Poloxamer-Based Thermoreversible Gel for Topical Delivery of Emodin: Influence of P407 and P188 on Solubility of Emodin and Its Application in Cellular Activity Screening
Abstract
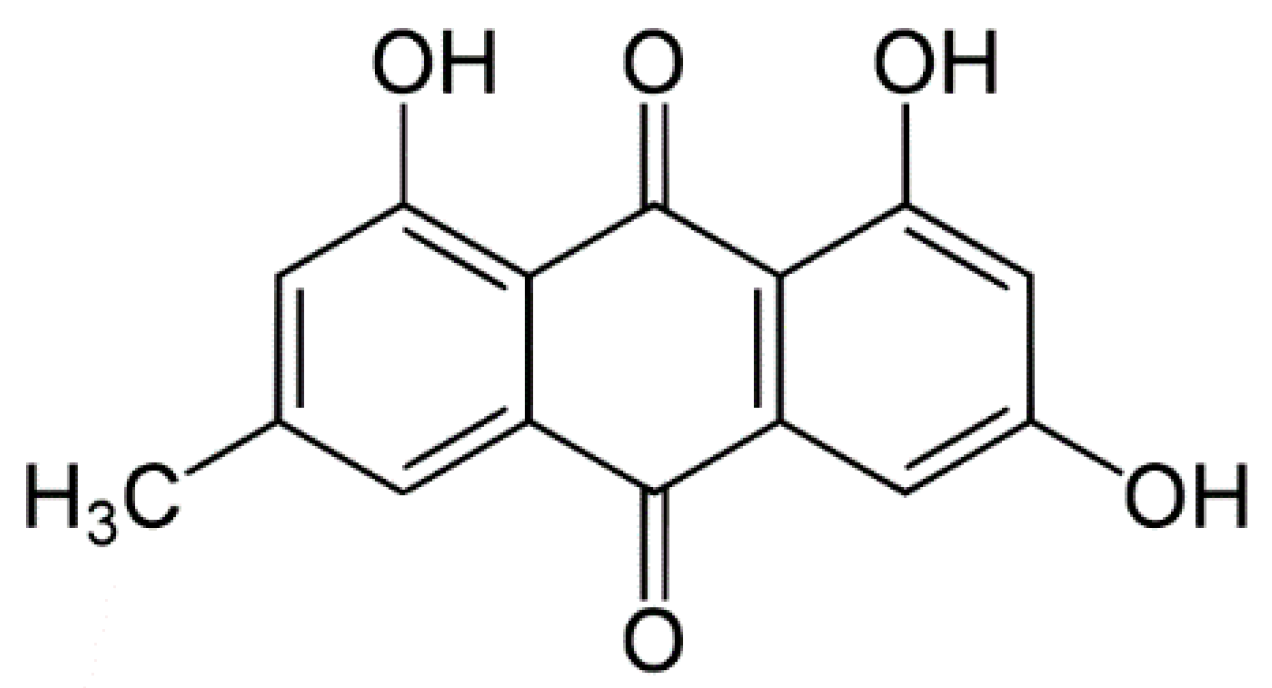
:1. Introduction
2. Results and Discussion
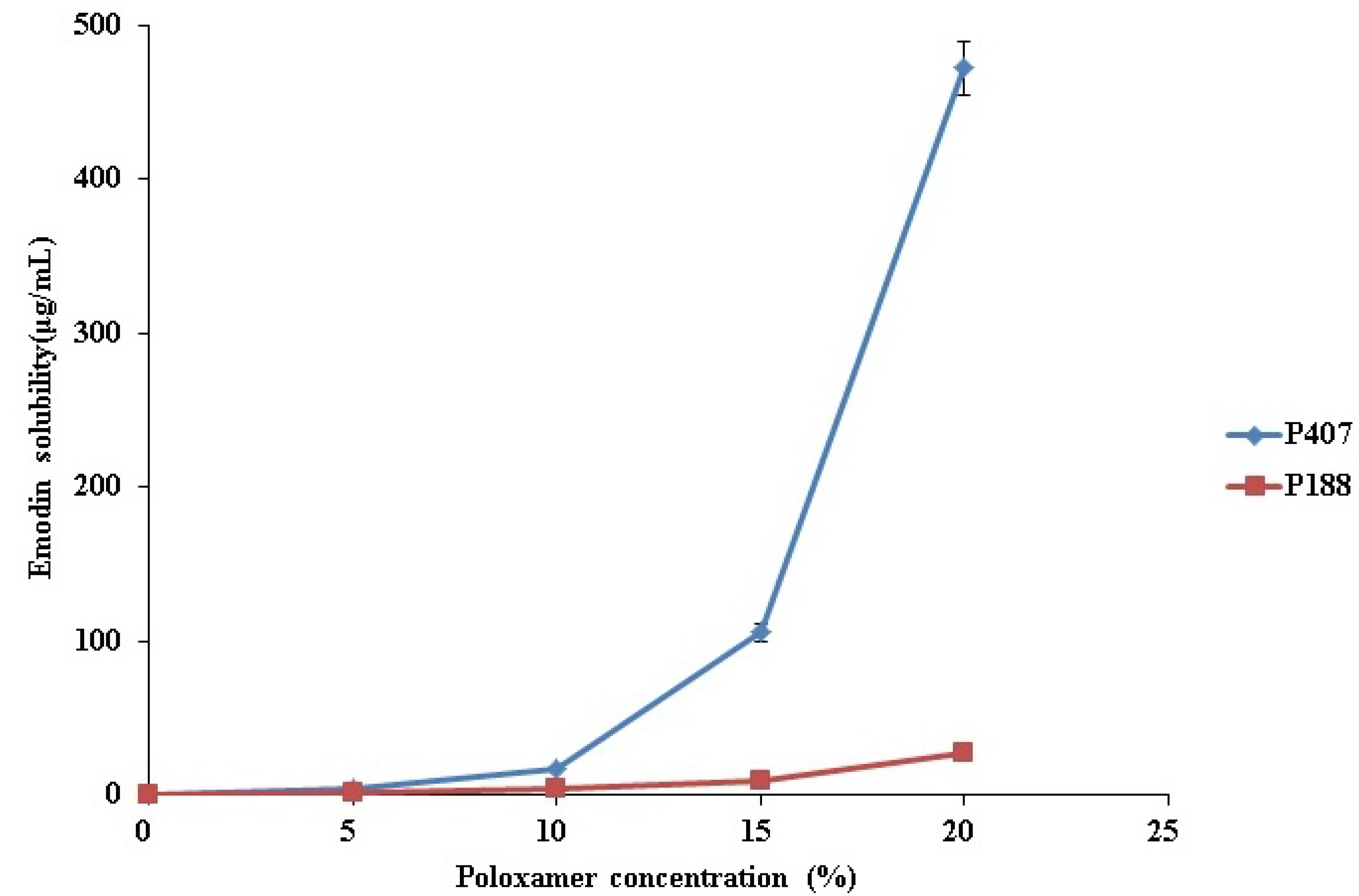
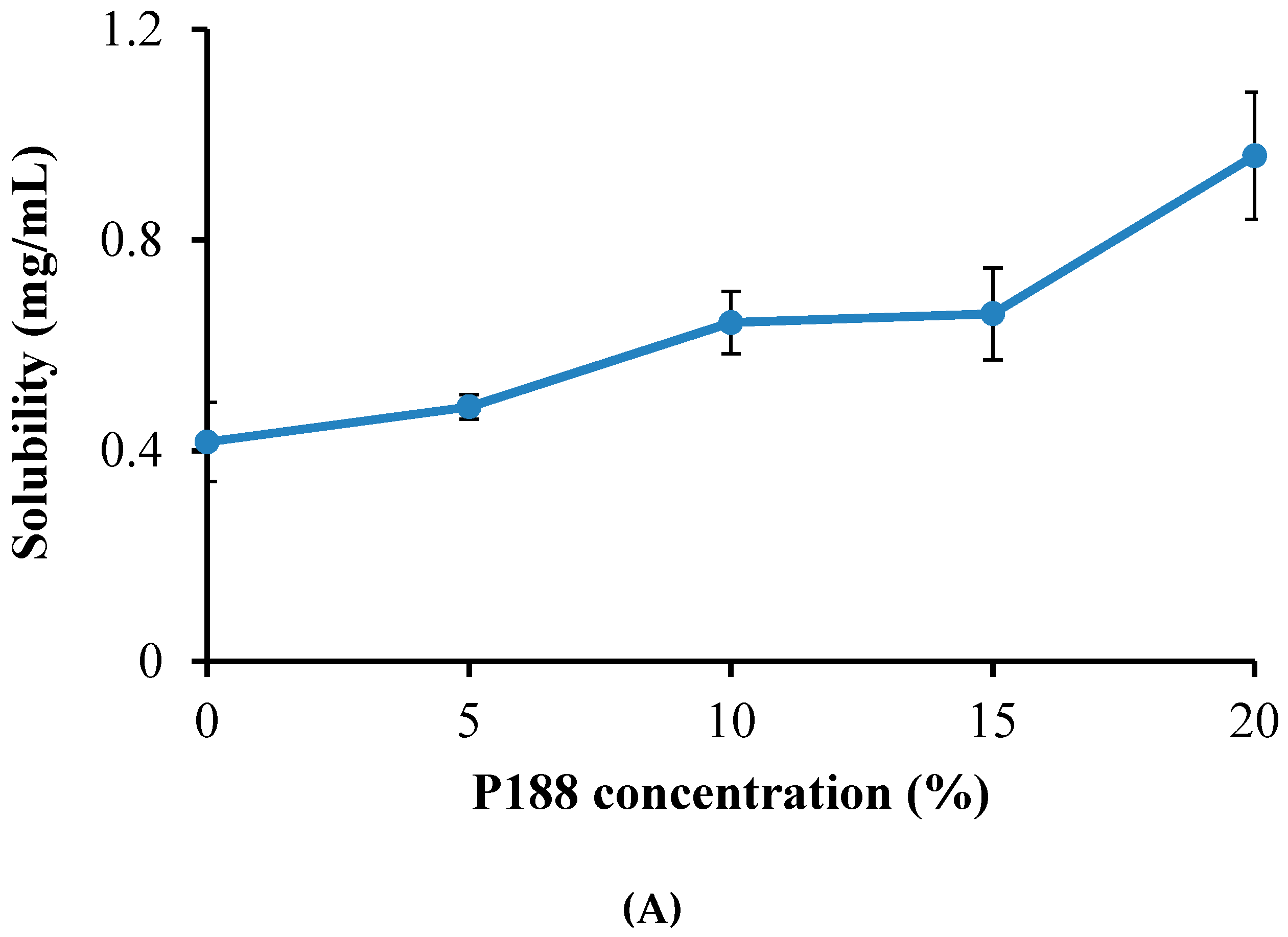
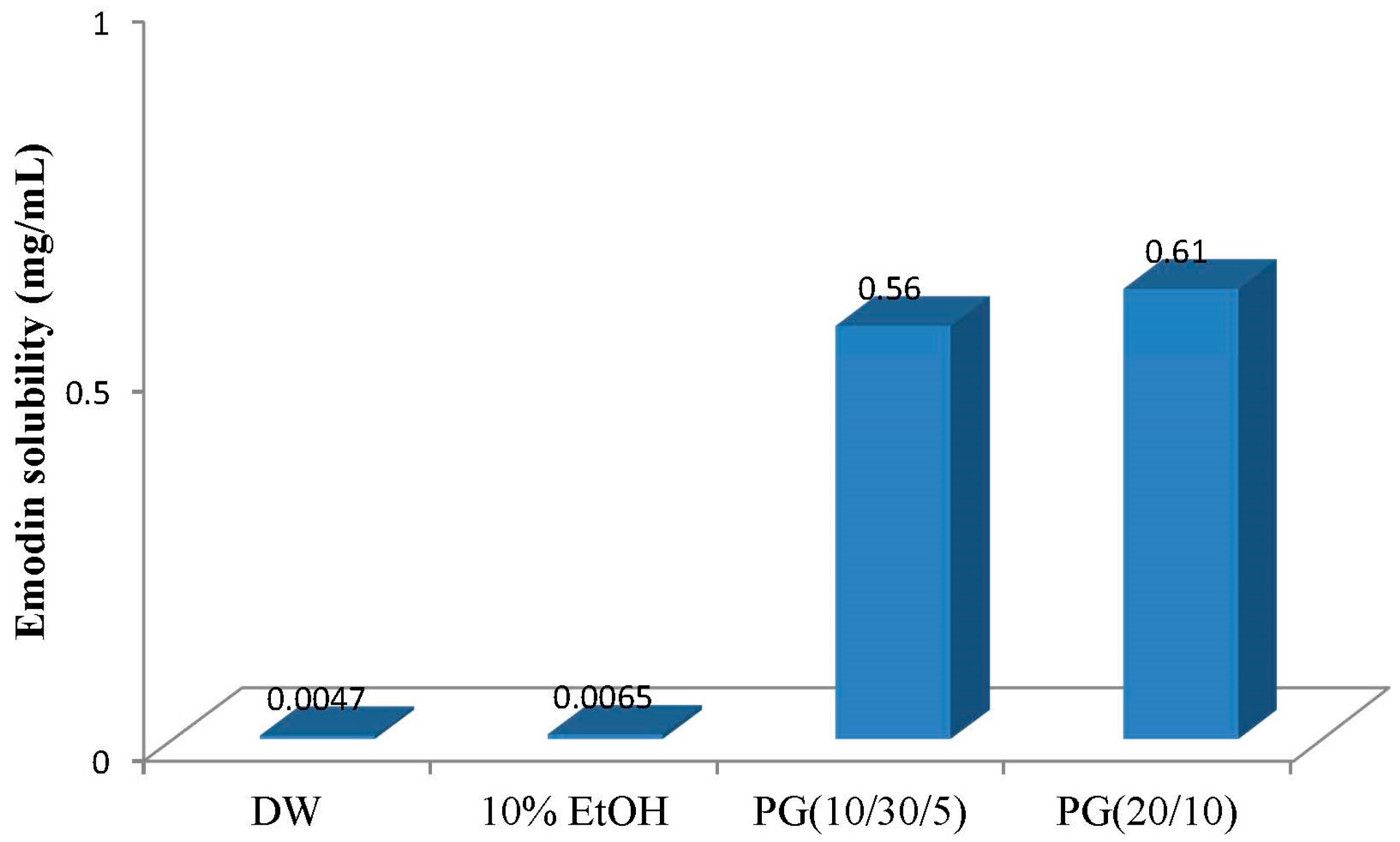
2.1. Comparison of P407 and P188 for the Solubilization of Emodin
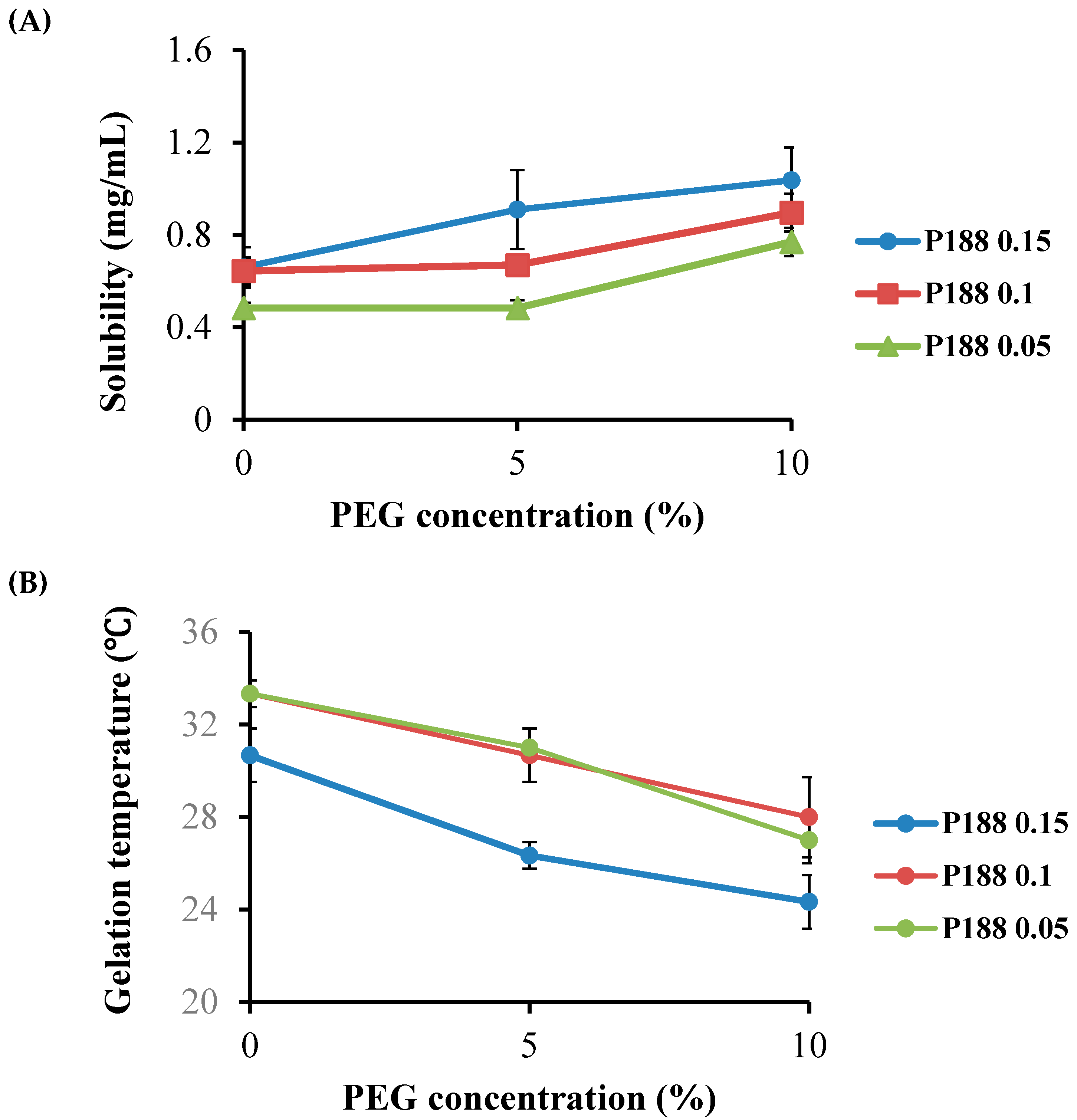
2.2. Effect of P407 and P188 on Emodin Solubility and Gelation Temperature in Thermoreversible PG
