Influence of Weak Base Addition to Hole-Collecting Buffer Layers in Polymer:Fullerene Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
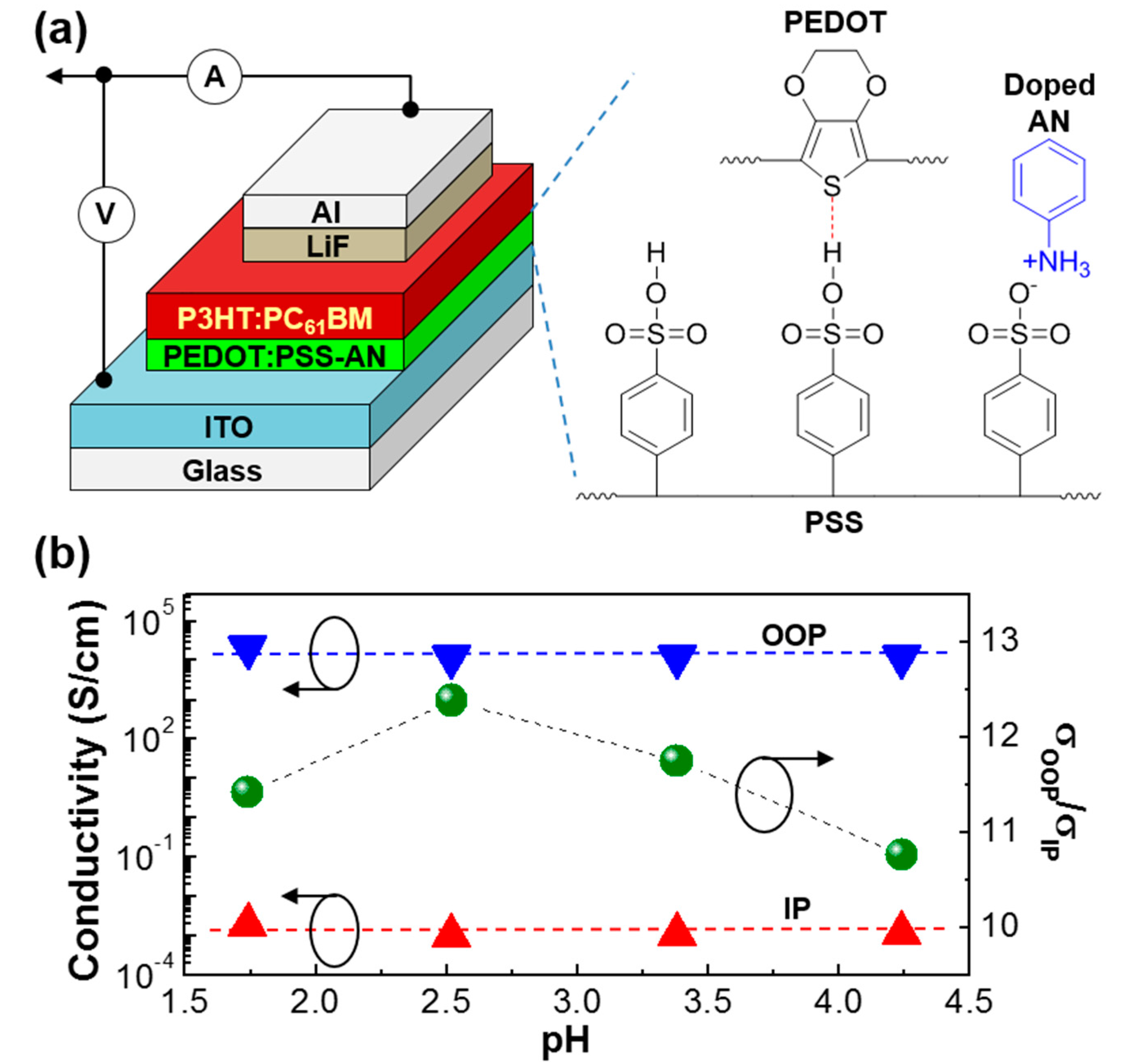
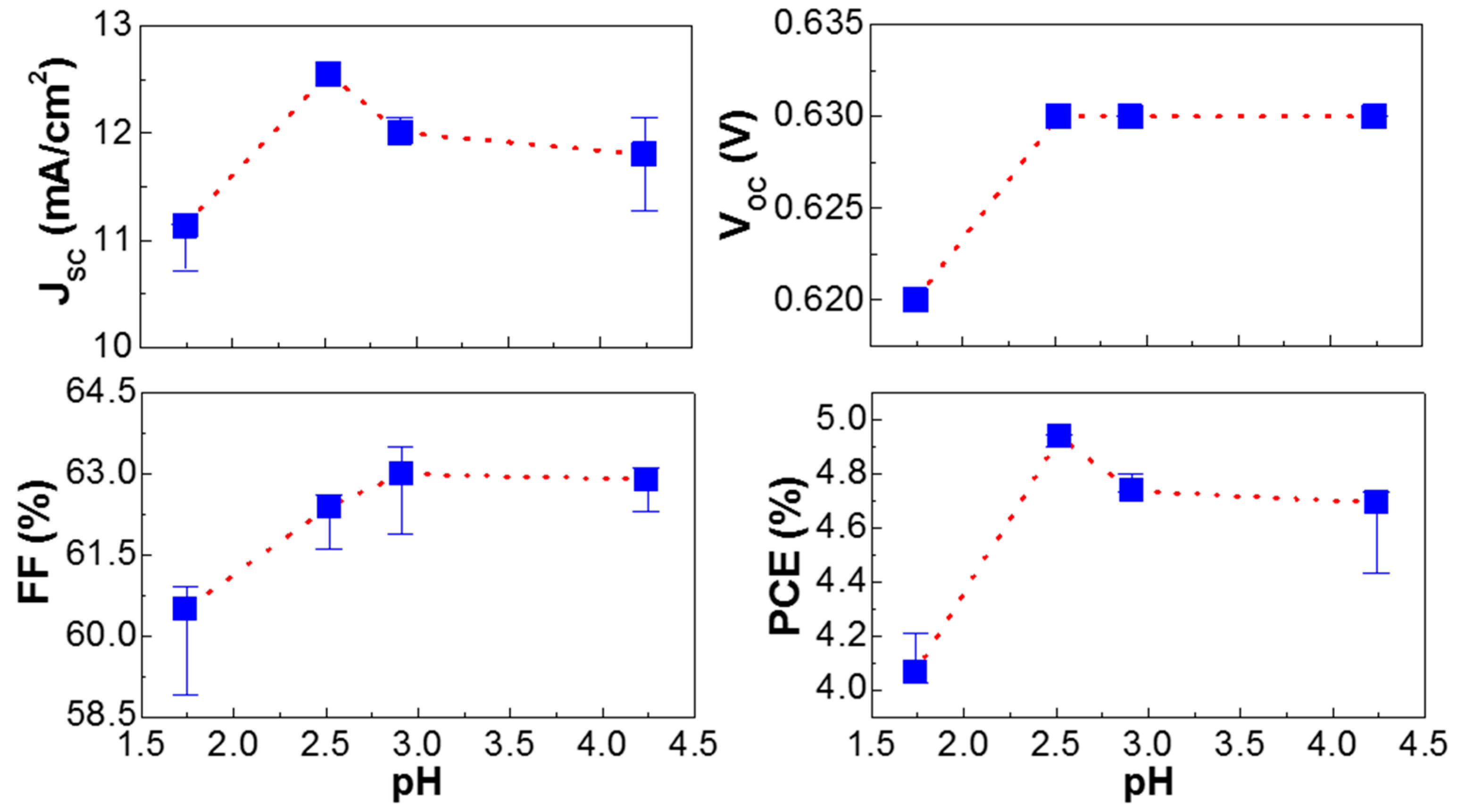
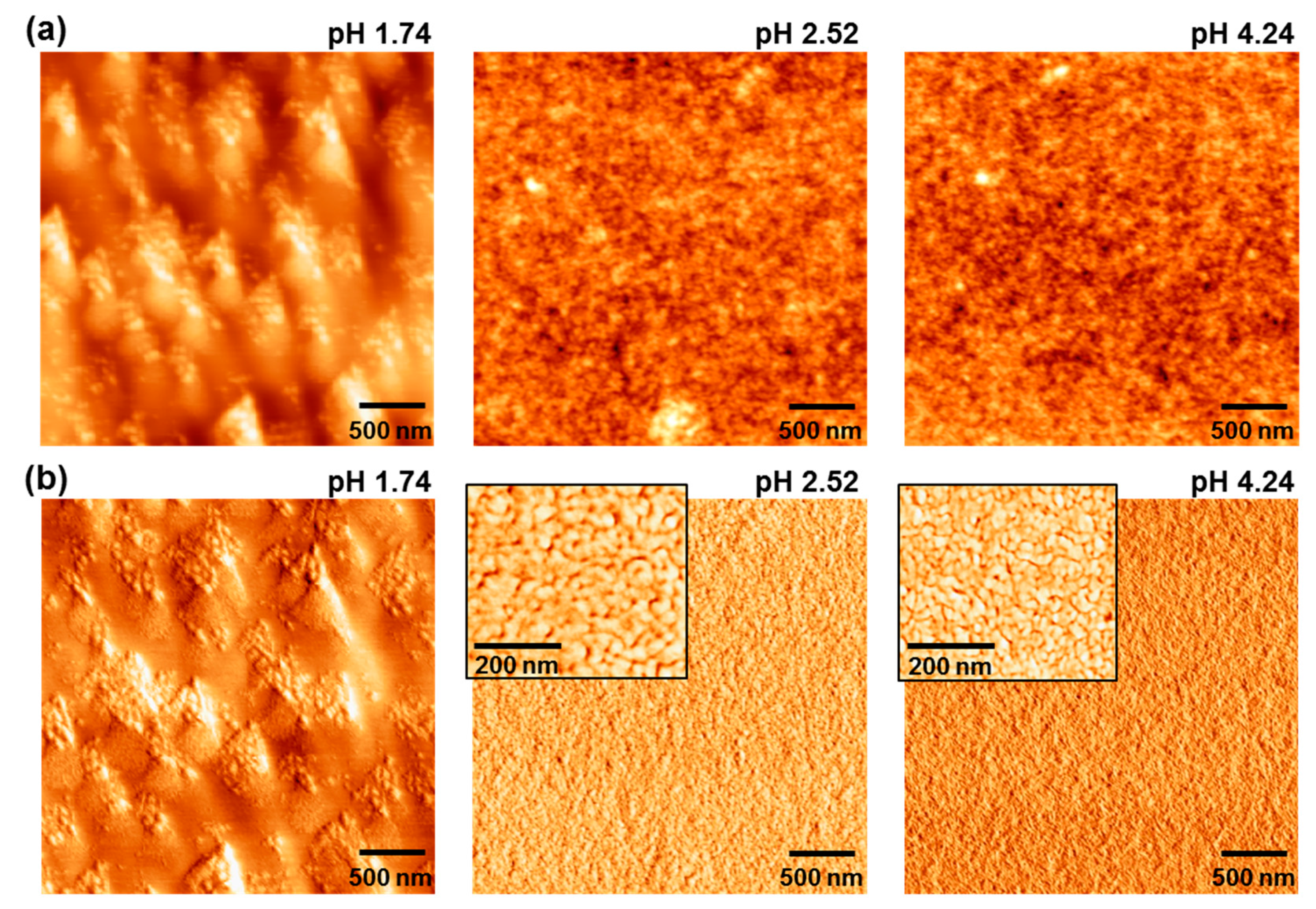
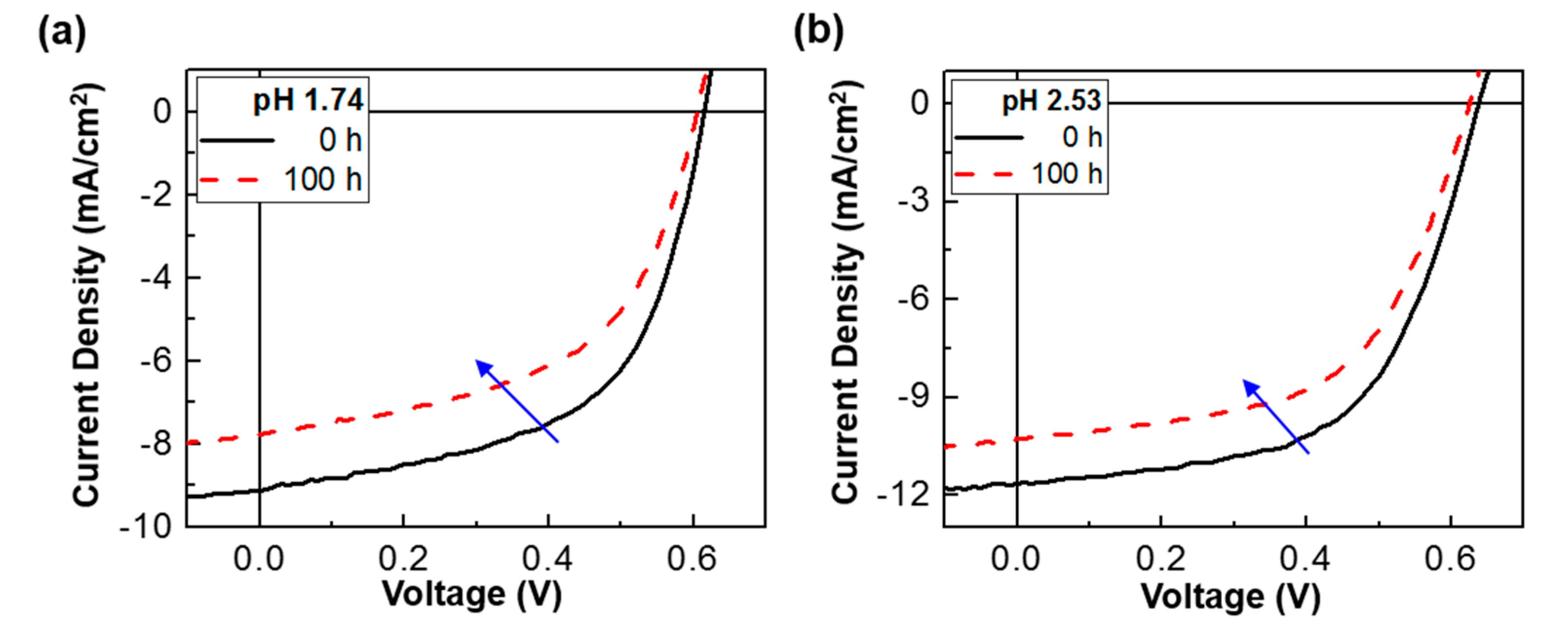
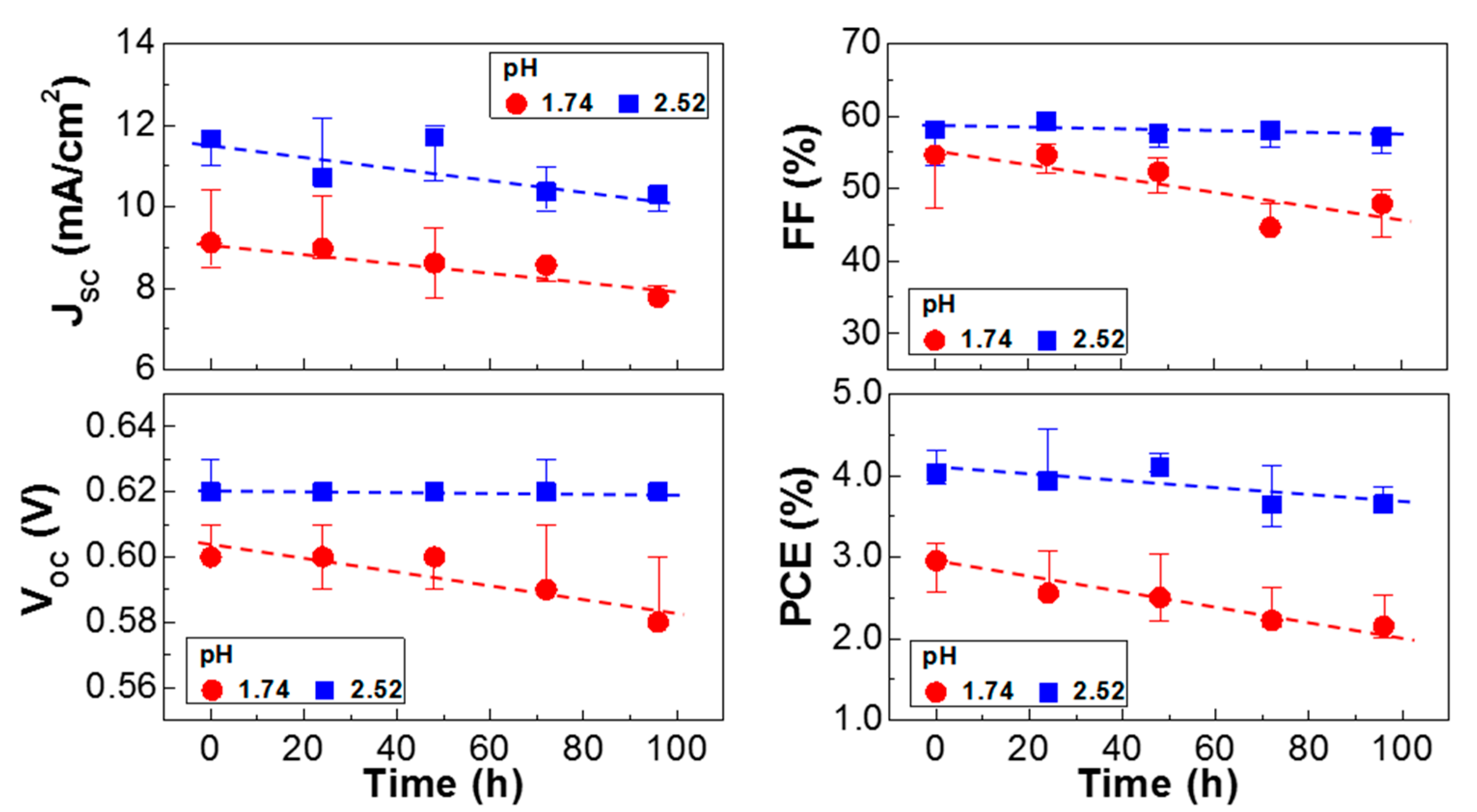
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Ameri, T.; Dennler, G.; Lungenschmied, C.; Brabec, C.J. Organic tandem solar cells: A review. Energy Environ. Sci. 2009, 2, 347–363. [Google Scholar] [CrossRef]
- Kim, H.; Nam, S.; Jeong, J.; Lee, S.; Seo, J.; Han, H.; Kim, H.; Kim, Y. Organic solar cells based on conjugated polymers: History and recent advances. Korean J. Chem. Eng. 2014, 31, 1095–1104. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.-L.; Jen, A.K.-Y. A review on the development of the inverted polymer solar cell architecture. Polym. Rev. 2010, 50, 474–510. [Google Scholar] [CrossRef]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Schrödner, M.; Sensfuss, S.; Schache, H.; Schultheis, K.; Welzel, T.; Heinemann, K.; Milker, R.; Marten, J.; Blankenburg, L. Reel-to-reel wet coating by variation of solvents and compounds of photoactive inks for polymer solar cell production. Sol. Energy Mater. Sol. Cells 2012, 107, 283–291. [Google Scholar] [CrossRef]
- Nam, S.; Shin, M.; Kim, H.; Ha, C.-S.; Ree, M.; Kim, Y. Improved performance of polymer:polymer solar cells by doping electron-accepting polymers with an organosulfonic acid. Adv. Funct. Mater. 2011, 21, 4527–4534. [Google Scholar] [CrossRef]
