Synthesis and Positive Inotropic Activity of [1,2,4]Triazolo[4,3-a] Quinoxaline Derivatives Bearing Substituted Benzylpiperazine and Benzoylpiperazine Moieties
Abstract
:1. Introduction
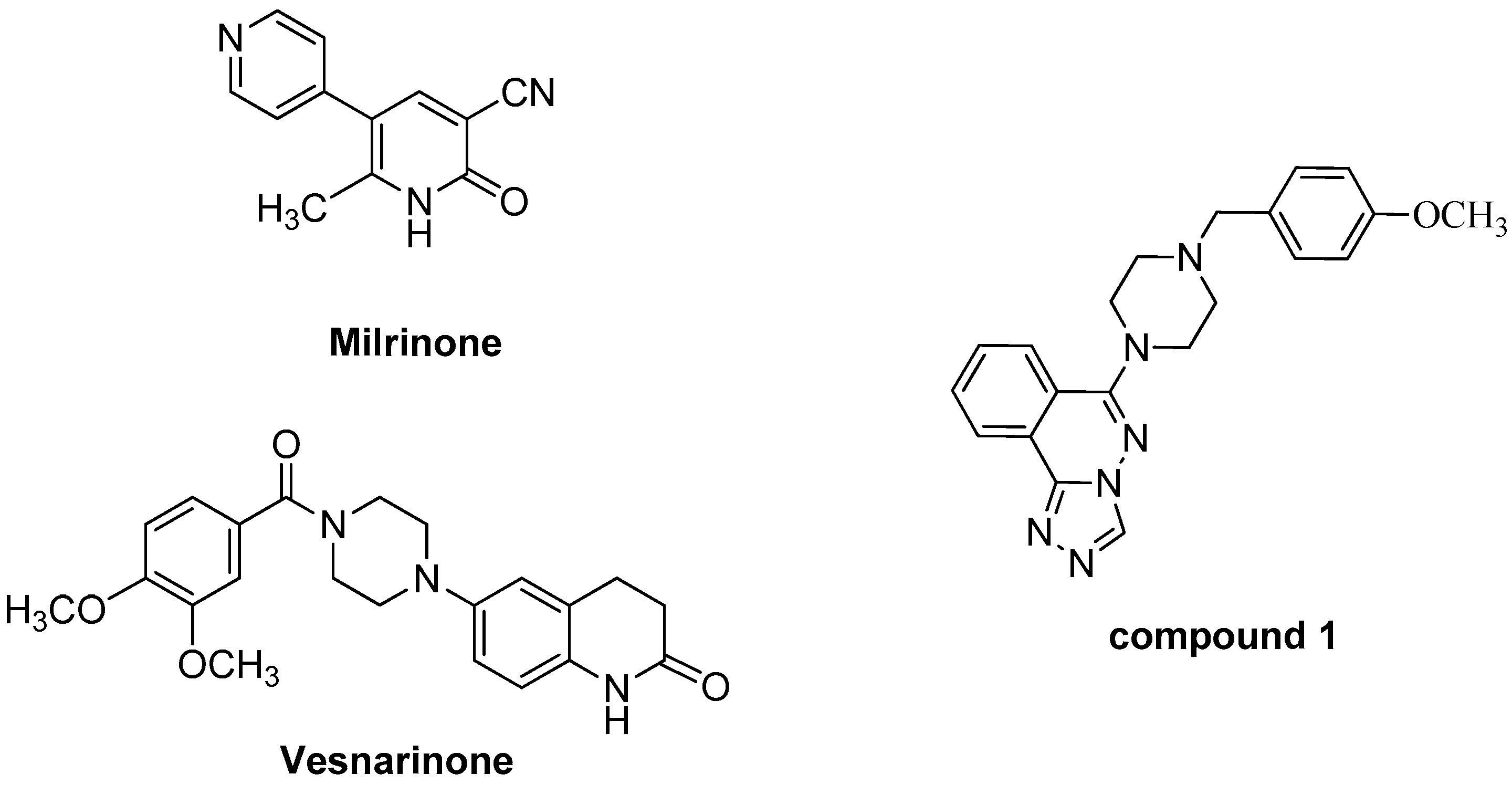
2. Results and Discussion
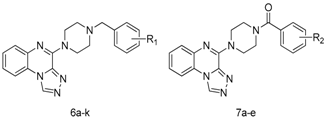
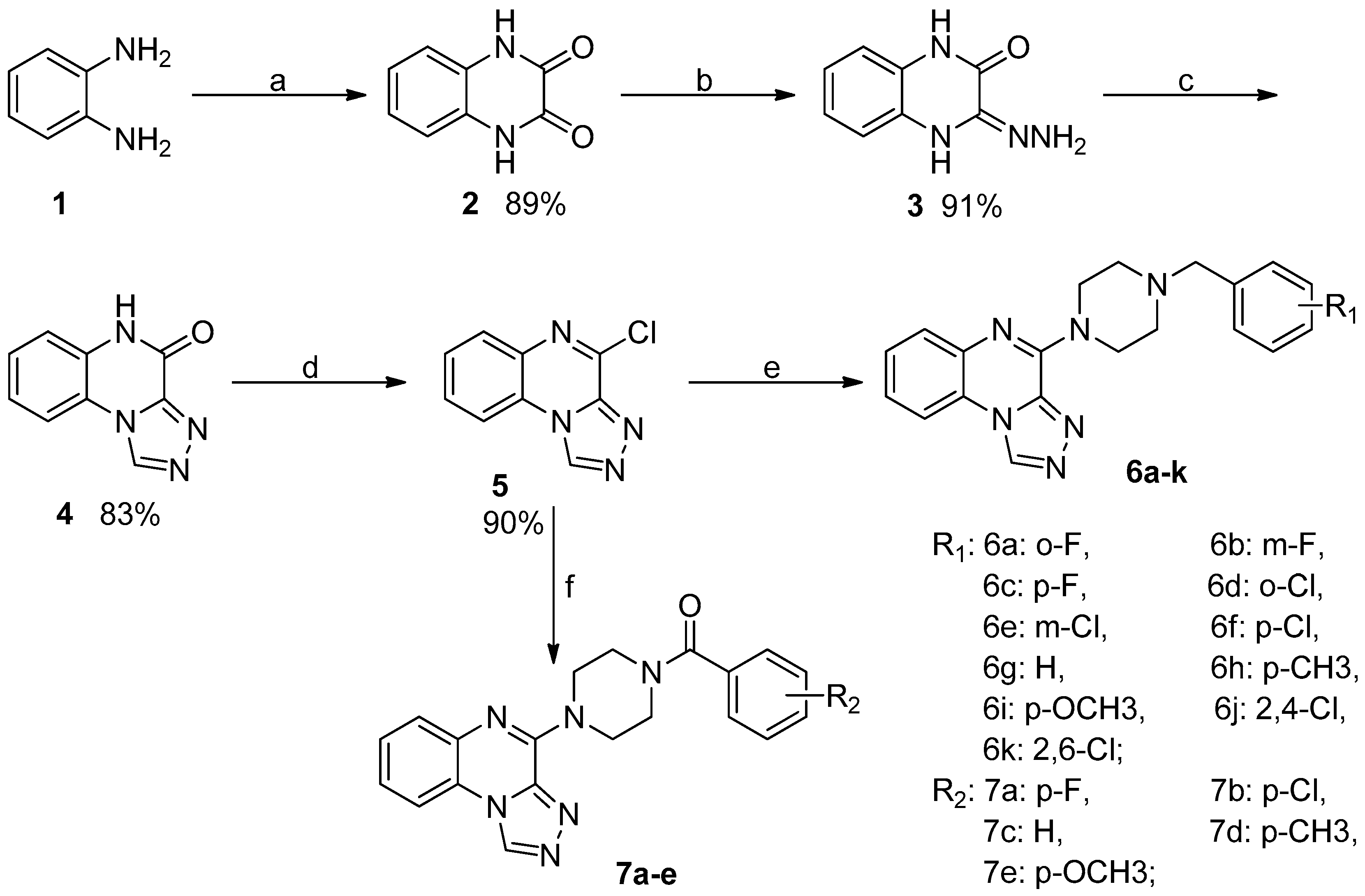
2.1. Synthesis
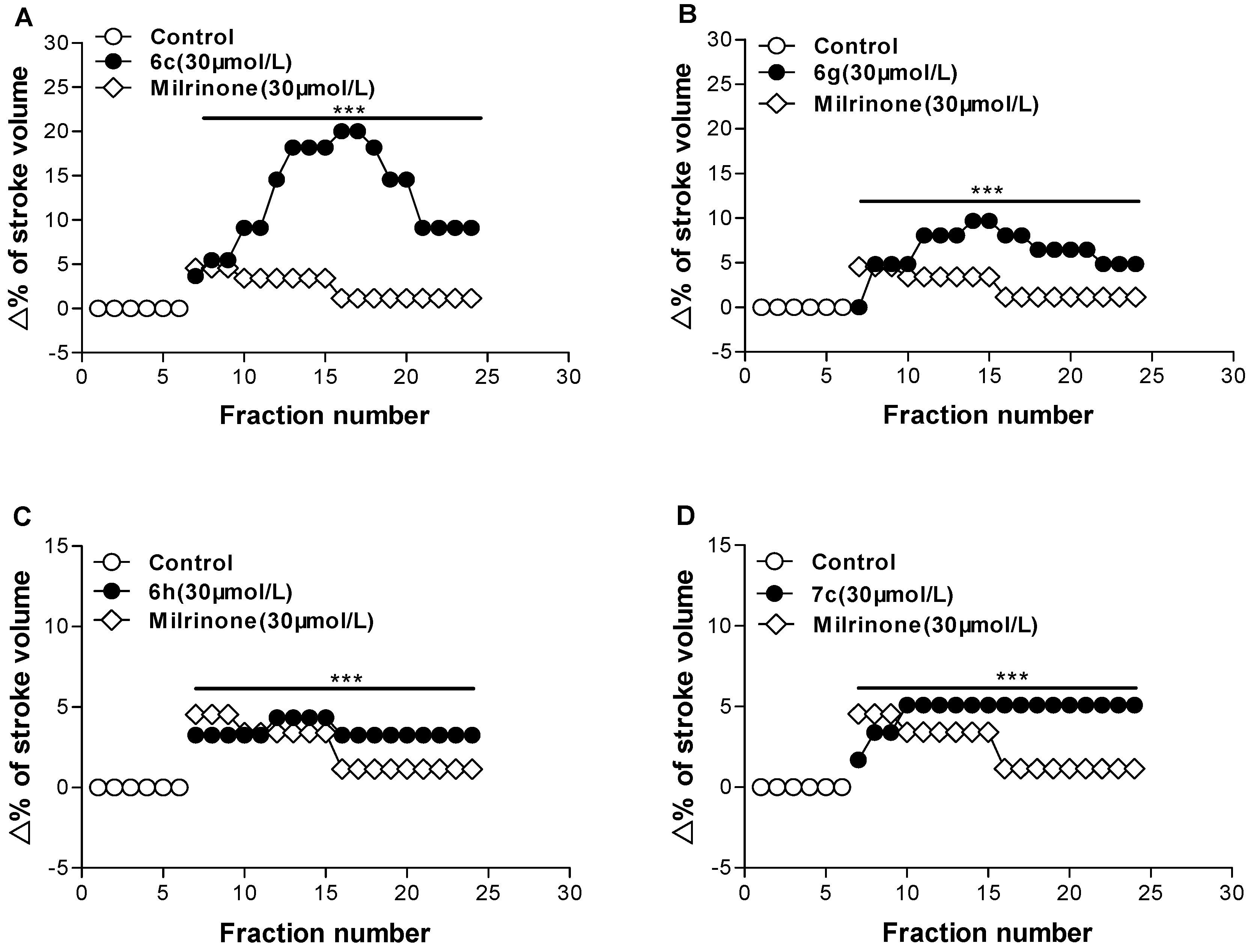
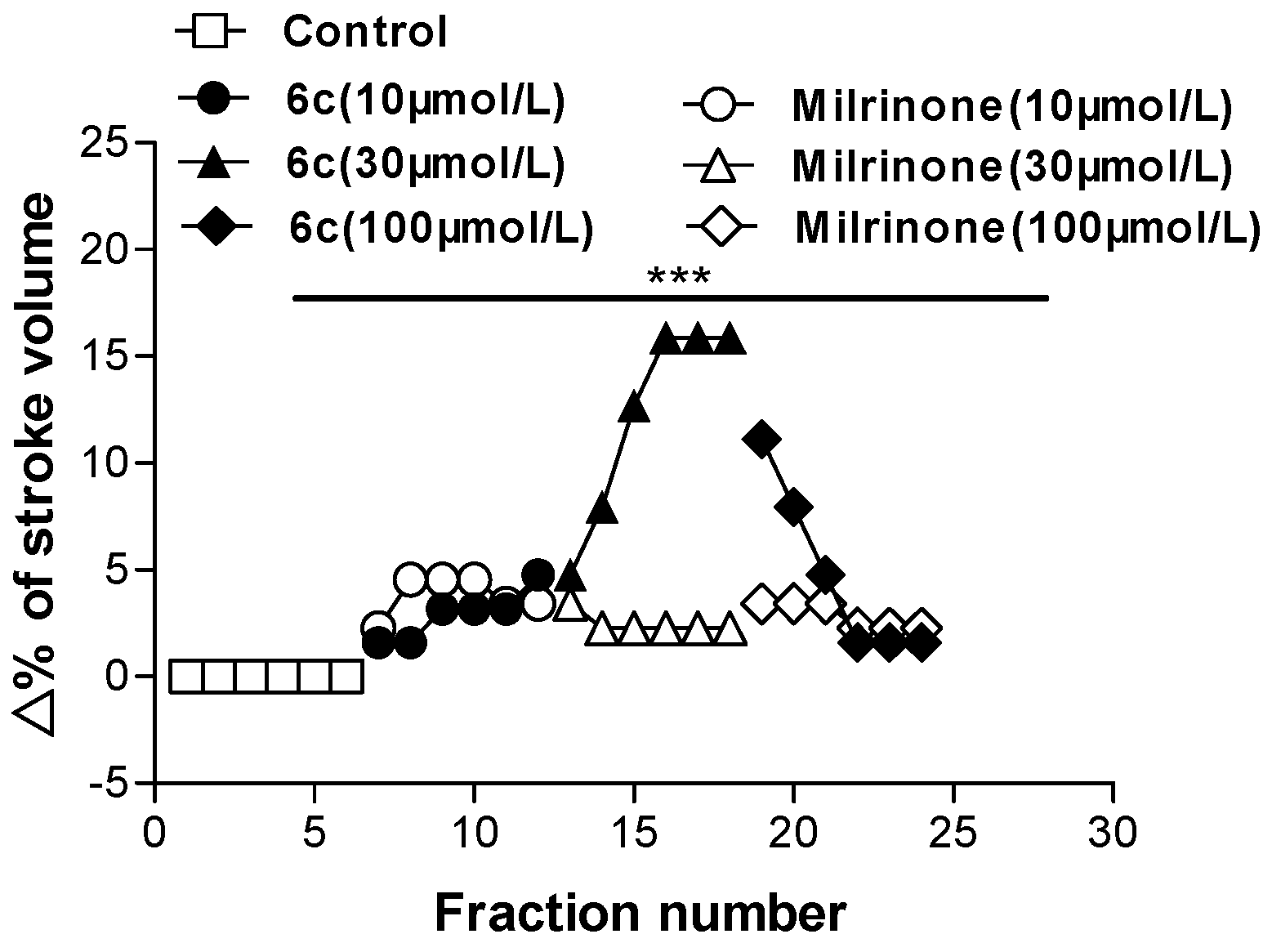
2.2. Biological Evaluation
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. General Experimental Procedure for the Synthesis of [1,2,4]Triazolo[4,3-a] Quinoxaline–Bearing Substituted Benzylpiperazine Moieties (6a–k)
3.2.2. [1,2,4]triazolo[4,3-a] quinoxaline Derivatives Bearing Substituted Benzoylpiperazine Moieties (7a–e)
3.3. Pharmacology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 1997, 336, 525–533. [Google Scholar]
- Fujioka, T.; Teramoto, S.; Mori, T.; Hosokawa, T.; Sumida, T.; Tominaga, M.; Yabuuchi, Y. Novel positive inotropic agents: Synthesis and biological activities of 6-(3-amino-2-hydroxypropoxy)-2(1H)-quinolinone derivatives. J. Med. Chem. 1992, 35, 3607–3612. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Carver, J.R.; Rodeheffer, R.J.; Ivanhoe, R.J.; DiBianco, R.; Zeldis, S.M.; Hendrix, G.H.; Bommer, W.J.; Elkayam, U.; Kukin, M.L.; et al. Effect of oral milrinone on mortality in severe chronic heart failure. N. Engl. J. Med. 1991, 325, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Oldstein, S.O.; Greenberg, B.H.; Lorell, B.H.; Bourge, R.C.; Jaski, B.E.; Gottlieb, S.O.; McGrew, F.D.; DeMets, L.; White, B.G. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N. Engl. J. Med. 1998, 339, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Supriatno, E.; Yoshida, H.; Sato, M. Vesnarinone inhibits angiogenesis and tumorigenicity of human oral squamous cell carcinoma cells by suppressing the expression of vascular endothelial growth factor and interleukin-8. Int. J. Oncol. 2005, 27, 1489–1497. [Google Scholar] [PubMed]
- Neubauer, S. The failing heart—An engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, L.X.; Che, J.; Sun, L.P.; Song, M.X.; Cui, X.; Piao, H.R. Synthesis and biological evaluation of [1,2,4]triazolo[3,4-a]phthalazine and tetrazolo[5,1-a]phthalazine derivatives bearing substituted benzylpiperazine moieties as positive inotropic Agents. Chem. Biol. Drug. Des. 2013, 81, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, X.Y.; Bai, M.; Ning, D.B.; Gong, P.; Han, J. Synthesis of XL765 as a phosphoinositide-3-kinase(PI3K) inhibitor. Chin. J. Med. Chem. 2011, 21, 290–294. [Google Scholar]
- Ubarhande, S.S.; Devhate, P.P.; Berad, B.N. Green synthesis of quinoxaline and substituted quinoxalines. Int. J. Chem. Sci. 2011, 9, 1768–1774. [Google Scholar]
- Ajani, O.O.; Obafemi, C.A.; Nwinyi, O.C.; Akinpelu, D.A. Microwave assisted synthesis and antimicrobial activity of 2-quinoxalinone-3-hydrazone derivatives. Bioorg. Med. Chem. 2010, 18, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.S.; Nimje, H.M.; Chaudhari, P.S.; Bayas, J.P.; Patel, E.K.; Karjikar, A.H.; Girme, P.D.; Oswal, R.J. Synthesis and Antimicrobial Activity of Novel Thiazolidinone and Azetidinone Derivatives. Asian J. Chem. 2011, 23, 4021–4023. [Google Scholar]
