Structure and Conformational Properties of d-Glucose/d-Galactose-Binding Protein in Crowded Milieu
Abstract
:1. Introduction
2. Results and Discussion
2.1. The GGBP Structure under Crowding Conditions
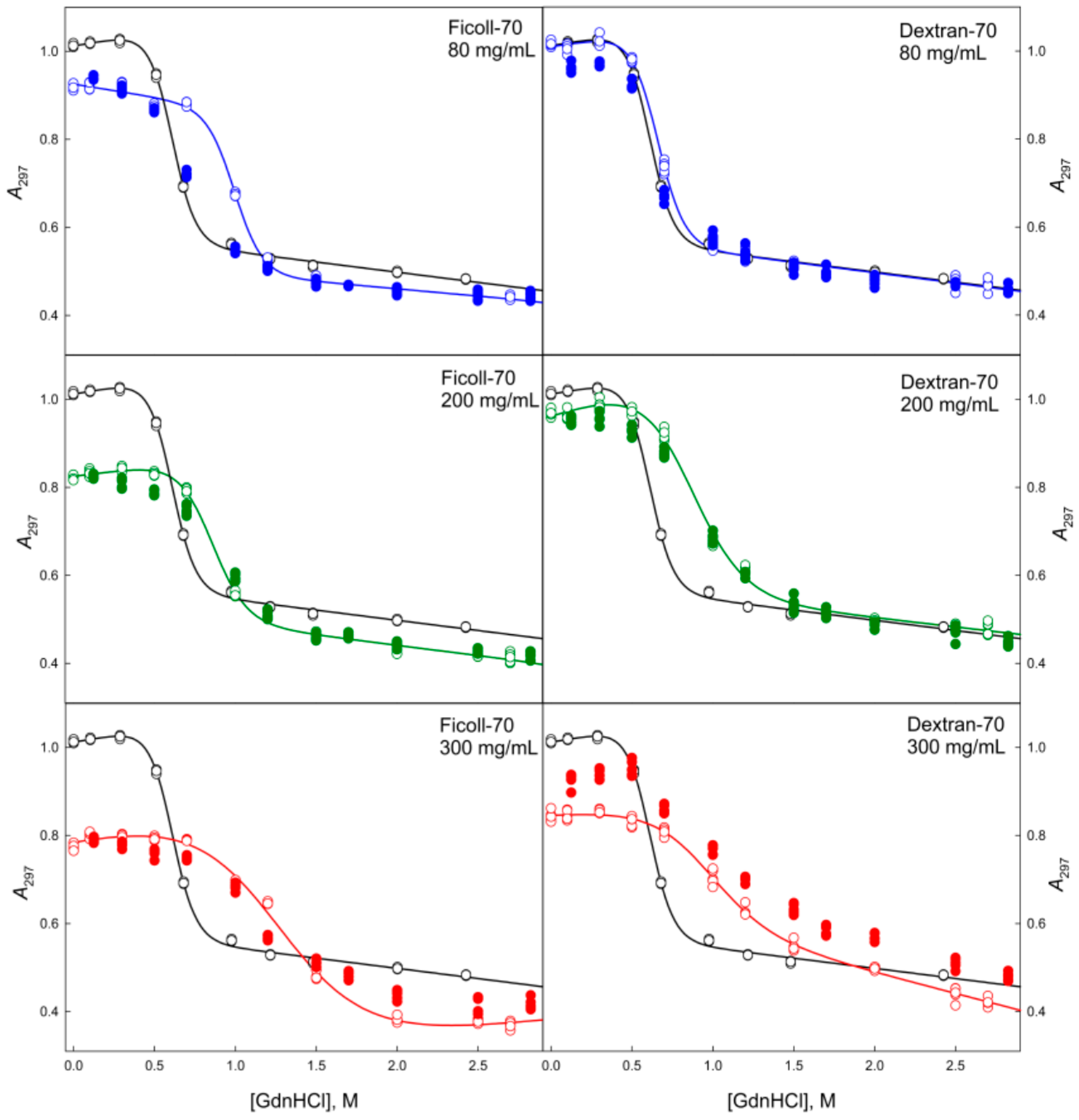
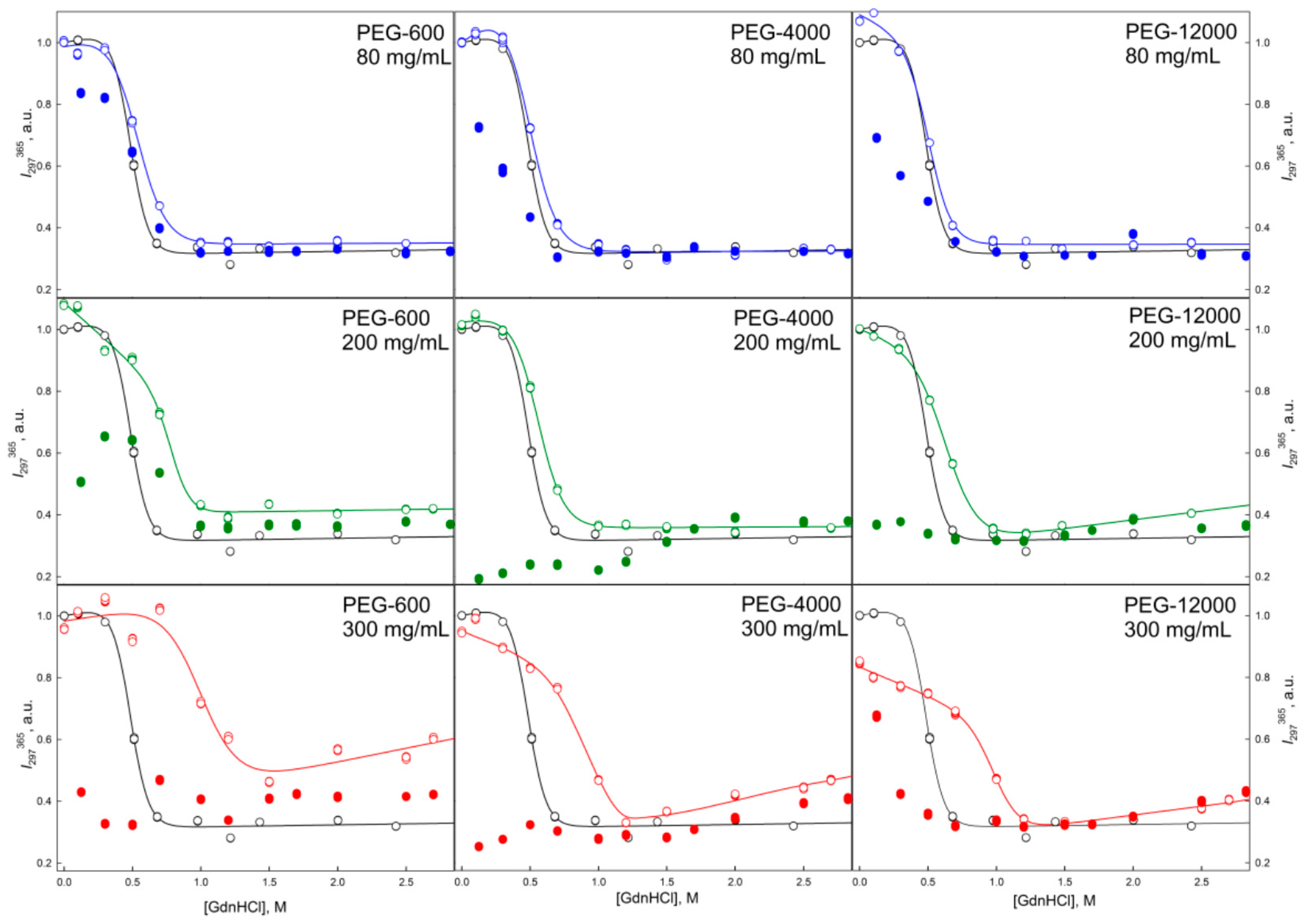
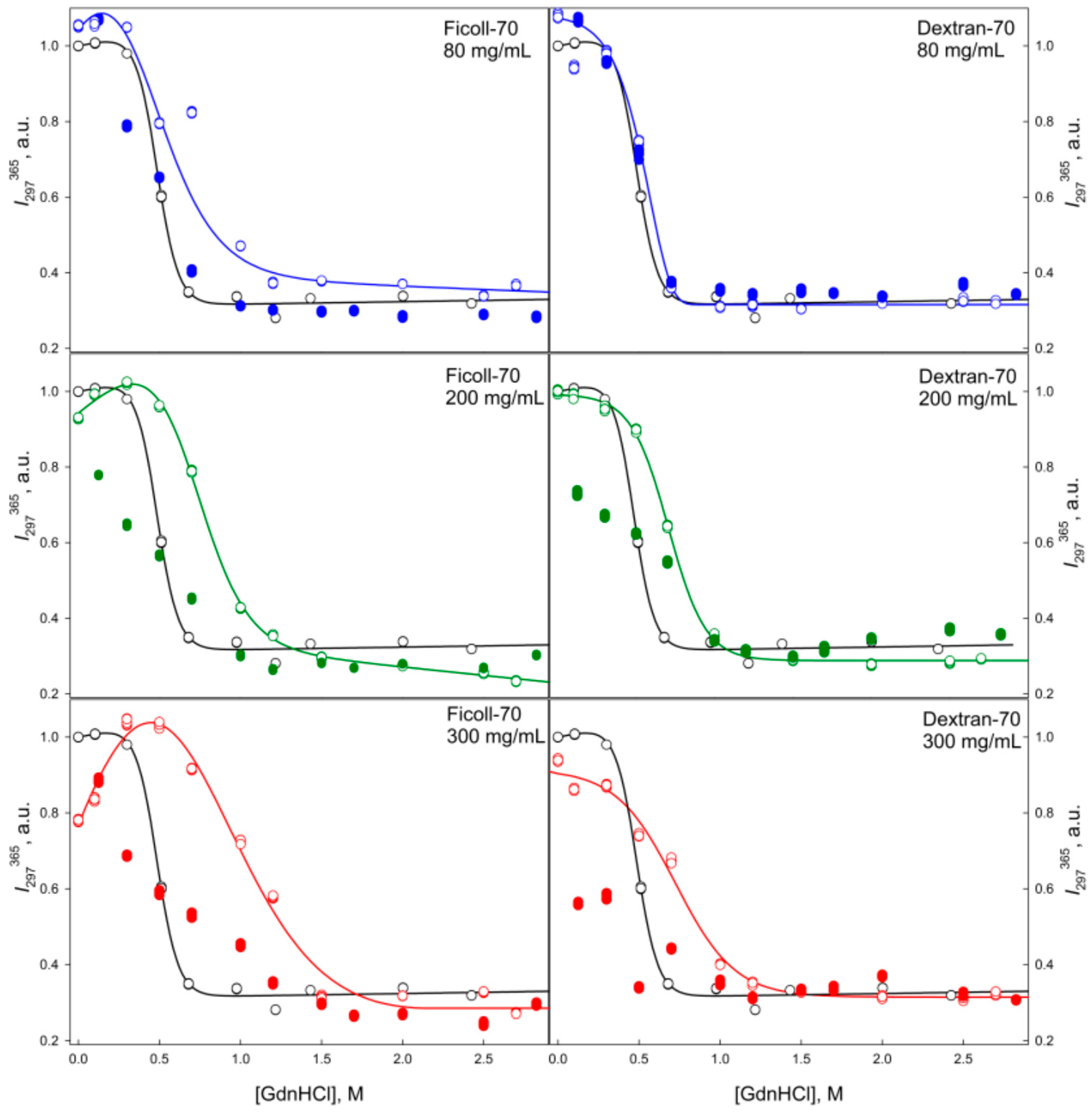
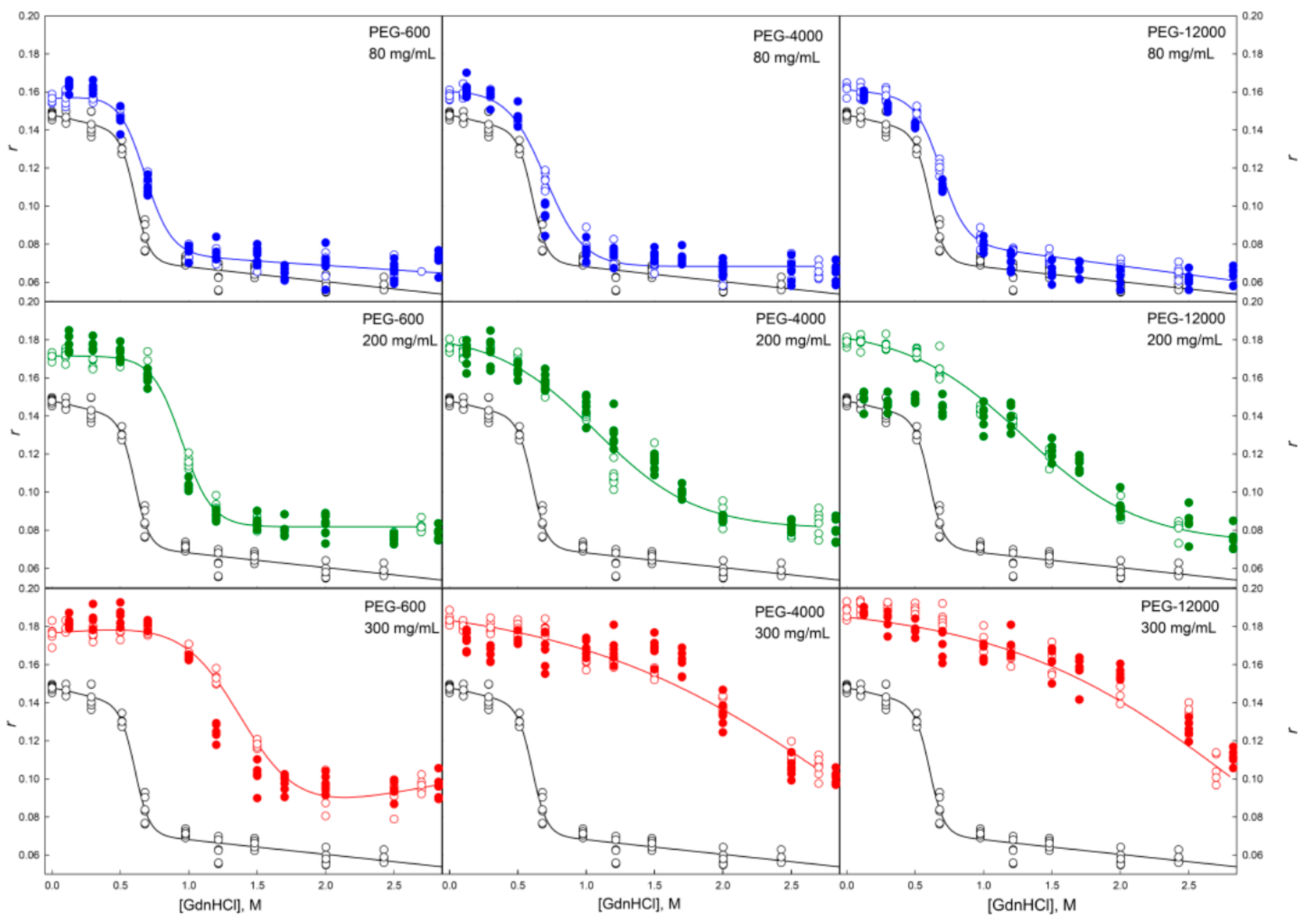
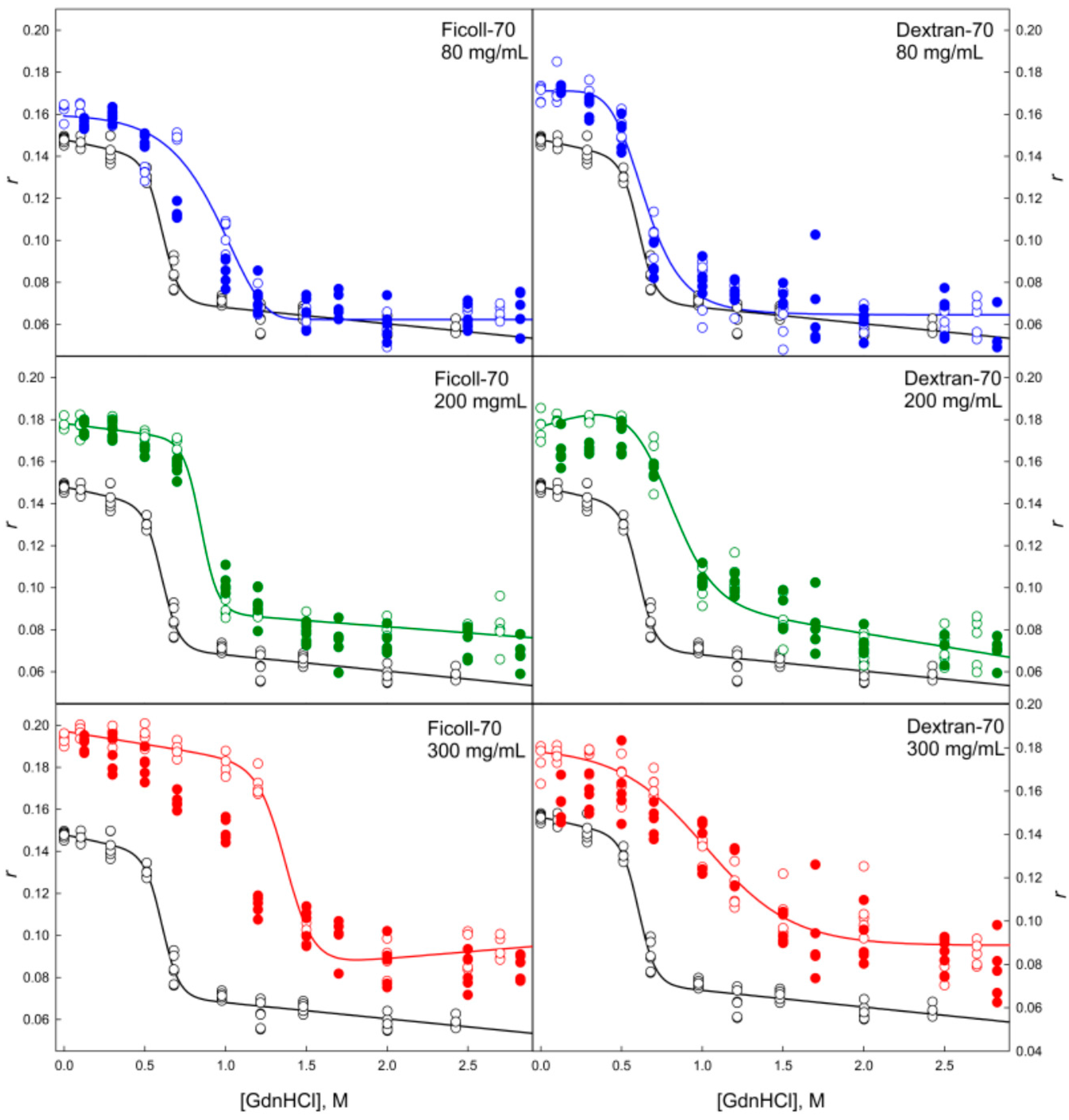
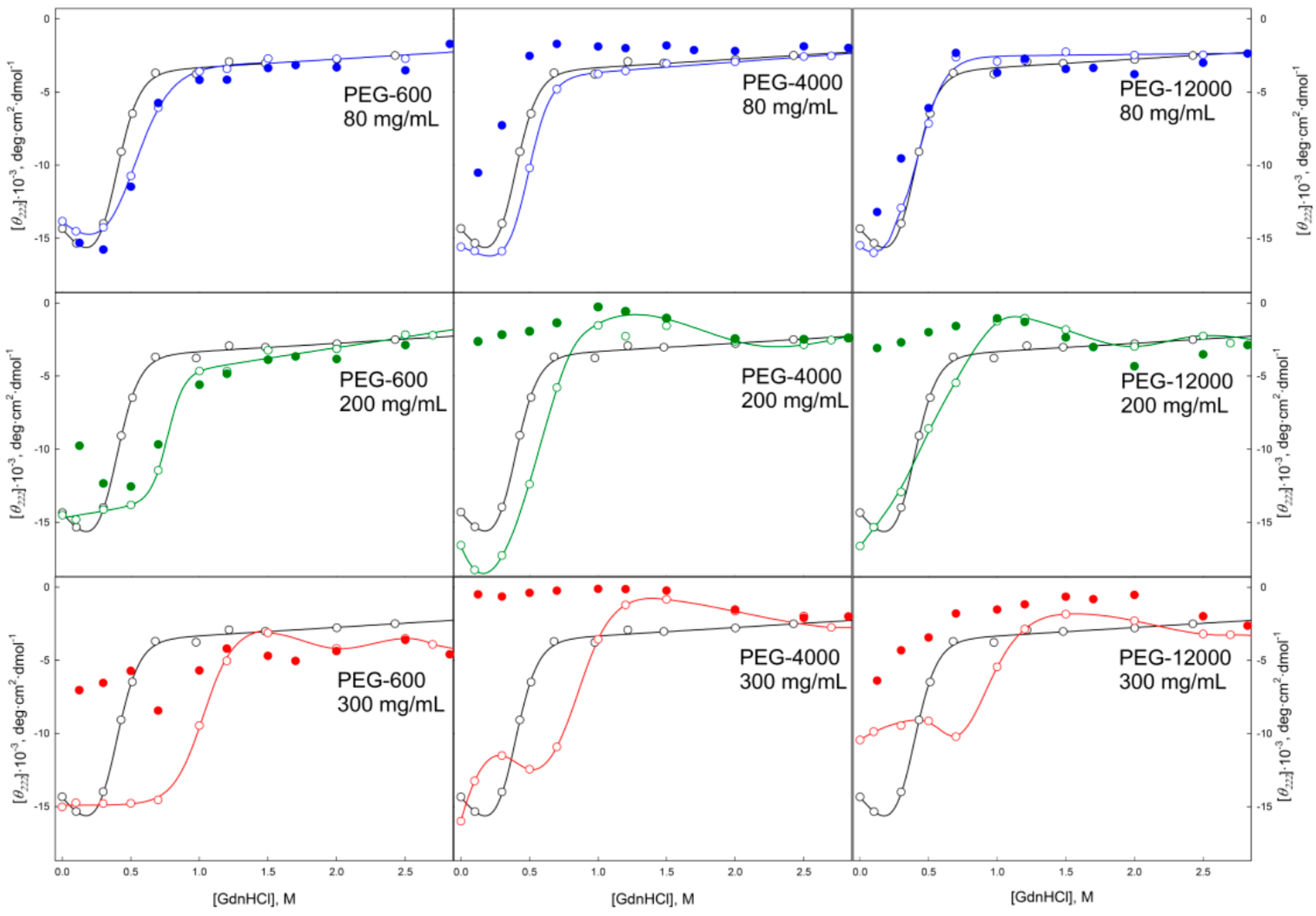
2.2. Unfolding/Refolding of GGBP in Crowded Milieu
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Steady-State Fluorescence Spectroscopy
3.2.2. Dynamic Quenching of Intrinsic Fluorescence
3.2.3. Circular Dichroism Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Radford, S.E. Protein folding: Progress made and promises ahead. Trends Biochem. Sci. 2000, 25, 611–618. [Google Scholar] [CrossRef]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 2014, 15, 23090–23140. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.A.; Filippov, D.O.; Kurganov, B.I. Effect of crowding on several stages of protein aggregation in test systems in the presence of alpha-crystallin. Int. J. Biol. Macromol. 2015, 80, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.A.; Kurganov, B.I.; Livanova, N.B. Biochemical effects of molecular crowding. Biochem. Biokhimiia 2004, 69, 1239–1251. [Google Scholar] [CrossRef]