- Cho, C.-K.; Hwang, W.-J.; Eun, K.; Choa, S.-H.; Na, S.-I.; Kim, H.-K. Mechanical flexibility of transparent PEDOT:PSS electrodes prepared by gravure printing for flexible organic solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 3269–3275. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Han, H.; Lee, H.; Nam, S.; Jeong, J.; Lee, I.; Kim, H.; Ha, C.-S.; Kim, Y. Poly(3-hexylthiophene-co-benzothiadiazole) (THBT) as an electron-accepting polymer for normal and inverted type all-polymer solar cells. Polym. Chem. 2013, 4, 2053–2061. [Google Scholar] [CrossRef]
- Su, M.-S.; Kuo, C.-Y.; Yuan, M.-C.; Jeng, U.-S.; Su, C.-J.; Wei, K.-H. Improving device efficiency of polymer/fullerene bulk heterojunction solar cells through enhanced crystallinity and reduced grain boundaries induced by solvent additives. Adv. Mater. 2011, 23, 3315–3319. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Lee, S.; Lee, I.; Shin, M.; Kim, H.; Kim, Y. Nanomorphology-driven two-stage hole mobility in blend films of regioregular and regiorandom polythiophenes. Nanoscale 2011, 3, 4261–4269. [Google Scholar] [CrossRef] [PubMed]
- Dante, M.; Peet, J.; Nguyen, T.-Q. Nanoscale charge transport and internal structure of bulk heterojunction conjugated polymer/fullerene solar cells by scanning probe microscopy. J. Phys. Chem. C 2008, 112, 7241–7249. [Google Scholar] [CrossRef]
