Immobilized Lipases on Functionalized Silica Particles as Potential Biocatalysts for the Synthesis of Fructose Oleate in an Organic Solvent/Water System
Abstract
:1. Introduction
2. Results and Discussion
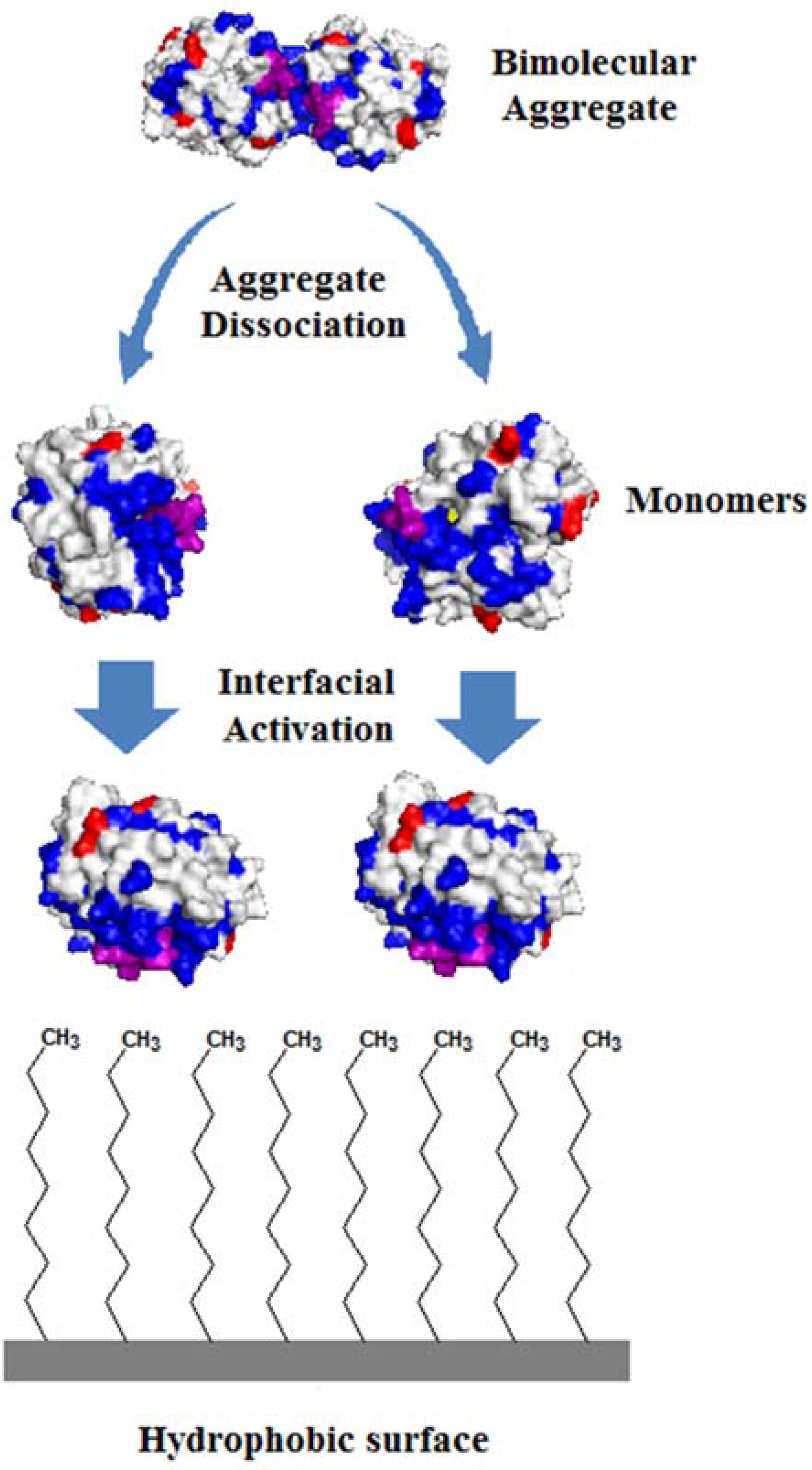
2.1. Immobilization of Lipases
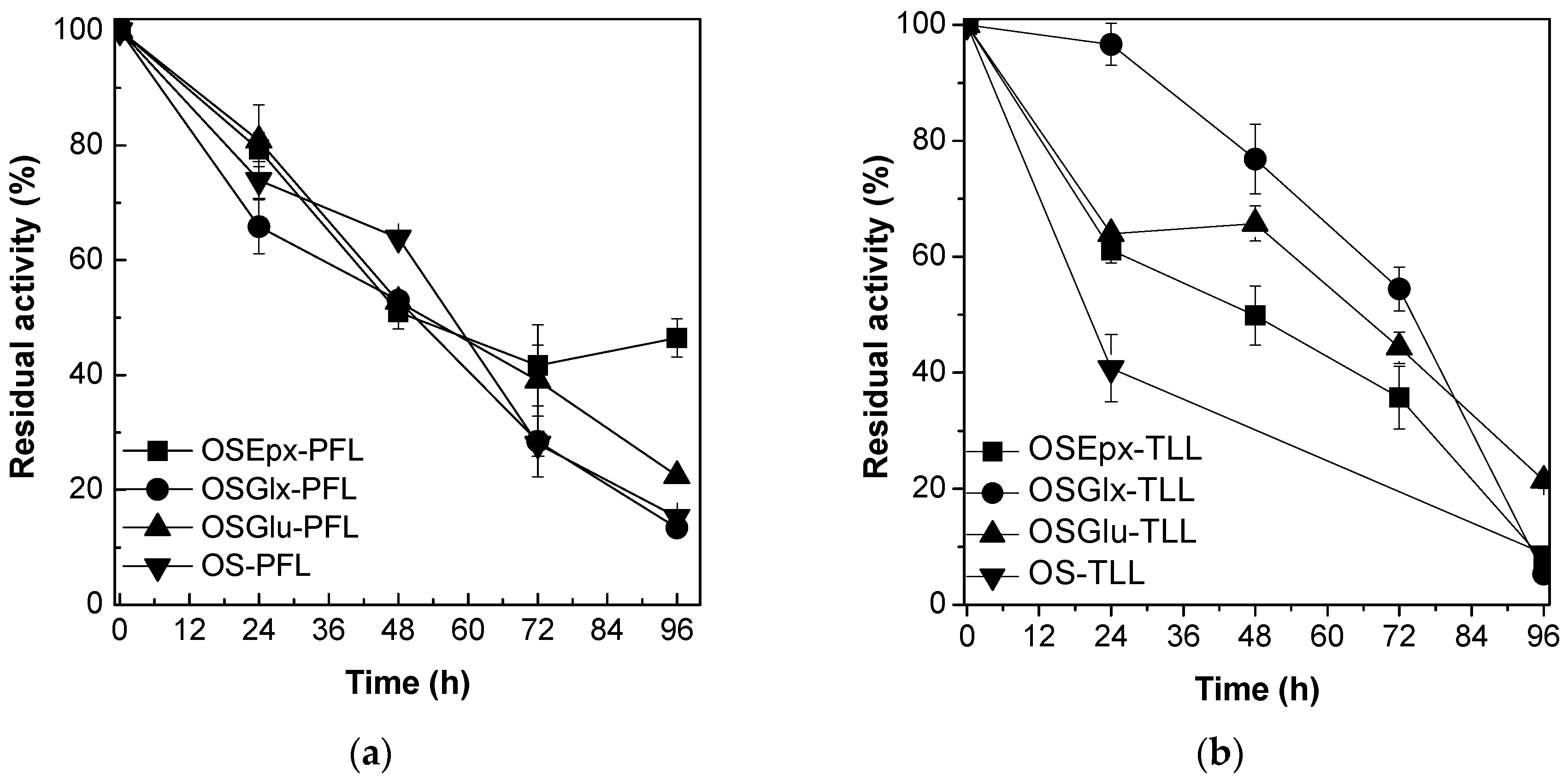
2.2. Stability of Immobilized Lipases Against tert-Butyl Alcohol
2.3. Solubility of Fructose in tert-Butyl Alcohol/Water System
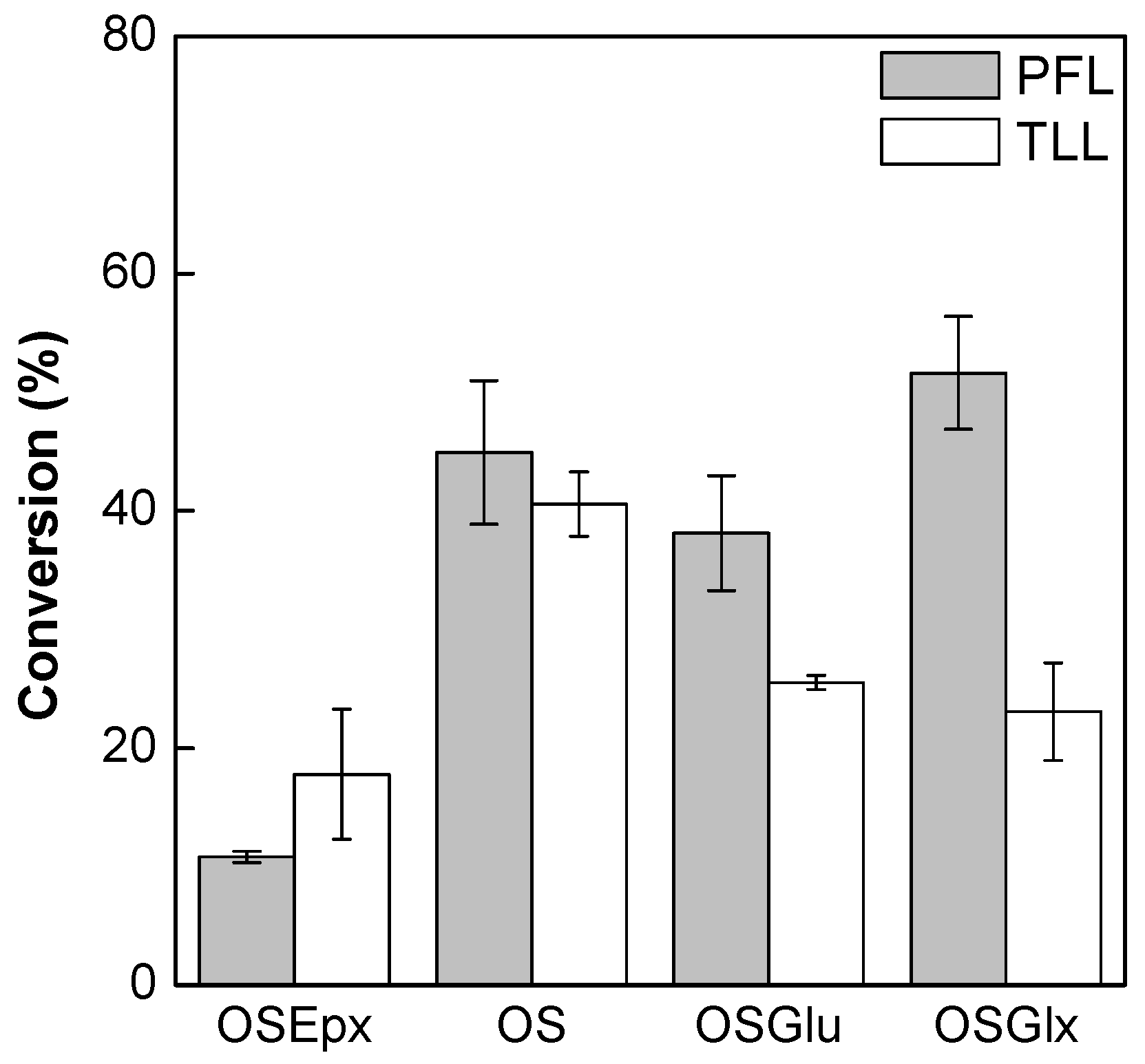
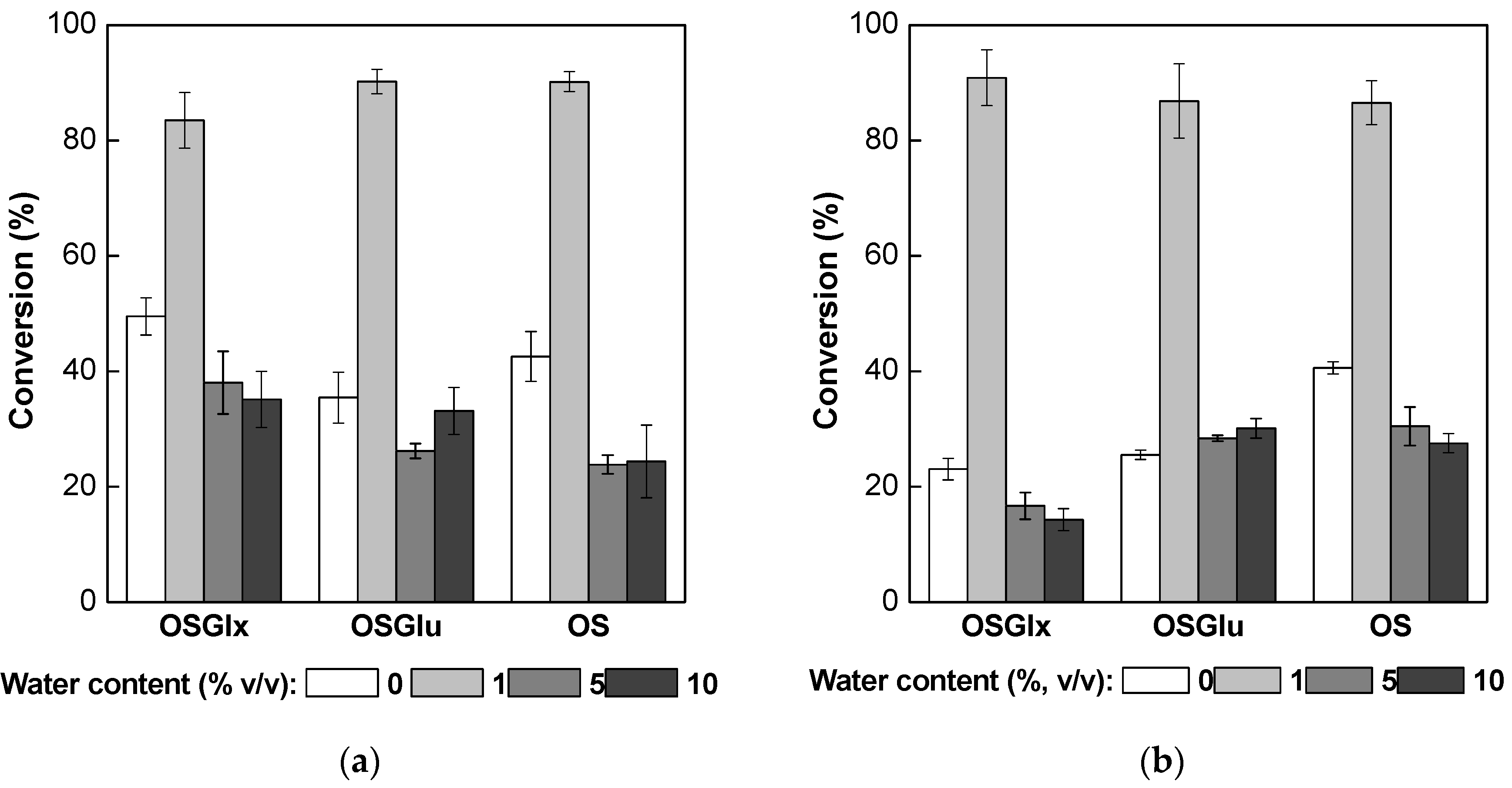
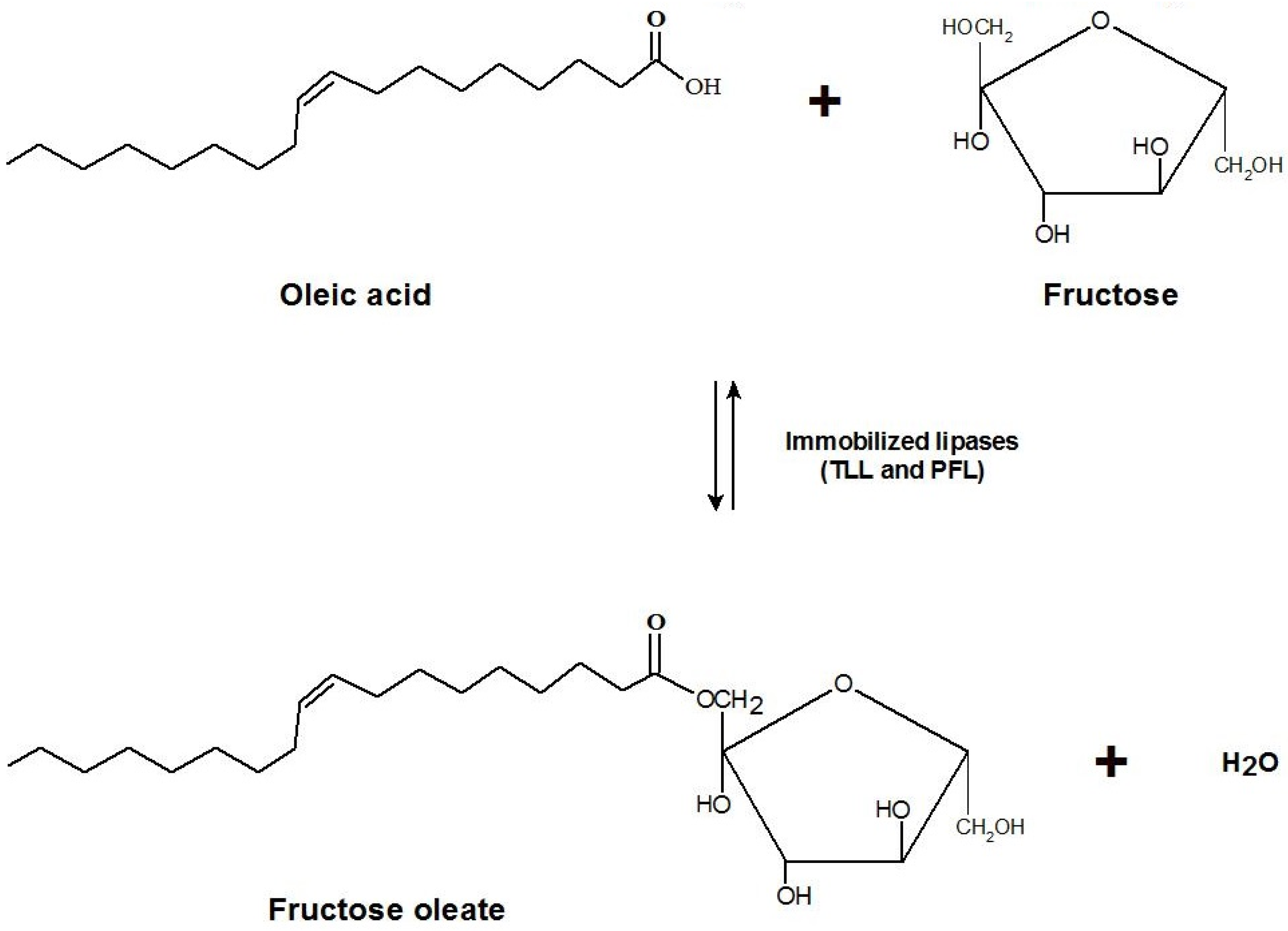
2.4. Synthesis of Fructose Oleate in tert-Butyl Alcohol/Water System
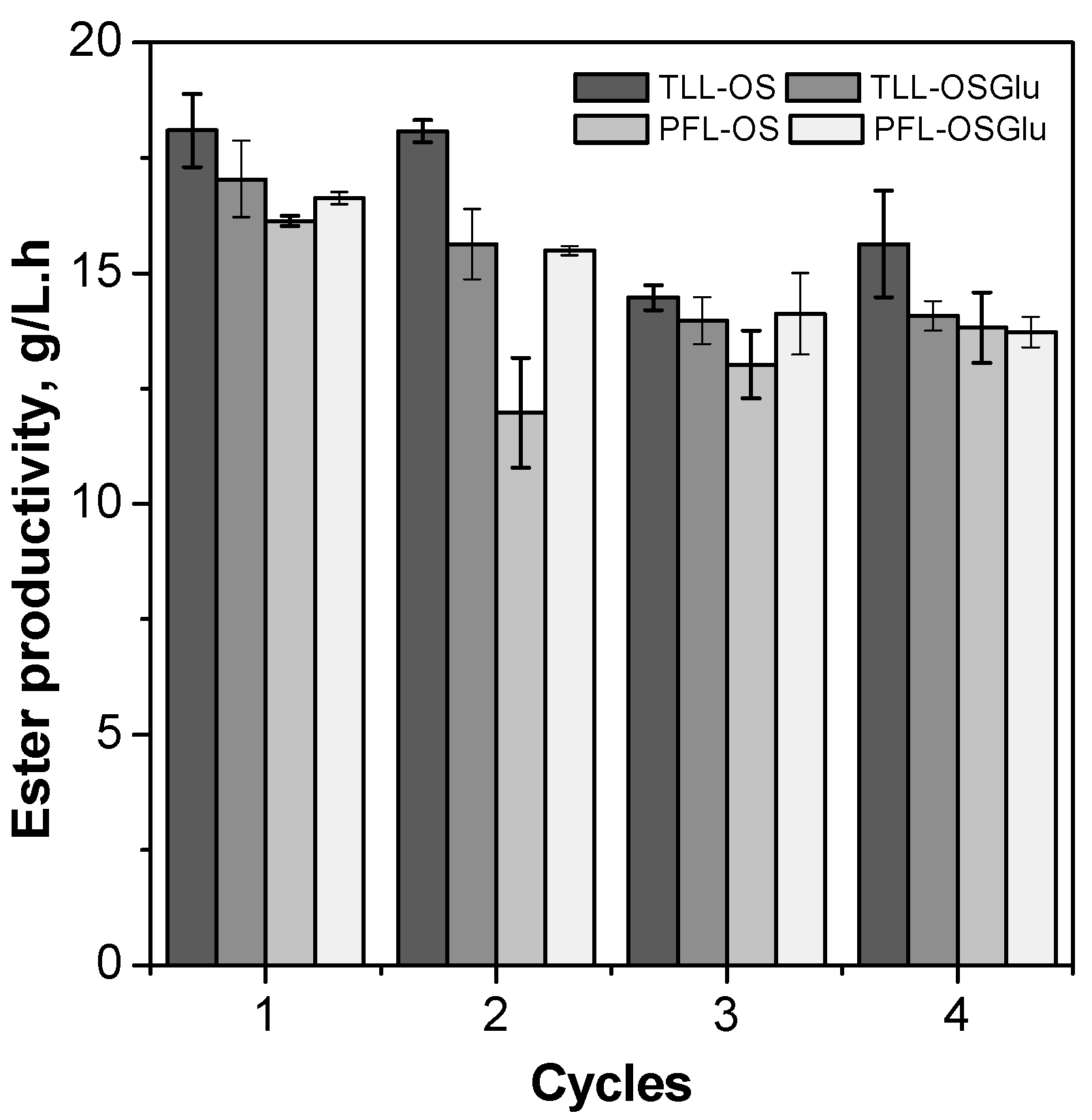
2.5. Operational Stability Tests
2.6. Literature Survey for the Enzymatic Synthesis of Fructose Oleate
3. Experimental Section
3.1. Materials
3.2. Methodology
3.2.1. Support Activation
3.2.2. Immobilization of TLL and PFL
3.2.3. p-NPB Activity Assay
3.2.4. Thermal Stability in tert-Butyl Alcohol
3.2.5. Solubility of Fructose
3.2.6. Synthesis of Fructose Oleate
3.2.7. Operational Stability Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dalla-Vecchia, R.; da Graça Nascimento, M.; Soldi, V. Aplicações sintéticas de lipases imobilizadas em polímeros. Quim. Nova 2004, 27, 623–630. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Palomo, J.M.; Ortiz, C.; Fernández-Lorente, G.; Fuentes, M.; Guisán, J.M.; Fernández-Lafuente, R. Lipase–lipase interactions as a new tool to immobilize and modulate the lipase properties. Enzyme Microb. Technol. 2005, 36, 447–454. [Google Scholar] [CrossRef]
- Lima, L.N.; Aragon, C.C.; Mateo, C.; Palomo, J.M.; Giordano, R.L.C.; Tardioli, P.W.; Guisan, J.M.; Fernandez-Lorente, G. Immobilization and stabilization of a bimolecular aggregate of the lipase from Pseudomonas fluorescens by multipoint covalent attachment. Process Biochem. 2013, 48, 118–123. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Vázquez-Duhalt, R.; Mateos-Díaz, J.C.; Guisán, J.M.; Favela-Torres, E. Carrier-free immobilization of lipase from Candida rugosa with polyethyleneimines by carboxyl-activated cross-linking. Biomacromolecules 2014, 15, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Piepenbreier, F.; Marthala, V.R.R.; Karbacher, K.; Hartmann, M. Immobilization of lipase in cage-type mesoporous organosilicas via covalent bonding and crosslinking. Catal. Today 2015, 243, 173–183. [Google Scholar] [CrossRef]