- Eronina, T.B.; Chebotareva, N.A.; Roman, S.G.; Kleymenov, S.Y.; Makeeva, V.F.; Poliansky, N.B.; Muranov, K.O.; Kurganov, B.I. Thermal denaturation and aggregation of apoform of glycogen phosphorylase b. Effect of crowding agents and chaperones. Biopolymers 2014, 101, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.G.; Chebotareva, N.A.; Kurganov, B.I. Anti-aggregation activity of small heat shock proteins under crowded conditions. Int. J. Biol. Macromol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, N.; Kinjo, M.; Negi, S.; Hoshino, M.; Goto, Y.; Urabe, I.; Yomo, T. Protein folding by the effects of macromolecular crowding. Protein Sci. 2004, 13, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Nölting, B. Calculation of the kinetic rate constants. In Protein Folding Kinetics; Springer: New York, NY, USA, 1999; pp. 27–49. [Google Scholar]
- Yuan, J.M.; Chyan, C.L.; Zhou, H.X.; Chung, T.Y.; Peng, H.; Ping, G.; Yang, G. The effects of macromolecular crowding on the mechanical stability of protein molecules. Protein Sci. 2008, 17, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, A.; Wang, Q.; Samiotakis, A.; Cheung, M.S.; Wittung-Stafshede, P. Factors defining effects of macromolecular crowding on protein stability: An in vitro/in silico case study using cytochrome c. Biochemistry 2010, 49, 6519–6530. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, X.; F Weise, C.; Sparrman, T.; Wolf-Watz, M.; Wittung-Stafshede, P. Macromolecular crowding extended to a heptameric system: The co-chaperonin protein 10. Biochemistry 2011, 50, 3034–3044. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Zhou, Z.; Bai, Y.; Choy, W.Y. 15n nmr spin relaxation dispersion study of the molecular crowding effects on protein folding under native conditions. J. Am. Chem. Soc. 2006, 128, 3916–3917. [Google Scholar] [CrossRef] [PubMed]
- Mikaelsson, T.; Aden, J.; Wittung-Stafshede, P.; Johansson, L.B. Macromolecular crowding effects on two homologs of ribosomal protein s16: Protein-dependent structural changes and local interactions. Biophys. J. 2014, 107, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kundu, J.; Mukherjee, S.K.; Chowdhury, P.K. Myoglobin unfolding in crowding and confinement. J. Phys. Chem. B 2012, 116, 12895–12904. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, B.; Ellis, R.J.; Dobson, C.M. Effects of macromolecular crowding on protein folding and aggregation. EMBO J. 1999, 18, 6927–6933. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.; Wang, C. Effects of macromolecular crowding on the refolding of glucose-6-phosphate dehydrogenase and protein disulfide isomerase. J. Biol. Chem. 2001, 276, 34396–34401. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.K.; Vyas, M.N.; Quiocho, F.A. Sugar and signal-transducer binding sites of the Escherichia coli galactose chemoreceptor protein. Science 1988, 242, 1290–1295. [Google Scholar] [CrossRef]
- Borrok, M.J.; Kiessling, L.L.; Forest, K.T. Conformational changes of glucose/galactose-binding protein illuminated by open, unliganded, and ultra-high-resolution ligand-bound structures. Protein Sci. 2007, 16, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Povarova, O.I.; Fonin, A.V.; Kuznetsova, I.M.; Turoverov, K.K.; Staiano, M.; Varriale, A.; D’Auria, S. New insight into protein-ligand interactions. The case of the d-galactose/d-glucose-binding protein from Escherichia coli. J. Phys. Chem. B 2011, 115, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Shilton, B.H.; Flocco, M.M.; Nilsson, M.; Mowbray, S.L. Conformational changes of three periplasmic receptors for bacterial chemotaxis and transport: The maltose-, glucose/galactose- and ribose-binding proteins. J. Mol. Biol. 1996, 264, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Miklos, A.C.; Sumpter, M.; Zhou, H.X. Competitive interactions of ligands and macromolecular crowders with maltose binding protein. PLoS ONE 2013, 8, e74969. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Fonin, A.V.; Stepanenko, O.V.; Staiano, M.; D’Auria, S.; Kuznetsova, I.M.; Turoverov, K.K. Tryptophan residue of the d-galactose/d-glucose-binding protein from E. coli localized in its active center does not contribute to the change in intrinsic fluorescence upon glucose binding. J. Fluoresc. 2015, 25, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Homouz, D.; Perham, M.; Samiotakis, A.; Cheung, M.S.; Wittung-Stafshede, P. Crowded, cell-like environment induces shape changes in aspherical protein. Proc. Natl. Acad. Sci. USA 2008, 105, 11754–11759. [Google Scholar] [CrossRef] [PubMed]
- Stagg, L.; Zhang, S.Q.; Cheung, M.S.; Wittung-Stafshede, P. Molecular crowding enhances native structure and stability of alpha/beta protein flavodoxin. Proc. Natl. Acad. Sci. USA 2007, 104, 18976–18981. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Samiotakis, A.; Ebbinghaus, S.; Nienhaus, L.; Homouz, D.; Gruebele, M.; Cheung, M.S. Structure, function, and folding of phosphoglycerate kinase are strongly perturbed by macromolecular crowding. Proc. Natl. Acad. Sci. USA 2010, 107, 17586–17591. [Google Scholar] [CrossRef] [PubMed]