- Collins, B.A.; Tumbleston, J.R.; Ade, H. Miscibility, crystallinity, and phase development in P3HT/PCBM solar cells: Toward an enlightened understanding of device morphology and stability. J. Phys. Chem. Lett. 2011, 2, 3135–3145. [Google Scholar] [CrossRef]
- Kim, H.; Shin, M.; Kim, Y. Distinct annealing temperature in polymer:fullerene:polymer ternary blend solar cells. J. Phys. Chem. C 2009, 113, 1620–1623. [Google Scholar] [CrossRef]
- Nam, S.; Woo, S.; Seo, J.; Kim, W.H.; Kim, H.; McNeill, C.R.; Shin, T.J.; Bradley, D.D.C.; Kim, Y. Pronounced cosolvent effects in polymer:polymer bulk heterojunction solar cells with sulfur-rich electron-donating and imide-containing electron-accepting polymers. ACS Appl. Mater. Interfaces 2015, 7, 15995–16002. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hansberg, B.; Sanyal, M.; Klein, M.F.G.; Pfaff, M.; Schnabel, N.; Jaiser, S.; Vorobiev, A.; Müller, E.; Colsmann, A.; Scharfer, P.; et al. Moving through the phase diagram:morphology formation in solution cast polymer-fullerene blend films for organic solar cells. ACS Nano 2011, 5, 8579–8590. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kim, H.; Park, J.; Nam, S.; Heo, K.; Ree, M.; Ha, C.-S.; Kim, Y. Abrupt morphology change upon thermal annealing in poly(3-hexylthiophene)/soluble fullerene blend films for polymer solar cells. Adv. Funct. Mater. 2010, 20, 748–754. [Google Scholar] [CrossRef]