- Lima, L.N.; Oliveira, G.C.; Rojas, M.J.; Castro, H.F.; Da Rós, P.C.M.; Mendes, A.A.; Giordano, R.L.C.; Tardioli, P.W. Immobilization of Pseudomonas fluorescens lipase on hydrophobic supports and application in biodiesel synthesis by transesterification of vegetable oils in solvent-free systems. J. Ind. Microbiol. Biotechnol. 2015, 42, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yuwen, L.-X.; Peng, L.-J. Parameters affecting the performance of immobilized enzyme. J. Chem. 2013, 2013, 946248. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Barbosa, O.; Hernandez, K.; Santos, J.C.S.; Rodrigues, R.C.; Fernandez-Lafuente, R. Evaluation of styrene-divinylbenzene beads as a support to immobilize lipases. Molecules 2014, 19, 7629–7645. [Google Scholar] [CrossRef] [PubMed]
- Martinelle, M.; Holmquist, M.; Hult, K. On the interfacial activation of Candida antarctica lipase A and B as compared with Humicola lanuginosa lipase. Biochim. Biophys. Acta 1995, 1258, 272–276. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl–Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19–20, 279–286. [Google Scholar] [CrossRef]
- Kopp, W.; Silva, F.A.; Lima, L.N.; Masunaga, S.H.; Tardioli, P.W.; Giordano, R.C.; Araújo-Moreira, F.M.; Giordano, R.L.C. Synthesis and characterization of robust magnetic carriers for bioprocess applications. Mater. Sci. Eng. B 2015, 193, 217–228. [Google Scholar] [CrossRef]
- Nigam, S.; Mehrotra, S.; Vani, B.; Mehrotra, R. Lipase immobilization techniques for biodiesel production: an overview. Int. J. Renew. Energy Biofuels 2014, 2014, 664708. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Hirata, D.B.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports. Enzyme Microb. Technol. 2017, 96, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of enzymes: A literature survey. In Methods in Molecular Biology; Guisan, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 1051, pp. 15–31. [Google Scholar]
