Exogenous and Endogenous Hydrogen Sulfide Protects Gastric Mucosa against the Formation and Time-Dependent Development of Ischemia/Reperfusion-Induced Acute Lesions Progressing into Deeper Ulcerations
Abstract
:1. Introduction
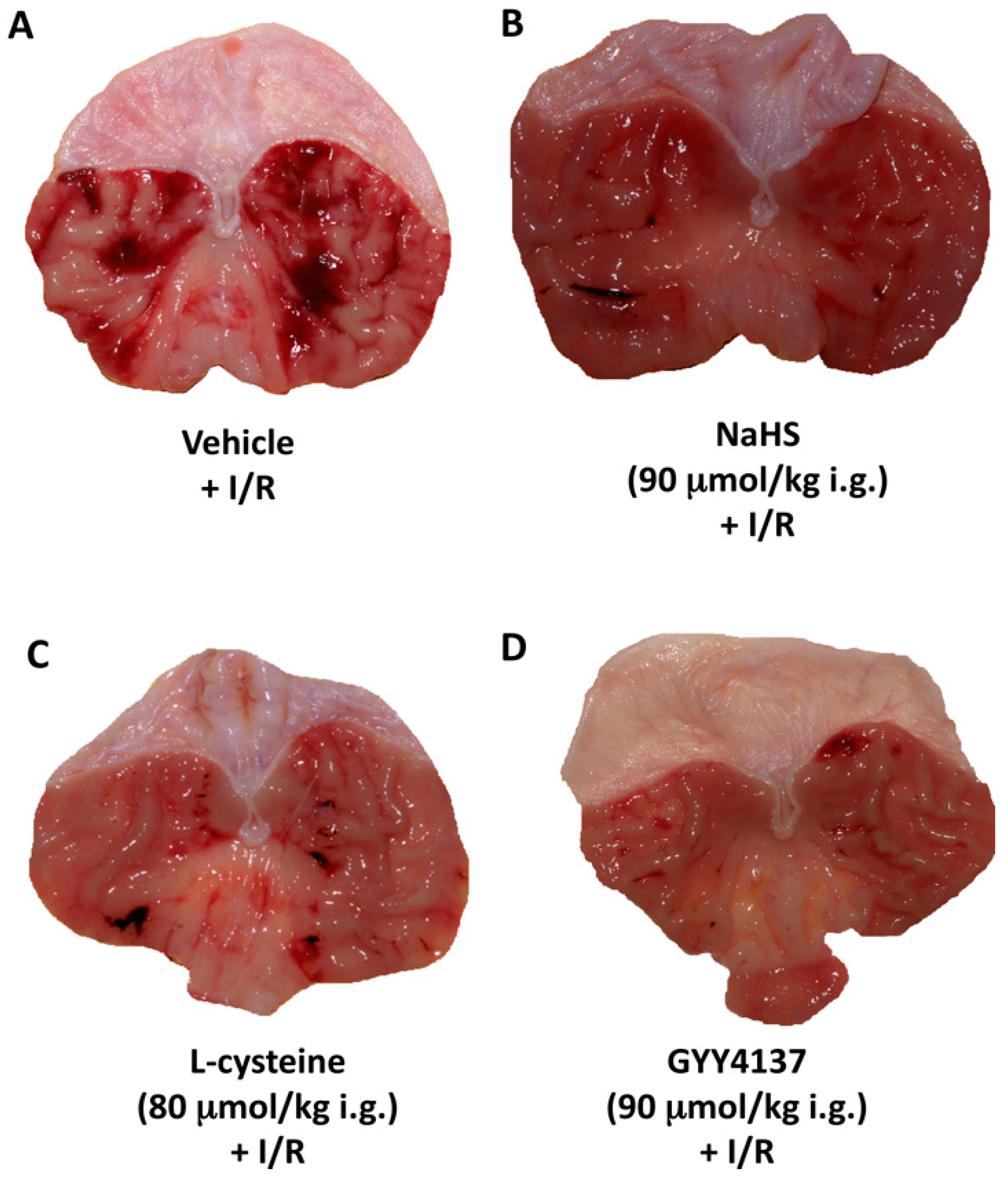
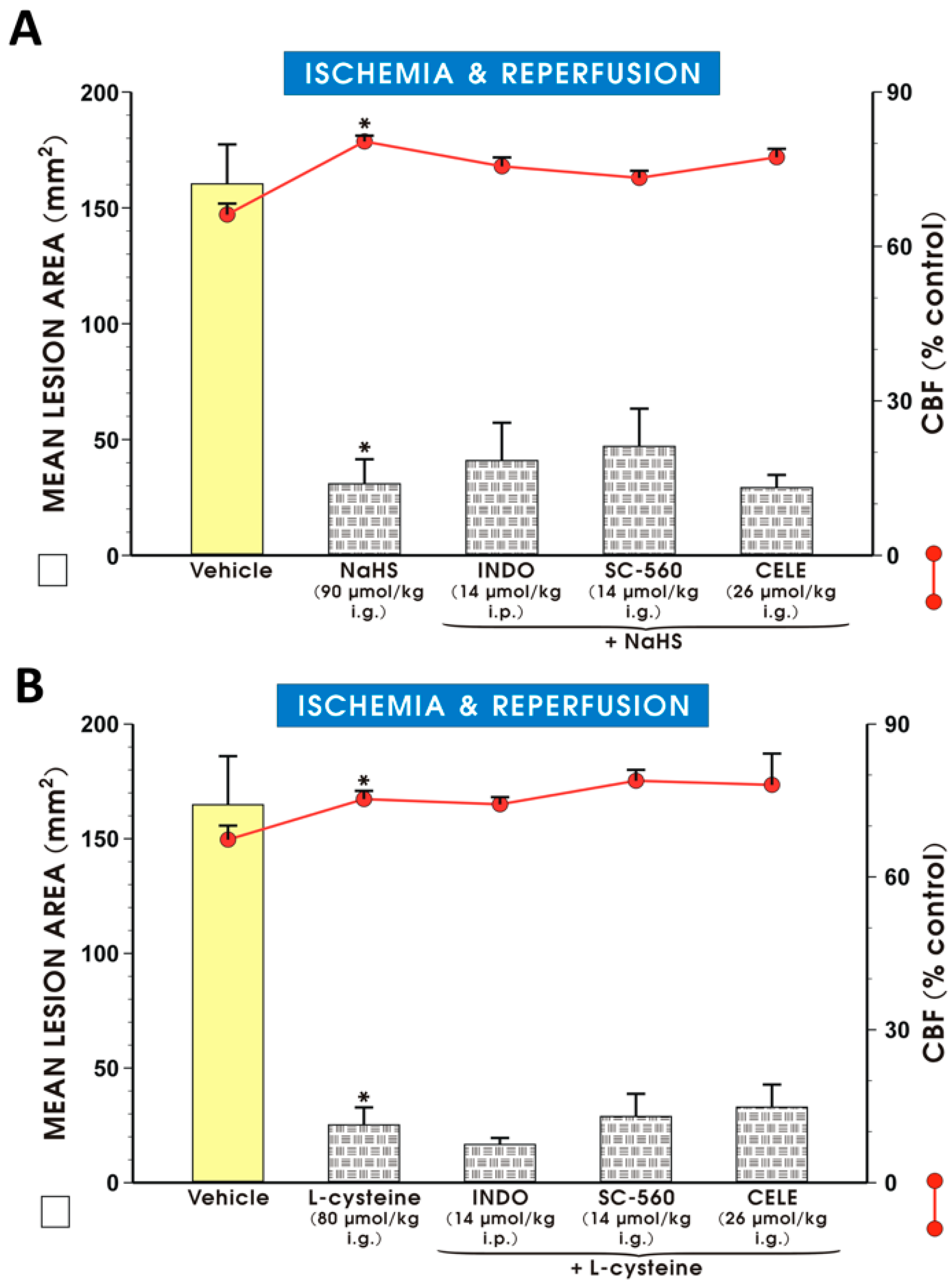