- Homouz, D.; Sanabria, H.; Waxham, M.N.; Cheung, M.S. Modulation of calmodulin plasticity by the effect of macromolecular crowding. J. Mol. Biol. 2009, 391, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Homouz, D.; Stagg, L.; Wittung-Stafshede, P.; Cheung, M.S. Macromolecular crowding modulates folding mechanism of alpha/beta protein apoflavodoxin. Biophys. J. 2009, 96, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, K.C.; Czader, A.; Waxham, M.N.; Cheung, M.S. The effect of macromolecular crowding, ionic strength and calcium binding on calmodulin dynamics. PLoS Comput. Biol. 2011, 7, e1002114. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.; Madeira, P.P.; Breydo, L.; Reichardt, C.; Uversky, V.N.; Zaslavsky, B.Y. Role of solvent properties of aqueous media in macromolecular crowding effects. J. Biomol. Struct. Dyn. 2016, 34, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Povarova, O.I.; Sulatskaya, A.I.; Ferreira, L.A.; Zaslavsky, B.Y.; Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. Protein unfolding in crowded milieu: What crowding can do to a protein undergoing unfolding? J. Biomol. Struct. Dyn. 2016, 34, 2155–2170. [Google Scholar] [CrossRef] [PubMed]
- Samiotakis, A.; Cheung, M.S. Folding dynamics of trp-cage in the presence of chemical interference and macromolecular crowding. I. J. Chem. Phys. 2011, 135, 175101. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.S. Where soft matter meets living matter--protein structure, stability, and folding in the cell. Curr. Opin. Struct. Biol. 2013, 23, 212–217. [Google Scholar] [CrossRef]
- Christiansen, A.; Wang, Q.; Cheung, M.S.; Wittung-Stafshede, P. Effects of macromolecular crowding agents on protein folding in vitro and in silico. Biophys. Rev. 2013, 5, 137–145. [Google Scholar] [CrossRef]
- Cheung, M.S.; Klimov, D.; Thirumalai, D. Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Kudlay, A.; Cheung, M.S.; Thirumalai, D. Crowding effects on the structural transitions in a flexible helical homopolymer. Phys. Rev. Lett. 2009, 102, 118101. [Google Scholar] [CrossRef] [PubMed]
- Kudlay, A.; Cheung, M.S.; Thirumalai, D. Influence of the shape of crowding particles on the structural transitions in a polymer. J. Phys. Chem. B 2012, 116, 8513–8522. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Fonin, A.V.; Sulatskaya, A.I.; Kuznetsova, I.M.; Turoverov, K.K. Fluorescence of dyes in solutions with high absorbance. Inner filter effect correction. PLoS ONE 2014, 9, e103878. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
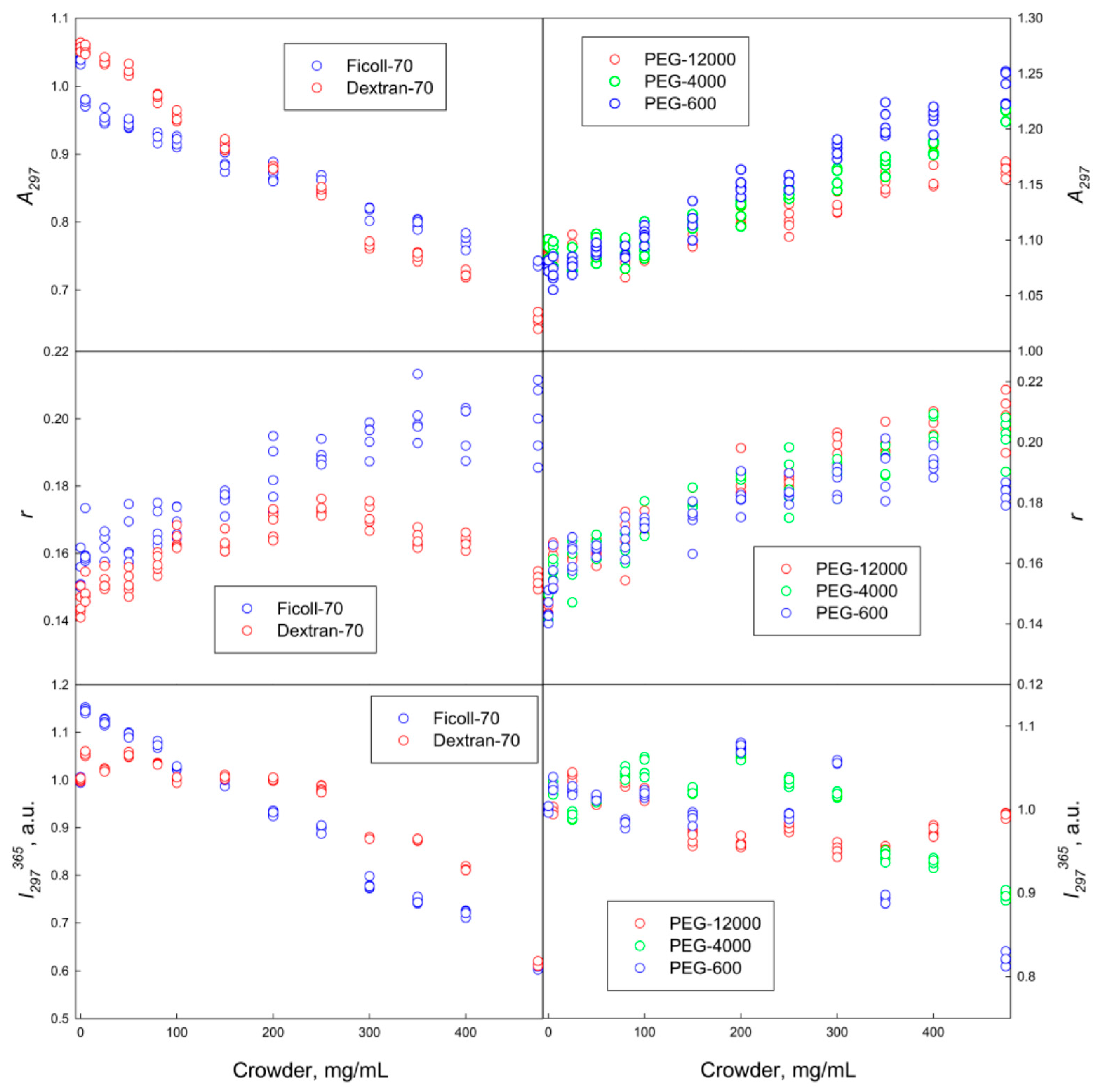
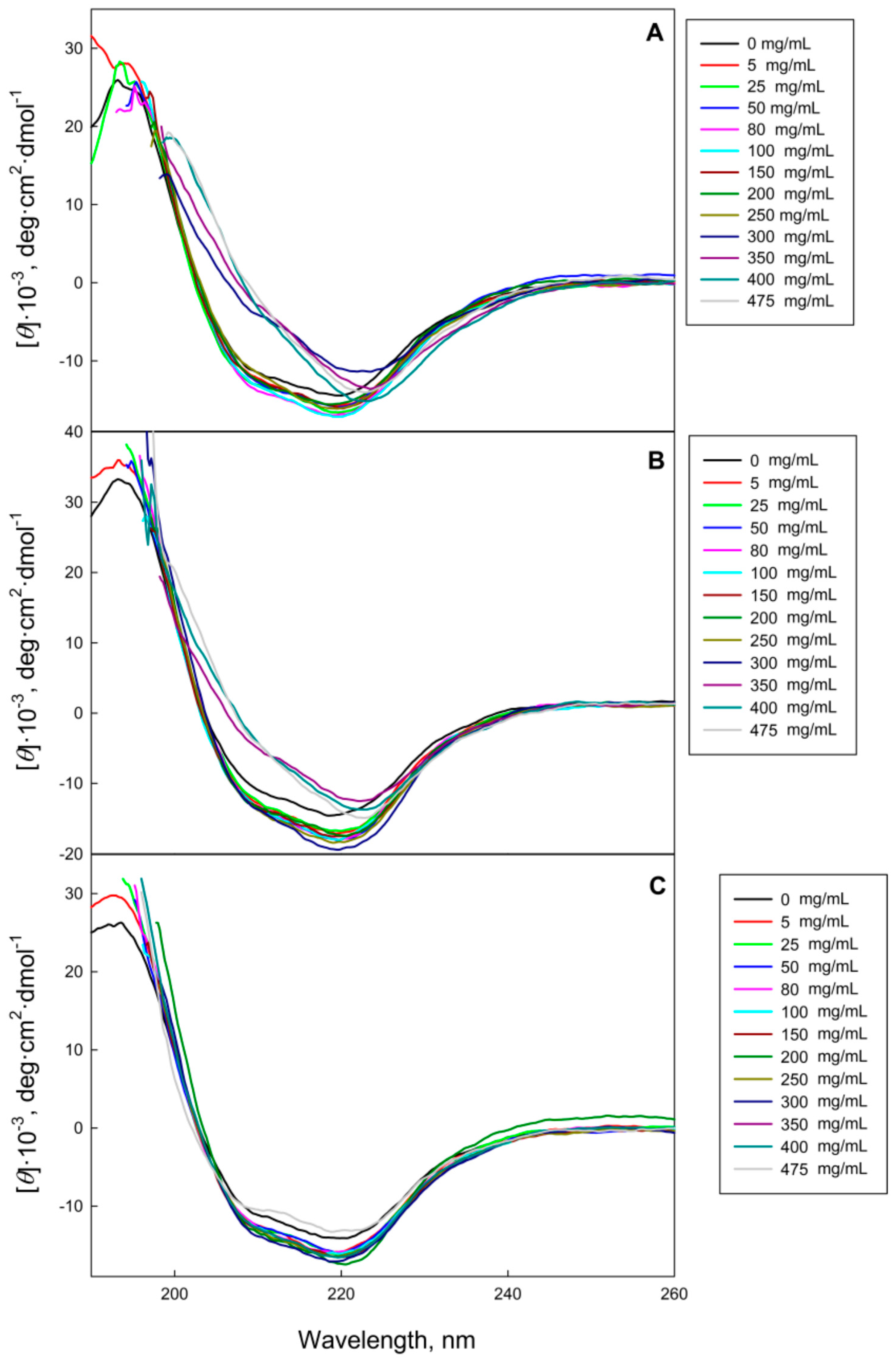
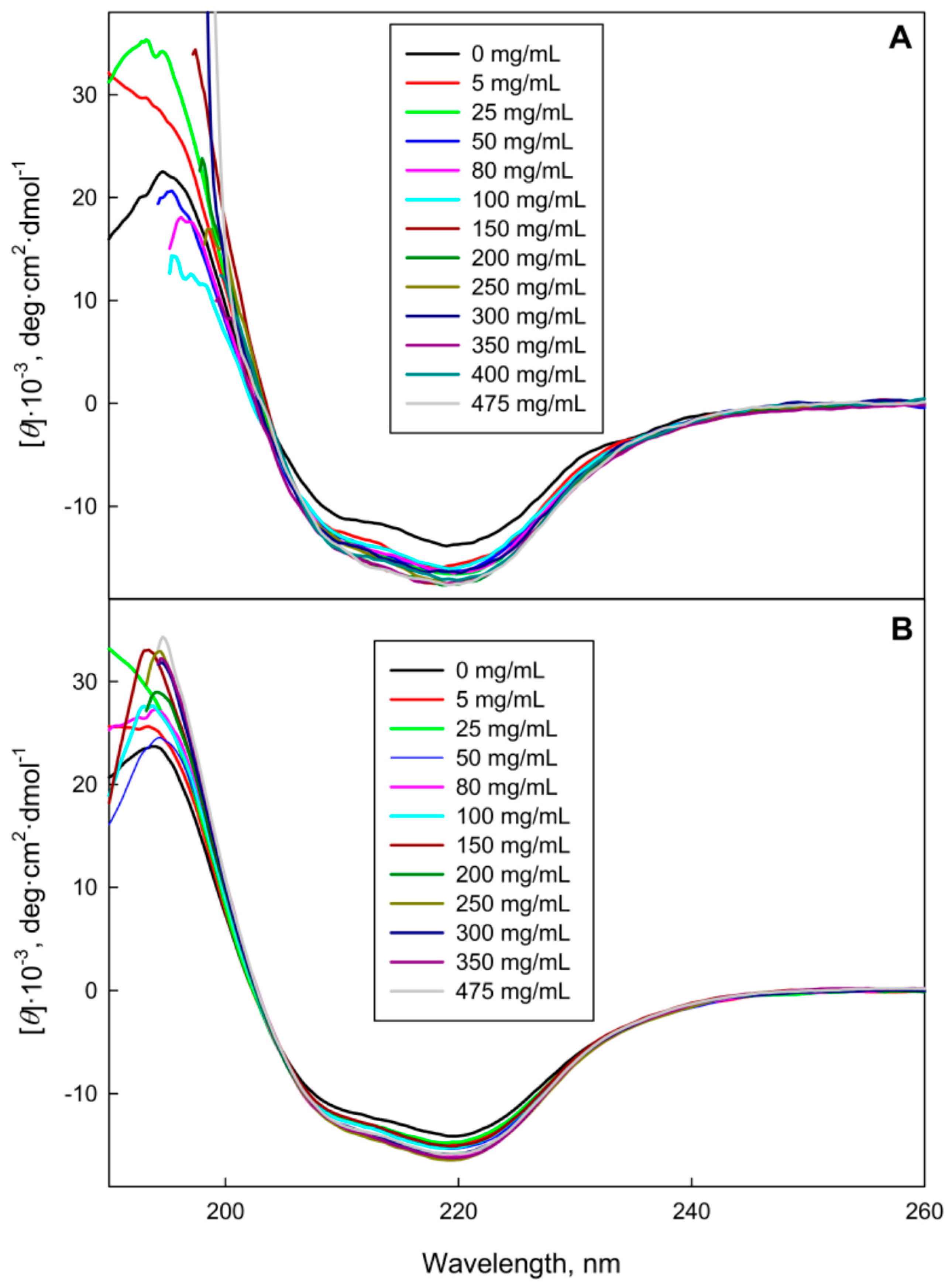