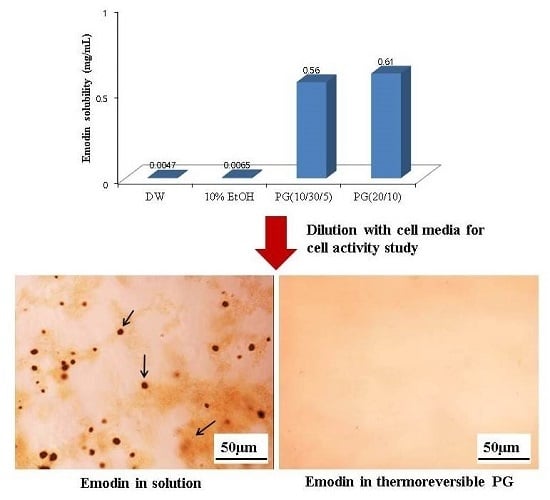
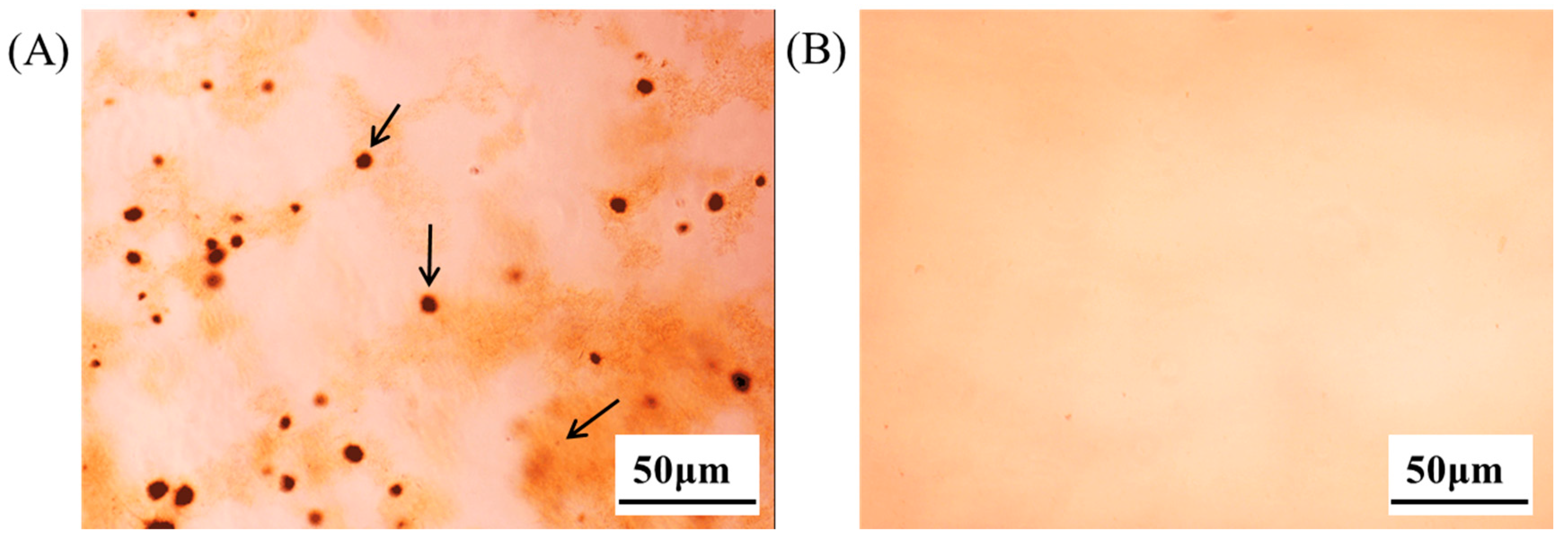
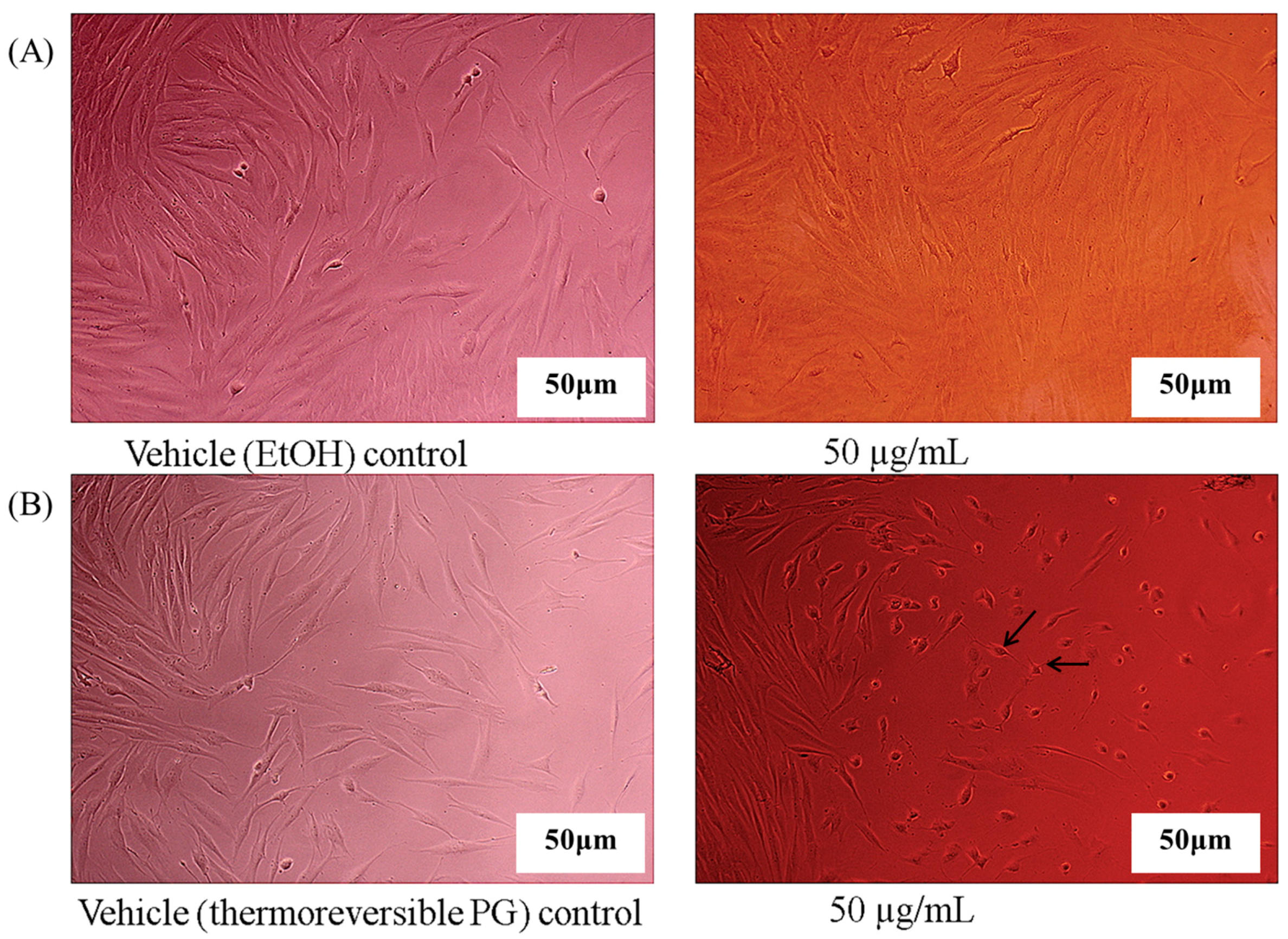
2.3. In Vitro Cell Activity Screening of Emodin Thermoreversible PG
3. Materials and Methods
3.1. Materials
3.2. Preparation of Emodin Loaded Thermoreversible Poloxamer Gel
3.3. Measurement of Gelation Temperature
3.4. Measurement of Solubility
3.5. Cell Culture Experiments
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuo, Y.C.; Tsai, W.J.; Meng, H.C.; Chen, W.P.; Yang, L.Y.; Lin, C.Y. Immune responses in human mesangial cells regulated