- Mateo, C.; Bolivar, J.M.; Godoy, C.A.; Rocha-Martin, J.; Pessela, B.C.; Curiel, J.A.; Muñoz, R.; Guisan, J.M.; Fernández-Lorente, G. Improvement of enzyme properties with a two-step immobilizaton process on novel heterofunctional supports. Biomacromolecules 2010, 11, 3112–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, C.; Fernandez-Lorente, G.; Rocha-Martin, J.; Bolivar, J.M.; Guisan, J.M. Oriented covalent immobilization of enzymes on heterofunctional-glyoxyl supports. Methods Mol. Biol. 2013, 1051, 73–88. [Google Scholar] [PubMed]
- Bernal, C.; Illanes, A.; Wilson, L. Heterofunctional hydrophilic-hydrophobic porous silica as support for multipoint covalent immobilization of lipases: Application to lactulose palmitate synthesis. Langmuir 2014, 30, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, V.; Kopp, W.; Guisán, J.M.; Giordano, R.L.C.; Mendes, A.A.; Tardioli, P.W. Improved catalytic properties of Candida antarctica lipase B multi-attached on tailor-made hydrophobic silica containing octyl and multifunctional amino-glutaraldehyde spacer arms. Process Biochem. 2016, 51, 2055–2066. [Google Scholar] [CrossRef]
- Mateo, C.; Fernández-Lorente, G.; Abian, O.; Fernández-Lafuente, R.; Guisán, J.M. Multifunctional epoxy supports: A new tool to improve the covalent immobilization of proteins. The promotion of physical adsorptions of proteins on the supports before their covalent linkage. Biomacromolecules 2000, 1, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Abaházi, E.; Boros, Z.; Poppe, L. Additives enhancing the catalytic properties of lipase from Burkholderia cepacia immobilized on mixed-function-grafted mesoporous silica gel. Molecules 2014, 19, 9818–9837. [Google Scholar] [CrossRef] [PubMed]
- Suescun, A.; Rueda, N.; Santos, J.C.S.; Castillo, J.J.; Ortiz, C.; Torres, R.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of lipases on glyoxyl–octyl supports: Improved stability and reactivation strategies. Process Biochem. 2015, 50, 1211–1217. [Google Scholar] [CrossRef]
- Rueda, N.; Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef]
