schmidt, J.M.; Leahy, J.G.; Rives, R. Antimicrobial activity of Artemisia douglasiana leaf essential oil. Fitoterapia 2004, 75, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Al-Ja’fari, A.H.; Vila, R.; Freixa, B.; Tomi, F.; Casanova, J.; Costa, J.; Cañigueral, S. Composition and antifungal activity of the essential oil from the rhizome and roots of Ferula hermonis. Phytochemistry 2011, 72, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Tellez, M.R.; Khan, I.A.; Kobaisy, M.; Schrader, K.K.; Dayan, F.E.; Osbrink, W. Composition of the essential oil of Lepidium meyenii (Walp.). Phytochemistry 2002, 61, 149–155. [Google Scholar] [CrossRef]
- Ronse, A.; de Pooter, H.; de Proft, M. Essential oils of Otacanthus. Phytochemistry 1997, 46, 1365–1368. [Google Scholar] [CrossRef]
- Babu, L.H.; Perumal, S.; Balasubramanian, M.P. Myrtenal attenuates diethylnitrosamine-induced hepatocellular carcinoma in rats by stabilizing intrinsic antioxidants and modulating apoptotic and anti-apoptotic cascades. Cell. Oncol. 2012, 35, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Babu, L.H.; Nandakumar, N.; Rengarajan, T.; Perumal, S.; Balasubramanian, M.P. Myrtenal ameliorates diethylnitrosamine-induced hepatocarcinogenesis through the activation of tumor suppressor protein p53 and regulation of lysosomal and mitochondrial enzymes. Fundam. Clin. Pharmacol. 2013, 27, 443–454. [Google Scholar]
- Babu, L.H.; Perumal, S.; Balasubramanian, M.P. Myrtenal, a natural monoterpene, down-regulates TNF-α expression and suppresses carcinogen-induced hepatocellular carcinoma in rats. Mol. Cell. Biochem. 2012, 369, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.; Dogra, A.K.; Wink, M. Myrtenal inhibits acetylcholinesterase, a known Alzheimer target. J. Pharm. Pharmacol. 2011, 63, 1368–1371. [Google Scholar] [CrossRef] [PubMed]
- Kamchonwongpaisan, S.; Nilanonta, C.; Tarnchompoo, B.; Thebtaranonth, C.; Thebtaranonth, Y.; Yuthavong, Y.; Kongsaeree, P.; Clardy, J. An antimalarial peroxide from Amomum krervanh Pierre. Tetrahedron lett. 1995, 36, 1821–1824. [Google Scholar] [CrossRef]
- Hardie, J.; Isaacs, R.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Methyl salicylate and (−)-(1R,5S)-myrtenal are plant-derived repellents for black bean aphid, Aphis fabae Scop. (Homoptera: Aphididae). J. Chem. Ecol. 1994, 20, 2847–2855. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yin, C.X.; Zhang, F.M.; Liu, Y.H.; Zhou, N. Synthesis and bioactivity determination of myrtenal. Chem. Bioeng. 2013, 30, 50–52. [Google Scholar]
- Yin, C.X.; Gao, Z.L.; Zhou, N.; Liu, F.C.; Qiao, Y. Synthesis and test of bioactivity on derivatives of oxygenated α-pinene as the aggregation pheromones of bark beetle. J. Yunnan Univ. (Nat. Sci. Ed.) 2001, 23, 132–134. [Google Scholar]
- Tachibana, S.; Ohno, Y.; Fujihara, Y.; Okada, Y.; Sugiura, M.; Takagi, S.; Nomura, M. Synthesis and physiological activities of monoterpene carboxylic acid esters with pyrones. J. Oleo Sci. 2006, 55, 181–189. [Google Scholar] [CrossRef]
- Nomura, M.; Hirokawa, T.; Fujihara, Y.; Takei, Y.; Yamamoto, R. Synthesis of terpenyl amides with monoterpene groups and their evaluation as mosquito repellents. Nippon Nogeikagaku Kaishi 1993, 67, 693–700. [Google Scholar] [CrossRef]
- Kasemura, K.; Nomura, M.; Tachibana, S.; Fujihara, Y. Herbicidal activity of monoterpenyl derivatives with dialkylamides residue (Part 2). J. Oleo Sci. Society 2000, 49, 501–504. [Google Scholar] [CrossRef]