by emodin from Polygonum hypoleucum Ohwi. Life Sci. 2001, 68, 1271–1286. [Google Scholar] [CrossRef]
- Basu, S.; Ghosh, A.; Hazra, B. Evaluation of the antibacterial activity of Ventilago madraspatana Gaertn, Rubia cordifolia Linn and Lantana camara Linn: Isolation of emodin and physcion as active antibacterial agents. Phytother. Res. 2005, 19, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Chung, J.G. Anticancer potential of emodin. Biomedicine 2012, 2, 108–116. [Google Scholar] [CrossRef]
- Liu, W.; Tang, L.; Ye, L.; Cai, Z.; Xia, B.; Zhang, J.; Hu, M.; Liu, Z. Species and gender differences affect the metabolism of emodin via glucuronidation. AAPS J. 2010, 12, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poor water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Martino, P.D. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Aller, M.; Guillarme, D.; Jean-Luc Veuthey, J.L.; Gurny, R. Strategies for formulating and delivering poorly water-soluble drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 342–351. [Google Scholar] [CrossRef]
- Bhat, P.A.; Dar, A.A.; Rather, G.M. Solubilization Capabilities of Some Cationic, Anionic, and Nonionic Surfactants toward the Poorly Water-Soluble Antibiotic Drug Erythromycin. J. Chem. Eng. Data 2008, 53, 1271–1277. [Google Scholar] [CrossRef]
- Kolašinac, N.; Kyriakos Kachrimanis, K.; Homšek, I.; Branka Grujić, B.; Zorica Ðurić, Z.; Ibrić, S. Solubility enhancement of desloratadine by solid dispersion in poloxamers. Int. J. Pharm. 2012, 436, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Khateb, K.A.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Radivojša, M.; Grabnar, I.; Grabnar, P.A. Thermoreversible in situ gelling poloxamer-based systems with chitosan nanocomplexes for prolonged subcutaneous delivery of heparin: Design and in vitro evaluation. Eur. J. Pharm. Sci. 2013, 50, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Kim, C.K. Design and evaluation of ondansetron liquid suppository for the treatment of emesis. Arch. Pharm. Res. 2013, 36, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Li, L.; Huang, C.; Li, W.; Wu, C. Optimization and physicochemical characterization of thermosensitive poloxamer gel containing puerarin for ophthalmic use. Chem. Pharm. Bull. 2006, 54, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Jang, D.J.; Kim, S.J.; Park, M.; Kim, A. Optimization of thermoreversible poloxamer gel system using QbD principle. Pharm. Dev. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Yin, L.; Yang, J.; Shan, G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur. J. Pharmacol. 2007, 567, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.G.; Dong, L.C.; Li, S.; Deng, Z. Combination of Pluronic/Vitamin E TPGS as a potential inhibitor of drug precipitation. Int. J. Pharm. 2008, 355, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are not available.