- Albuquerque, T.L.; Rueda, N.; Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Binay, B.; Özdemir, E.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Easy stabilization of interfacially activated lipases using heterofunctional divinyl sulfone activated-octyl agarose beads. Modulation of the immobilized enzymes by altering their nanoenvironment. Process Biochem. 2016, 51, 865–874. [Google Scholar] [CrossRef]
- Rueda, N.; Santos, C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J. Mol. Catal. B Enzym. 2016, 128, 10–18. [Google Scholar] [CrossRef]
- Rueda, N.; Albuquerque, T.L.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Torres, R.; Ortiz, C.; Santos, J.C.S.; Barbosa, O.; Fernandez-Lafuente, R. Reversible immobilization of lipases on heterofunctional octyl-amino agarose beads prevents enzyme desorption. Molecules 2016, 21, 646–663. [Google Scholar] [CrossRef] [PubMed]
- Hirata, D.B.; Albuquerque, T.L.; Rueda, N.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Fernandez-Lafuente, R. Evaluation of different immobilized lipases in transesterification reactions using tributyrin: Advantages of the heterofunctional octyl agarose beads. J. Mol. Catal. B Enzym. 2016, 133, 117–123. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Luo, G.; Zhu, S. Immobilization of penicillin G acylase on macro-mesoporous silica spheres. Bioresour. Technol. 2011, 102, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.; Sierra, L.; Mesa, M. Improvement of thermal stability of β-galactosidase from Bacillus circulans by multipoint covalent immobilization in hierarchical macro-mesoporous silica. J. Mol. Catal. B Enzym. 2012, 84, 166–172. [Google Scholar] [CrossRef]
- Bernal, C.; Illanes, A.; Wilson, L. Improvement of efficiency in the enzymatic synthesis of lactulose palmitate. J. Agric. Food Chem. 2015, 63, 3716–3724. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Asymmetric hydrolysis of dimethyl-3-phenylglutarate in sequential batch reactor operation catalyzed by immobilized Geobacillus thermocatenulatus lipase. Catal. Today 2015, 255, 21–26. [Google Scholar] [CrossRef]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Selectivity of R-α-monobenzoate glycerol synthesis catalyzed by Candida antarctica lipase B immobilized on heterofunctional supports. Process Biochem. 2015, 50, 1870–1877. [Google Scholar] [CrossRef]