- Galal, S.A.; Abdelsamie, A.S.; Soliman, S.M.; Mortier, J.; Wolber, G.; Ali, M.M.; Tokuda, H.; Suzuki, N.; Lida, A.; Ramadan, R.A. Design, synthesis and structure–activity relationship of novel quinoxaline derivatives as cancer chemopreventive agent by inhibition of tyrosine kinase receptor. Eur. J. Med. Chem. 2013, 69, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Negishi, N.; Nagaya, H. Design, The role of the nigrotegmental GABAergic pathway in the propagation of pentylenetetrazol-induced seizures. Brain Res. 1989, 480, 383–387. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
| Compound | R | Increased Stroke Volume (%) a |
|---|---|---|
| 6a | o-F | — b |
| 6b | m-F | — |
| 6c | p-F | 12.53 ± 0.30 |
| 6d | o-Cl | — |
| 6e | m-Cl | — |
| 6f | p-Cl | 1.01 ± 0.06 |
| 6g | H | 6.36 ± 0.13 |
| 6h | p-CH3 | 3.50 ± 0.03 |
| 6i | p-OCH3 | 0.63 ± 0.05 |
| 6j | 2,4-Cl | — |
| 6k | 2,6-Cl | — |
| 7a | p-F | 0.99 ± 0.06 |
| 7b | p-Cl | — |
| 7c | H | 4.71 ± 0.05 |