| Poloxamer (%, w/w) | PEG400 (%, w/w) | Gelation Temperature (°C) | Solubility (mg/mL) | |
|---|---|---|---|---|
| P407 | P188 | |||
| 20 | 0 | 0 | 23.67 ± 1.15 | 0.42 ± 0.05 |
| 20 | 5 | 0 | 33.33 ± 0.58 | 0.47 ± 0.00 |
| 20 | 5 | 5 | 31.00 ± 0.00 | 0.50 ± 0.03 |
| 20 | 5 | 10 | 27.00 ± 1.00 | 0.74 ± 0.01 |
| 20 | 10 | 0 | 33.33 ± 0.58 | 0.61 ± 0.01 |
| 20 | 10 | 5 | 30.67 ± 1.15 | 0.66 ± 0.01 |
| 20 | 10 | 0 | 28.00 ± 1.73 | 0.85 ± 0.01 |
| 20 | 15 | 0 | 30.67 ± 1.15 | 0.63 ± 0.01 |
| 20 | 15 | 5 | 26.33 ± 0.58 | 0.85 ± 0.07 |
| 20 | 15 | 20 | 24.33 ± 1.15 | 0.96 ± 0.00 |
| 20 | 20 | 0 | 25.67 ± 0.58 | 0.90 ± 0.00 |
| Poloxamer (%, w/w) | PEG400 (%, w/w) | Gelation Temperature (°C) | Solubility (mg/mL) | |
|---|---|---|---|---|
| P407 | P188 | |||
| 10 | 30 | 0 | 36.5 ± 0.6 | 0.54 ± 0.06 |
| 10 | 30 | 5 | 34.5 ± 0.5 | 0.56 ± 0.07 |
| 10 | 30 | 10 | <25.0 | 0.69 ± 0.05 |
| 5 | 35 | 0 | 38.7 ± 0.4 | 0.50 ± 0.05 |
| 5 | 35 | 5 | 35.5 ± 0.9 | 0.52 ± 0.06 |
| 5 | 35 | 10 | 30.5 ± 0.4 | 0.61 ± 0.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, E.; Park, M.; Jeong, S.; Kwon, T.; Kim, E.-H.; Jung, K.; Kim, A. Poloxamer-Based Thermoreversible Gel for Topical Delivery of Emodin: Influence of P407 and P188 on Solubility of Emodin and Its Application in Cellular Activity Screening. Molecules 2017, 22, 246. https://doi.org/10.3390/molecules22020246
Ban E, Park M, Jeong S, Kwon T, Kim E-H, Jung K, Kim A. Poloxamer-Based Thermoreversible Gel for Topical Delivery of Emodin: Influence of P407 and P188 on Solubility of Emodin and Its Application in Cellular Activity Screening. Molecules. 2017; 22(2):246. https://doi.org/10.3390/molecules22020246
Chicago/Turabian StyleBan, Eunmi, Mijung Park, Seonghee Jeong, Taekhyun Kwon, Eun-Hee Kim, Kiwon Jung, and Aeri Kim. 2017. "Poloxamer-Based Thermoreversible Gel for Topical Delivery of Emodin: Influence of P407 and P188 on Solubility of Emodin and Its Application in Cellular Activity Screening" Molecules 22, no. 2: 246. https://doi.org/10.3390/molecules22020246