- Holliday, S.; Ashraf, R.S.; Wadsworth, A.; Baran, D.; Yousaf, S.A.; Nielsen, C.B.; Tan, C.-H.; Dimitrov, S.D.; Shang, Z.; Gasparini, N.; et al. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 2016, 7, 11585. [Google Scholar] [CrossRef] [PubMed]
- Kumari, T.; Lee, S.M.; Kang, S.-H.; Chen, S.; Yang, C. Ternary solar cells with a mixed face-on and edge-on orientation enable an unprecedented efficiency of 12.1%. Energy Environ. Sci. 2016. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Jeong, J.; Seo, J.; Nam, S.; Han, H.; Kim, H.; Anthopoulos, T.D.; Bradley, D.D.C.; Kim, Y. Significant stability enhancement in high-efficiency polymer:fullerene bulk heterojunction solar cells by blocking ultraviolet photons from solar light. Adv. Sci. 2016, 3, 1500269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qian, D.; Zhang, S.; Li, S.; Inganäs, O.; Gao, F.; Hou, J. Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.-G.; Bin, H.; Chen, S.; Gao, L.; Xue, L.; Yang, C.; Li, Y. Side-chain isomerization on an n-type organic semiconductor ITIC acceptor makes 11.77% high efficiency polymer solar cells. J. Am. Chem. Soc. 2016, 138, 15011–15018. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Seo, J.; Woo, S.; Kim, W.H.; Kim, H.; Bradley, D.D.C.; Kim, Y. Inverted polymer fullerene solar cells exceeding 10% efficiency with poly(2-ethyl-2-oxazoline) nanodots on electron-collecting buffer layers. Nat. Commun. 2015, 6, 8929. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, K.; Wan, X.; Zhang, Q.; Kan, B.; Xia, R.; Liu, F.; Yang, X.; Feng, H.; Ni, W.; et al. Solution-processed organic tandem solar cells with power conversion efficiencies >12%. Nat. Photonics 2016. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, K.; Xia, R.; Wu, Z.; Sun, C.; Cao, J.; Qian, L.; Li, W.; Liu, S.; Huang, F.; et al. High-performance polymer tandem solar cells employing a new n-type conjugated polymer as an interconnecting layer. Adv. Mater. 2016, 28, 4817–4823. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, W.; Li, S.; Mukherjee, S.; Carpenter, J.H.; Awartani, O.; Jiao, X.; Hou, J.; Ade, H. High-efficiency nonfullerene organic solar cells: Critical factors that affect complex multi-length scale morphology and device performance. Adv. Energy Mater. 2016. [Google Scholar] [CrossRef]
- Woo, S.; Kim, W.H.; Kim, H.; Yi, Y.; Lyu, H.-K.; Kim, Y. 8.9% Single-stack inverted polymer solar cells with electron-rich polymer nanolayer-modified inorganic electron-collecting buffer layers. Adv. Energy Mater. 2014, 4, 1301692. [Google Scholar] [CrossRef]
- Nam, S.; Seo, J.; Han, H.; Kim, H.; Hahm, S.G.; Ree, M.; Gal, Y.-S.; Anthopoulos, T.D.; Bradley, D.D.C.; Kim, Y. >10% Efficiency polymer:fullerene solar cells with polyacetylene-based polyelectrolyte interlayers. Adv. Mater. Interfaces 2016, 3, 1600415. [Google Scholar] [CrossRef]