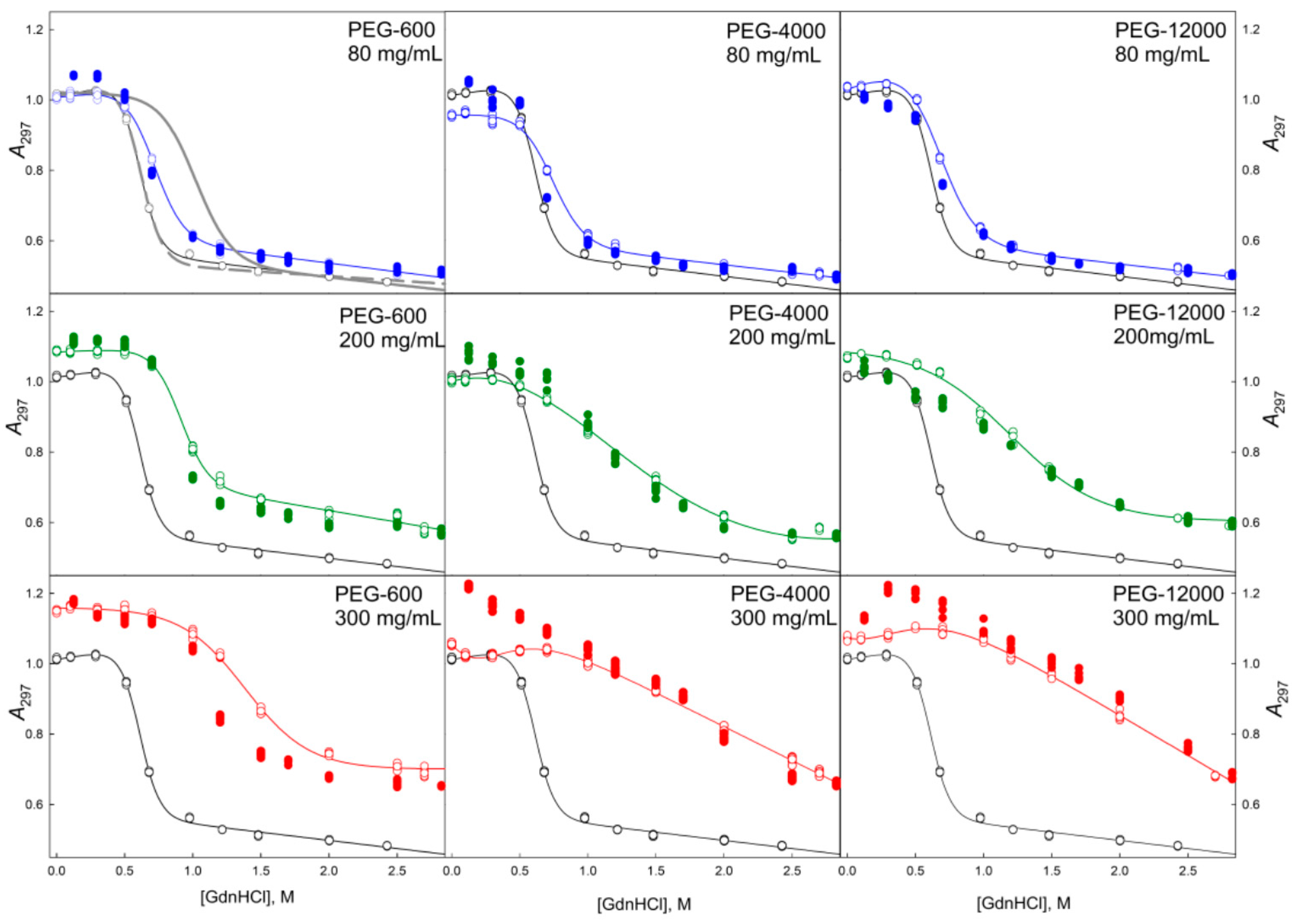

| Crowding Agent | KSV, M−1 | A297 | r | [θ]222·10−3, deg·cm2·dmol−1 |
|---|---|---|---|---|
| PEG-12000 | 2.1 | 1.12 | 0.20 | −11 |
| PEG-4000 | 2.2 | 1.16 | 0.18 | −18 |
| PEG-600 | 1.7 | 1.18 | 0.18 | −16 |
| Ficoll-70 | 1.3 | 0.82 | 0.19 | −16 |
| Dextran-70 | 1.7 | 0.77 | 0.17 | −15 |
| No crowding agents * | 4.7 | 1 | 0.15 | −14 |
| Glucose * | 1.9 | 1 | 0.16 | −15 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonin, A.V.; Silonov, S.A.; Sitdikova, A.K.; Kuznetsova, I.M.; Uversky, V.N.; Turoverov, K.K. Structure and Conformational Properties of d-Glucose/d-Galactose-Binding Protein in Crowded Milieu. Molecules 2017, 22, 244. https://doi.org/10.3390/molecules22020244
Fonin AV, Silonov SA, Sitdikova AK, Kuznetsova IM, Uversky VN, Turoverov KK. Structure and Conformational Properties of d-Glucose/d-Galactose-Binding Protein in Crowded Milieu. Molecules. 2017; 22(2):244. https://doi.org/10.3390/molecules22020244
Chicago/Turabian StyleFonin, Alexander V., Sergey A. Silonov, Asiya K. Sitdikova, Irina M. Kuznetsova, Vladimir N. Uversky, and Konstantin K. Turoverov. 2017. "Structure and Conformational Properties of d-Glucose/d-Galactose-Binding Protein in Crowded Milieu" Molecules 22, no. 2: 244. https://doi.org/10.3390/molecules22020244