- Ferrer, M.; Cruces, M.A.; Plou, F.J.; Pastor, E.; Fuentes, G.; Bernabé, M.; Parra, J.L.; Ballesteros, A. Chemical versus enzymatic catalysis for the regioselective synthesis of sucrose esters of fatty acids. Stud. Surf. Sci. Catal. 2000, 130, 509–514. [Google Scholar]
- Ferrer, M.; Soliveri, J.; Plou, F.J.; López-Cortés, N.; Reyes-Duarte, D.; Christensen, M.; Copa-Patiño, J.L.; Ballesteros, A. Synthesis of sugar esters in solvent mixtures by lipases from Thermomyces lanuginosus and Candida antarctica B, and their antimicrobial properties. Enzyme Microb. Technol. 2005, 36, 391–398. [Google Scholar] [CrossRef]
- Li, L.; Ji, F.; Wang, J.; Li, Y.; Bao, Y. Esterification degree of fructose laurate exerted by Candida antarctica lipase B in organic solvents. Enzyme Microb. Technol. 2015, 69, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T.; Chisti, Y. Lipase mediated synthesis of sugar fatty acid esters. Process Biochem. 2011, 46, 2079–2090. [Google Scholar] [CrossRef]
- Matte, C.R.; Bussamara, R.; Dupont, J.; Rodrigues, R.C.; Hertz, P.F.; Ayub, M.A.Z. Immobilization of Thermomyces lanuginosus lipase by different techniques on Immobead 150 support: Characterization and applications. Appl. Biochem. Biotechnol. 2014, 172, 2507–2520. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.B.; Friedrich, J.L.R.; Cavalheiro, J.C.; Garcia-Galan, C.; Barbosa, O.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Improved production of butyl butyrate with lipase from Thermomyces lanuginosus immobilized on styrene-divinylbenzene beads. Bioresour. Technol. 2013, 134, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Self-assembly of Pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Guisán, J. Aldehyde-agarose gels as activated supports for immobilization-stabilization of enzymes. Enzyme Microb. Technol. 1988, 10, 375–382. [Google Scholar] [CrossRef]