- Barbuceanu, S.-F.; Saramet, G.; Almajan, G.L.; Draghici, C.; Barbuceanu, F.; Bancescu, G. New heterocyclic compounds from 1,2,4-triazole and 1,3,4-thiadiazole class bearing diphenylsulfone moieties. Synthesis, characterization and antimicrobial activity evaluation. Eur. J. Med. Chem. 2012, 49, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Al-Omar, M.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 5-(1-adamantyl)-4-arylideneamino-3-mercapto-1,2,4-triazoles and related derivatives. Molecules 2010, 15, 2526–2550. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Zhang, J.; Yu, S.; Hu, H.; Zou, Y.; Zhao, Q.; Dan, Z.; Zhang, D.; Wu, Q. Design, synthesis, and biological evaluation of novel 1-(1H-1,2,4-triazole-1-yl)-2-(2,4-difluorophenyl)-3-substituted benzylamino-2-propanols. Bioorg. Med. Chem. Lett. 2009, 19, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.S.; Poojary, B.; Prasad, D.J.; Naik, P.; Holla, B.S. Synthesis and antitumor activity studies of some new fused 1,2,4-triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety. Eur. J. Med. Chem. 2009, 44, 5066–5070. [Google Scholar] [CrossRef] [PubMed]
- Uzgoren-Baran, A.; Tel, B.C.; Sarigol, D.; Ozturk, E.I.; Kazkayasi, I.; Okay, G.; Ertan, M.; Tozkoparan, B. Thiazolo[3,2-b]-1,2,4-triazole-5(6H)-one substituted with ibuprofen: Novel non-steroidal anti-inflammatory agents with favorable gastrointestinal tolerance. Eur. J. Med. Chem. 2012, 57, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Tatar, E.; Küçükgüzel, Ş.G.; Karakuş, S.; Clercq, E.D.; Andrei, G.; Snoeck, R.; Pannecouque, C.; Öktem Okullu, S.; Ünübol, N.; Kocagöz, T. Synthesis and biological evaluation of some new 1,3,4-thiadiazole and 1,2,4-triazole derivatives from l-methionine as antituberculosis and antiviral agents. Marmara Pharm. J. 2015, 2, 88–102. [Google Scholar] [CrossRef]
- Liu, X.H.; Xu, X.Y.; Tan, C.X.; Weng, J.Q.; Xin, J.H.; Chen, J. Synthesis, crystal structure, herbicidal activities and 3D-QSAR study of some novel 1,2,4-triazolo[4,3-a]pyridine derivatives. Pest Manag. Sci. 2015, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Duan, W.; Lin, G.; Liu, L.; Zhang, R.; Li, D. Synthesis and antifungal activity of dehydroabietic acid-based 1,3,4-thiadiazole-thiazolidinone compounds. Mol. Divers. 2016, 20, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Li, F.Y.; Duan, W.G.; Liao, J.N.; Lin, Z. d.; Lin, G.S.; Cen, B.; Lei, F.H. Synthesis and antifungal activity of camphoric acid-based acylhydrazone compounds. Holzforschung 2014, 68, 889–895. [Google Scholar] [CrossRef]
- Li, F.Y.; Mo, Q.J.; Duan, W.G.; Lin, G.S.; Cen, B.; Chen, N.Y.; Yang, Z.Q. Synthesis and insecticidal activities of N-(5-dehydroabietyl-1,3,4-thiadiazol-2-yl)-benzenesulfonamides. Med. Chem. Res. 2014, 23, 4420–4426. [Google Scholar] [CrossRef]
- Lin, G.S.; Ma, C.H.; Duan, W.G.; Cen, B.; Lei, F.H.; Yang, Z.Q. Synthesis and biological activities of α-pinene-based dithiadiazoles. Holzforschung 2014, 68, 75–83. [Google Scholar] [CrossRef]
- Huang, D.Y.; Duan, W.G.; Lin, G.S.; Bai, X.; Xiao, H.; Yang, Z.Q. Synthesis and antifungal activities of 2-sustituted acylamino-5-(α-campholenicaldehyde)-based-1,3,4-thiadiazole compounds. Chem. Ind. For. Prod. 2016, 36, 61–69. [Google Scholar]
- Lin, G.S.; Zou, R.X.; Duan, W.G.; Ma, X.L.; Cen, B.; Lei, F.H. Synthesis and herbicidal activities of novel pinic acid-based diacylhydrazone compounds. Chin. J. Synth. Chem. 2013, 21, 513–517. [Google Scholar]
- Coxon, J.M.; Hydes, G.J.; Steel, P.J. Carbon-13 nuclear magnetic resonance spectra of pinane monoterpenoids. J. Chem. Soc. Perk 1984, 1351–1355. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2, 3, 5, and 6 are available from the authors.