- Cowan, S.R.; Schulz, P.; Giordano, A.J.; Garcia, A.; MacLeod, B.A.; Marder, S.R.; Kahn, A.; Ginley, D.S.; Ratcliff, E.L.; Olson, D.C. Chemically controlled reversible and irreversible extraction barriers via stable interface modification of zinc oxide electron collection layer in polycarbazole-based organic solar cells. Adv. Funct. Mater. 2014, 24, 4671–4680. [Google Scholar] [CrossRef]
- Kang, H.; Hong, S.; Lee, J.; Lee, K. Electrostatically self-assembled nonconjugated polyelectrolytes as an ideal interfacial layer for inverted polymer solar cells. Adv. Mater. 2012, 24, 3005–3009. [Google Scholar] [CrossRef] [PubMed]
- Po, R.; Carboner, C.; Bernardi, A.; Camaioni, N. The role of buffer layers in polymer solar cells. Energy Environ. Sci. 2011, 4, 285–310. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, H.-R.; Kang, Y.-J.; Kim, D.-H.; Kang, J.-W. Organic solar cells employing magnetron sputtered p-type nickel oxide thin film as the anode buffer layer. Sol. Energy Mater. Sol. Cells 2010, 94, 2332–2336. [Google Scholar] [CrossRef]
- Zhao, W.; Ye, L.; Zhang, S.; Fan, B.; Sun, M.; Hou, J. Ultrathin polyaniline-based buffer layer for highly efficient polymer solar cells with wide applicability. Sci. Rep. 2014, 4, 6570. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Luo, Z.; Xu, T.; Yu, L. Cooperative plasmonic effect of Ag and Au nanoparticles on enhancing performance of polymer solar cells. Nano Lett. 2013, 13, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.J.; Ananthanarayanan, K.; Luther, J.; Ho, G.W. Influence of a novel fluorosurfactant modified PEDOT:PSS hole transport layer on the performance of inverted organic solar cells. J. Mater. Chem. 2012, 22, 25057–25064. [Google Scholar] [CrossRef]