| 7d | p-CH3 | — |
| A | — | 9.92 ± 0.09 |
| milrinone | 2.46 ± 0.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-K.; Ma, L.-X.; Wei, Z.-Y.; Cui, X.; Zhan, S.; Yin, X.-M.; Piao, H.-R. Synthesis and Positive Inotropic Activity of [1,2,4]Triazolo[4,3-a] Quinoxaline Derivatives Bearing Substituted Benzylpiperazine and Benzoylpiperazine Moieties. Molecules 2017, 22, 273. https://doi.org/10.3390/molecules22020273
Liu X-K, Ma L-X, Wei Z-Y, Cui X, Zhan S, Yin X-M, Piao H-R. Synthesis and Positive Inotropic Activity of [1,2,4]Triazolo[4,3-a] Quinoxaline Derivatives Bearing Substituted Benzylpiperazine and Benzoylpiperazine Moieties. Molecules. 2017; 22(2):273. https://doi.org/10.3390/molecules22020273
Chicago/Turabian StyleLiu, Xue-Kun, Long-Xu Ma, Zhi-Yu Wei, Xun Cui, Shi Zhan, Xiu-Mei Yin, and Hu-Ri Piao. 2017. "Synthesis and Positive Inotropic Activity of [1,2,4]Triazolo[4,3-a] Quinoxaline Derivatives Bearing Substituted Benzylpiperazine and Benzoylpiperazine Moieties" Molecules 22, no. 2: 273. https://doi.org/10.3390/molecules22020273