- Mateo, C.; Grazú, V.; Pessela, B.C.C.; Montes, T.; Palomo, J.M.; Torres, R.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisán, J.M. Advances in the design of new epoxy supports for enzyme immobilization-stabilization. Biochem. Soc. Trans. 2007, 35, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Lage, F.A.P.; Bassi, J.J.; Corradini, M.C.C.; Todero, L.M.; Luiz, J.H.H.; Mendes, A.A. Preparation of a biocatalyst via physical adsorption of lipase from Thermomyces lanuginosus on hydrophobic support to catalyze biolubricant synthesis by esterification reaction in a solvent-free system. Enzyme Microb. Technol. 2016, 84, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, T.; Torres-Salas, P.; Antonioli, N.; Morelli, C.F.; Speranza, G.; Terreni, M. Regioselective deacetylation of disaccharides via immobilized Aspergillus niger esterase(s)-catalyzed hydrolysis in aqueous and non-aqueous media. ChemCatChem 2013, 5, 2925–2931. [Google Scholar] [CrossRef]
- Bavaro, T.; Torres-Salas, P.; Ubiali, D.; Terreni, M. Regioselective enzymatic hydrolysis of hexa-O-acetyl-lactal in a green non-aqueous medium. RSC Adv. 2013, 3, 7355–7359. [Google Scholar] [CrossRef]
- Yan, Y.; Bornscheuer, U.T.; Cao, L.; Schmid, R.D. Lipase-catalyzed solid-phase synthesis of sugar fatty acid esters: Removal of byproducts by azeotropic distillation. Enzyme Microb. Technol. 1999, 25, 725–728. [Google Scholar] [CrossRef]
- Neta, N.S.; Teixeira, J.A.; Rodrigues, L.R. Sugar ester surfactants: Enzymatic synthesis and applications in food industry. Crit. Rev. Food Sci. Nutr. 2015, 55, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, M.; Knez, Z. The influence of water on the synthesis of n-butyl oleate by immobilized Mucor miehei lipase. J. Am. Oil Chem. Soc. 1990, 67, 775–778. [Google Scholar] [CrossRef]
- Cui, F.-J.; Zhao, H.-X.; Sun, W.-J.; Wei, Z.; Yu, S.-L.; Zhou, Q.; Dong, Y. Ultrasound-assisted lipase-catalyzed synthesis of D-isoascorbyl palmitate: process optimization and kinetic evaluation. Chem. Cent. J. 2013, 7, 1–10. [Google Scholar] [CrossRef]
- Ferrer, M.; Cruces, M.A.; Bernabé, M.; Ballesteros, A.; Plou, F.J. Lipase-catalyzed regioselective acylation of sucrose in two-solvent mixtures. Biotechnol. Bioeng. 1999, 65, 10–16. [Google Scholar] [CrossRef]