- Jeong, J.; Woo, S.; Park, S.; Kim, H.; Lee, S.W.; Kim, Y. Wide range thickness effect of hole-collecting buffer layers for polymer:fullerene solar cells. Org. Electron. 2013, 14, 2889–2895. [Google Scholar] [CrossRef]
- McCarthy, J.E.; Hanley, C.A.; Brennan, L.J.; Lambertini, V.G.; Gun’ko, Y.K. Fabrication of highly transparent and conducting PEDOT:PSS films using a formic acid treatment. J. Mater. Chem. C 2014, 2, 764–770. [Google Scholar] [CrossRef]
- Ouyang, J. Solution-processed PEDOT:PSS films with conductivities as indium tin oxide through a treatment with mild and weak organic acids. ACS Appl. Mater. Interfaces 2013, 5, 13082–13088. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-C.; Chang, H.-C.; Chen, C.-H.; Huang, Y.-C.; Whang, W.-T. A facile dedoping approach for effectively tuning thermoelectricity and acidity of PEDOT:PSS films. Org. Electron. 2014, 15, 641–645. [Google Scholar] [CrossRef]
- Kim, H.; Nam, S.; Lee, H.; Woo, S.; Ha, C.-S.; Ree, M.; Kim, Y. Influence of controlled acidity of hole-collecting buffer layers on the performance and lifetime of polymer:fullerene solar cells. J. Phys. Chem. C 2011, 115, 13502–13510. [Google Scholar] [CrossRef]
- Lee, W.; Song, M.; Park, S.; Nam, S.; Seo, J.; Kim, H.; Kim, Y. Acidity-controlled conducting polymer films for organic thermoelectric devices with horizontal and vertical architectures. Sci. Rep. 2016, 6, 33795. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.