| Compounds | Relative Inhibition Rate (%) against the Fungi | ||||
|---|---|---|---|---|---|
| F. oxysporum f. sp. cucumerinum | C. arachidicola | P. piricola | A. solani | G. zeae | |
| 6a (R = Et) | 30.9 | 36.7 | 90.7 | 28.9 | 27.6 |
| 6b (R = n-Pr) | 35.6 | 46.7 | 54.6 | 47.8 | 30.6 |
| 6c (R = i-Pr) | 37.9 | 36.7 | 98.2 | 28.9 | 40.9 |
| 6d (n-Bu) | 47.2 | 46.7 | 36.8 | 47.8 | 36.5 |
| 6e (R = Bn) | 20.5 | 33.3 | 43.3 | 38.6 | 45.6 |
| 6f (o-Me Bn) | 20.5 | 33.3 | 70.5 | 32.9 | 60.2 |
| 6g (m-Me Bn) | 23.2 | 36.7 | 48.1 | 47.1 | 49.0 |
| 6h (p-Me Bn) | 30.0 | 25.4 | 20.0 | 18.3 | 33.3 |
| 6i (o-Cl Bn) | 40.0 | 33.1 | 30.0 | 26.7 | 50.0 |
| 6j (m-Cl Bn) | 30.0 | 40.8 | 30.0 | 18.3 | 56.7 |
| 6k (p-Cl Bn) | 20.0 | 25.4 | 20.0 | 18.3 | 36.7 |
| 6l (R = o-NO2 Bn) | 37.9 | 46.7 | 96.4 | 45.1 | 42.4 |
| 6m (R = m-NO2 Bn) | 58.8 | 36.7 | 51.1 | 50.5 | 37.9 |
| 6n (R = p-NO2 Bn) | 30.9 | 26.7 | 36.8 | 42.4 | 30.6 |
| Myrtenal | 25.0 | 28.2 | 37.8 | 41.3 | 31.7 |
| Azoxystrobin | 87.5 | 92.3 | 96.0 | 91.3 | 90.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.-S.; Duan, W.-G.; Yang, L.-X.; Huang, M.; Lei, F.-H. Synthesis and Antifungal Activity of Novel Myrtenal-Based 4-Methyl-1,2,4-triazole-thioethers. Molecules 2017, 22, 193. https://doi.org/10.3390/molecules22020193
Lin G-S, Duan W-G, Yang L-X, Huang M, Lei F-H. Synthesis and Antifungal Activity of Novel Myrtenal-Based 4-Methyl-1,2,4-triazole-thioethers. Molecules. 2017; 22(2):193. https://doi.org/10.3390/molecules22020193
Chicago/Turabian StyleLin, Gui-Shan, Wen-Gui Duan, Lin-Xiao Yang, Min Huang, and Fu-Hou Lei. 2017. "Synthesis and Antifungal Activity of Novel Myrtenal-Based 4-Methyl-1,2,4-triazole-thioethers" Molecules 22, no. 2: 193. https://doi.org/10.3390/molecules22020193