- Reyes-Duarte, D.; López-Cortés, N.; Ferrer, M.; Plou, F.J.; Ballesteros, A. Parameters affecting productivity in the lipase-catalysed synthesis of sucrose palmitate. Biocatal. Biotransform. 2005, 23, 19–27. [Google Scholar] [CrossRef]
- Coulon, D.; Girardin, M.; Rovel, B.; Ghoul, M. Comparison of direct esterification and transesterification of fructose by Candida antarctica lipase. Biotechnol. Lett. 1995, 17, 183–186. [Google Scholar] [CrossRef]
- Coulon, D.; Girardin, M.; Engasser, J.M.; Ghoul, M. Investigation of keys parameters of fructose oleate enzymatic synthesis catalyzed by an immobilized lipase. Ind. Crops Prod. 1997, 6, 375–381. [Google Scholar] [CrossRef]
- Ducret, A.; Giroux, A.; Trani, M.; Lortie, R. Characterization of enzymatically prepared biosurfactants. J. Am. Oil Chem. Soc. 1996, 73, 109–113. [Google Scholar] [CrossRef]
- Patil, D.; de Leonardis, A.; Nag, A. Synthesis of biosurfactants from natural resources. J. Food Biochem. 2011, 35, 747–758. [Google Scholar] [CrossRef]
- Ye, R.; Hayes, D.G.; Burton, R.; Liu, A.; Harte, F.M.; Wang, Y. Solvent-free lipase-catalyzed synthesis of technical-grade sugar esters and evaluation of their physicochemical and bioactive properties. Catalysts 2016, 6, 78. [Google Scholar] [CrossRef]
- Khaled, N.; Montet, D.; Pina, M.; Graille, J. Fructose oleate synthesis in a fixed catalyst bed reactor. Biotechnol. Lett. 1991, 13, 167–172. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
| Protein Loading = 1 mg/g of Support | ||||||
| Support | TLL | PFL | ||||
| IY d (%) | RA e (%) | HA f (IU/g) | IY d (%) | RA e (%) | HA f (IU/g) | |
| OS a | 88.6 ± 1.0 | 110.4 ± 0.4 | 104.7 ± 0.3 | 92.0 ± 1.8 | 208.1 ± 4.1 | 20.3 ± 0.7 |
| OSGlu a | 89.2 ± 1.6 | 36.9 ± 1.3 | 35.2 ± 1.1 | 87.5 ± 0.6 | 110.3 ± 5.7 | 9.8 ± 0.5 |
| OSEpx b | 84.3 ± 0.1 | 58.6 ± 1.3 | 75.5 ± 0.8 | 80.4 ± 3.6 | 157.1 ± 7.0 | 13.4 ± 0.9 |
| OSGlx c | 86.9 ± 0.4 | 50.5 ± 3.5 | 43.8 ± 2.6 | 90.0 ± 1.5 | 139.7 ± 2.4 | 13.3 ± 1.1 |
| Protein Loading = 10 mg/g of Support | ||||||
| Support | TLL | PFL | ||||
| IY d (%) | RA e (%) | HA f (IU/g) | IY d (%) | RA e (%) | HA f (IU/g) | |