| Parameters | pH | |||
|---|---|---|---|---|
| 1.74 | 2.52 | 2.91 | 4.24 | |
| VOC (V) | 0.62 (±0.01) | 0.63 (±0.01) | 0.63 (±0.01) | 0.62 (±0.01) |
| JSC (mA/cm2) | 11.15 (±0.41) * (10.89) | 12.54 (±0.09) * (12.15) | 12.01 (±0.08) * (11.80) | 12.14 (±0.52) * (11.67) |
| FF (%) | 60.90 (±1.60) | 62.60 (±0.80) | 63.50 (±1.10) | 62.9 (±0.60) |
| PCE (%) | 4.21 (±0.14) | 4.95 (±0.05) | 4.80 (±0.07) | 4.73 (±0.30) |
| RS (Ω cm2) | 110 (±17.0) | 100 (±11.0) | 90 (±11.0) | 90 (±10.0) |
| RSH (kΩ cm2) | 5.7 (±0.01) | 5.9 (±0.02) | 10.3 (±0.02) | 12.5 (±0.01) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.; Park, S.; Song, M.; Jeong, J.; Lee, C.; Kim, H.; Kim, Y. Influence of Weak Base Addition to Hole-Collecting Buffer Layers in Polymer:Fullerene Solar Cells. Molecules 2017, 22, 262. https://doi.org/10.3390/molecules22020262
Seo J, Park S, Song M, Jeong J, Lee C, Kim H, Kim Y. Influence of Weak Base Addition to Hole-Collecting Buffer Layers in Polymer:Fullerene Solar Cells. Molecules. 2017; 22(2):262. https://doi.org/10.3390/molecules22020262
Chicago/Turabian StyleSeo, Jooyeok, Soohyeong Park, Myeonghun Song, Jaehoon Jeong, Chulyeon Lee, Hwajeong Kim, and Youngkyoo Kim. 2017. "Influence of Weak Base Addition to Hole-Collecting Buffer Layers in Polymer:Fullerene Solar Cells" Molecules 22, no. 2: 262. https://doi.org/10.3390/molecules22020262