| OS a | 96.9 ± 0.3 | 93.3 ± 0.1 | 169.1 ± 9.2 | 89.6 ± 0.5 | 55.2 ± 0.1 | 132.7 ± 2.1 |
| OSGlu a | 94.9 ± 0.2 | 47.6 ± 0.1 | 84.6 ± 0.6 | 90.3 ± 1.1 | 39.8 ± 0.1 | 96.4 ± 0.6 |
| OSEpx b | 96.7 ± 0.8 | 56.1 ± 0.1 | 101.5 ± 1.6 | 88.0 ± 0.9 | 5.3 ± 1.2 | 12.6 ± 0.2 |
| OSGlx c | 95.5 ± 0.6 | 33.9 ± 0.2 | 60.7 ± 1.6 | 90.3 ± 1.4 | 6.0 ± 1.0 | 14.6 ± 0.2 |
| Water Content (% v/v) | Soluble Fructose Concentration | |
|---|---|---|
| g/L | mM | |
| 0 | 9.5 ± 0.1 | 52.7 ± 0.6 |
| 1 | 12.7 ± 0.3 | 70.5 ± 1.7 |
| 5 | 37.2 ± 0.8 | 206.5 ± 4.4 |
| 10 | 42.2 ± 2.1 | 234.2 ± 11.6 |
| Biocatalysts | Productivity (g/L.h) as Function of Water Amount (% v/v) in the Reaction Medium | |||
|---|---|---|---|---|
| 0% | 1% | 5% | 10% | |
| PFL-OSEpx | 0.06 ± 0.01 | |||
| PFL-OSGlx | 0.39 ± 0.03 | 0.88 ± 0.05 | 1.18 ± 0.17 | 1.24 ± 0.17 |
| PFL-OSGlu | 0.28 ± 0.04 | 0.95 ± 0.02 | 0.81 ± 0.04 | 1.17 ± 0.14 |
| PFL-OS | 0.34 ± 0.03 | 0.95 ± 0.02 | 0.74 ± 0.05 | 0.86 ± 0.22 |
| TLL-OSEpx | 0.14 ± 0.01 | |||
| TLL-OSGlx | 0.18 ± 0.01 | 0.96 ± 0.05 | 0.52 ± 0.07 | 0.50 ± 0.07 |
| TLL-OSGlu | 0.20 ± 0.01 | 0.92 ± 0.07 | 0.88 ± 0.02 | 1.06 ± 0.06 |
| TLL-OS | 0.32 ± 0.01 | 0.92 ± 0.04 | 0.94 ± 0.10 | 0.97 ± 0.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vescovi, V.; Giordano, R.L.C.; Mendes, A.A.; Tardioli, P.W. Immobilized Lipases on Functionalized Silica Particles as Potential Biocatalysts for the Synthesis of Fructose Oleate in an Organic Solvent/Water System. Molecules 2017, 22, 212. https://doi.org/10.3390/molecules22020212
Vescovi V, Giordano RLC, Mendes AA, Tardioli PW. Immobilized Lipases on Functionalized Silica Particles as Potential Biocatalysts for the Synthesis of Fructose Oleate in an Organic Solvent/Water System. Molecules. 2017; 22(2):212. https://doi.org/10.3390/molecules22020212
Chicago/Turabian StyleVescovi, Vinicius, Raquel L. C. Giordano, Adriano A. Mendes, and Paulo W. Tardioli. 2017. "Immobilized Lipases on Functionalized Silica Particles as Potential Biocatalysts for the Synthesis of Fructose Oleate in an Organic Solvent/Water System" Molecules 22, no. 2: 212. https://doi.org/10.3390/molecules22020212