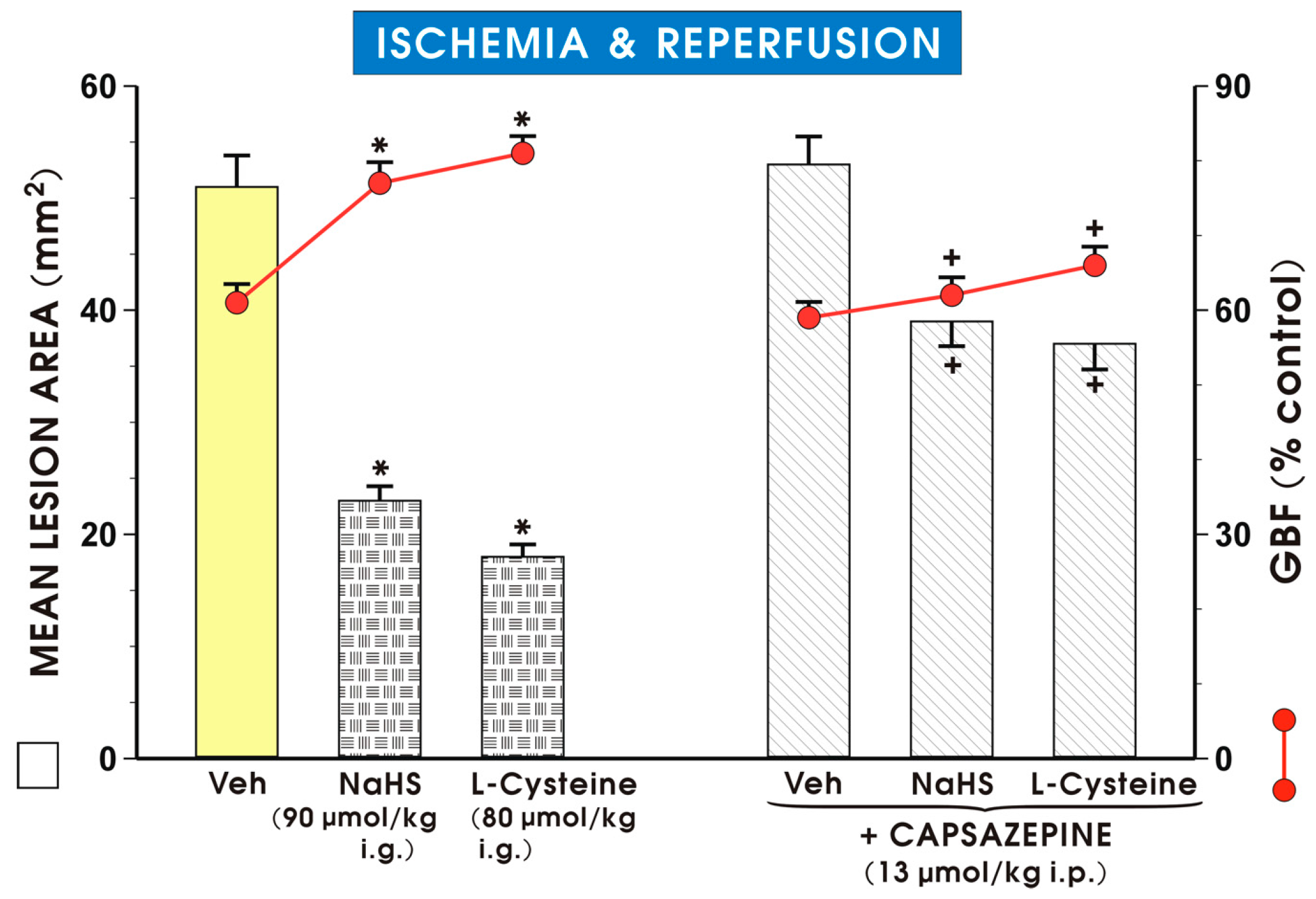
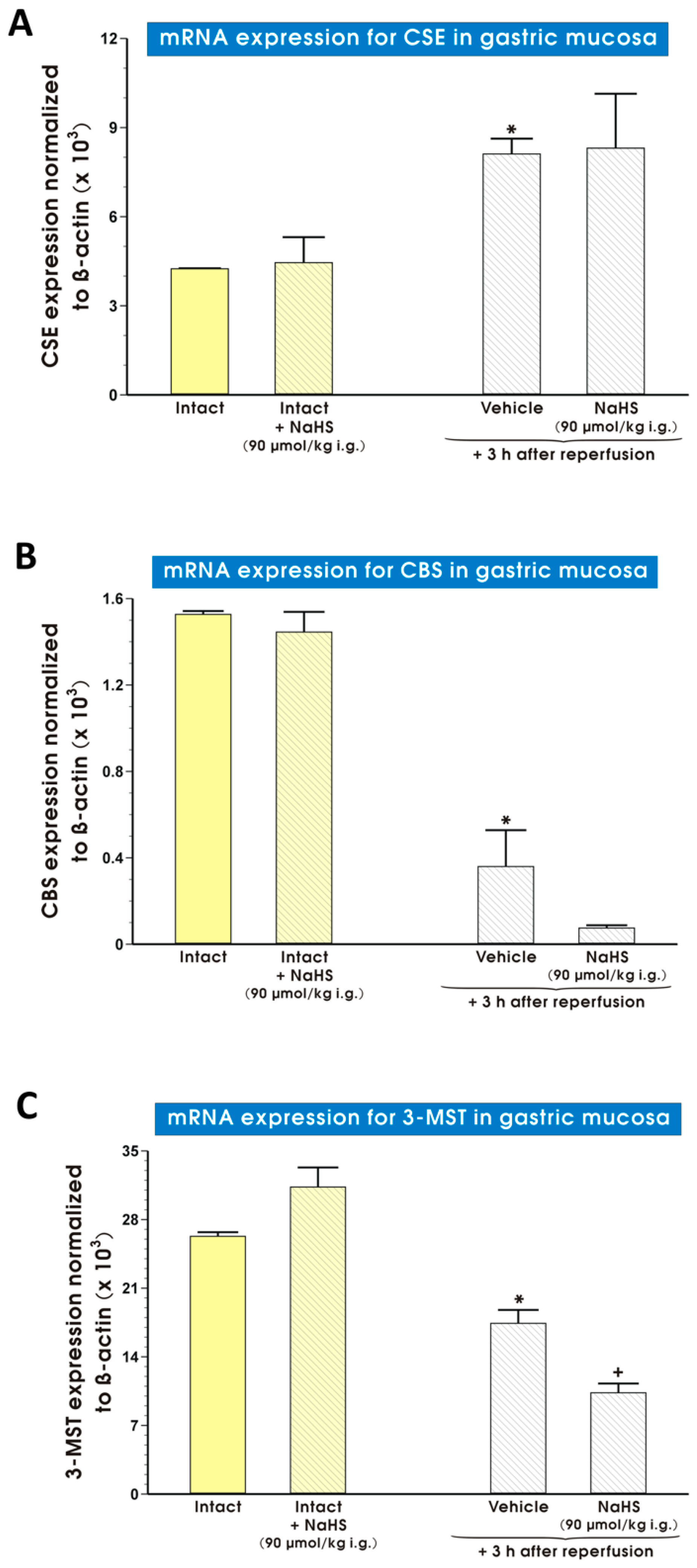
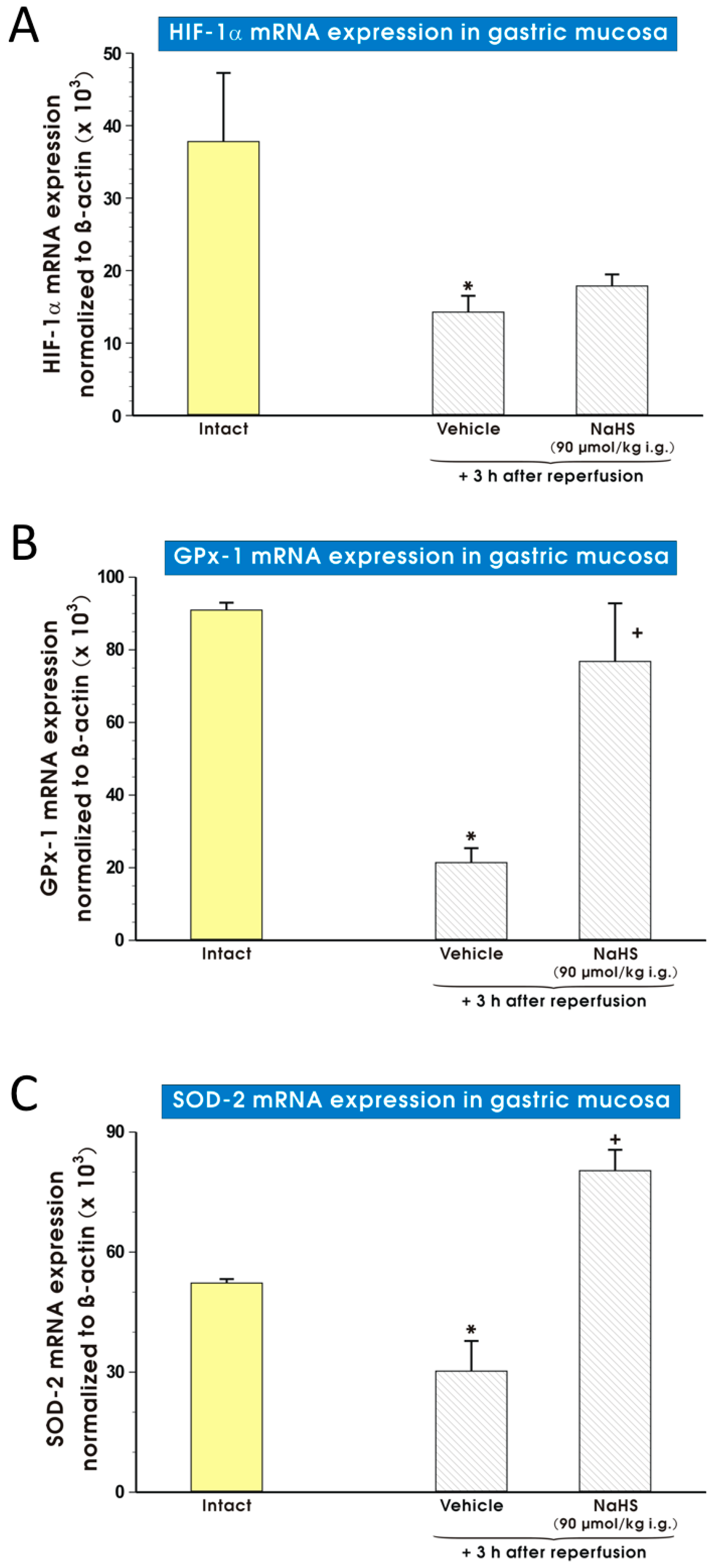
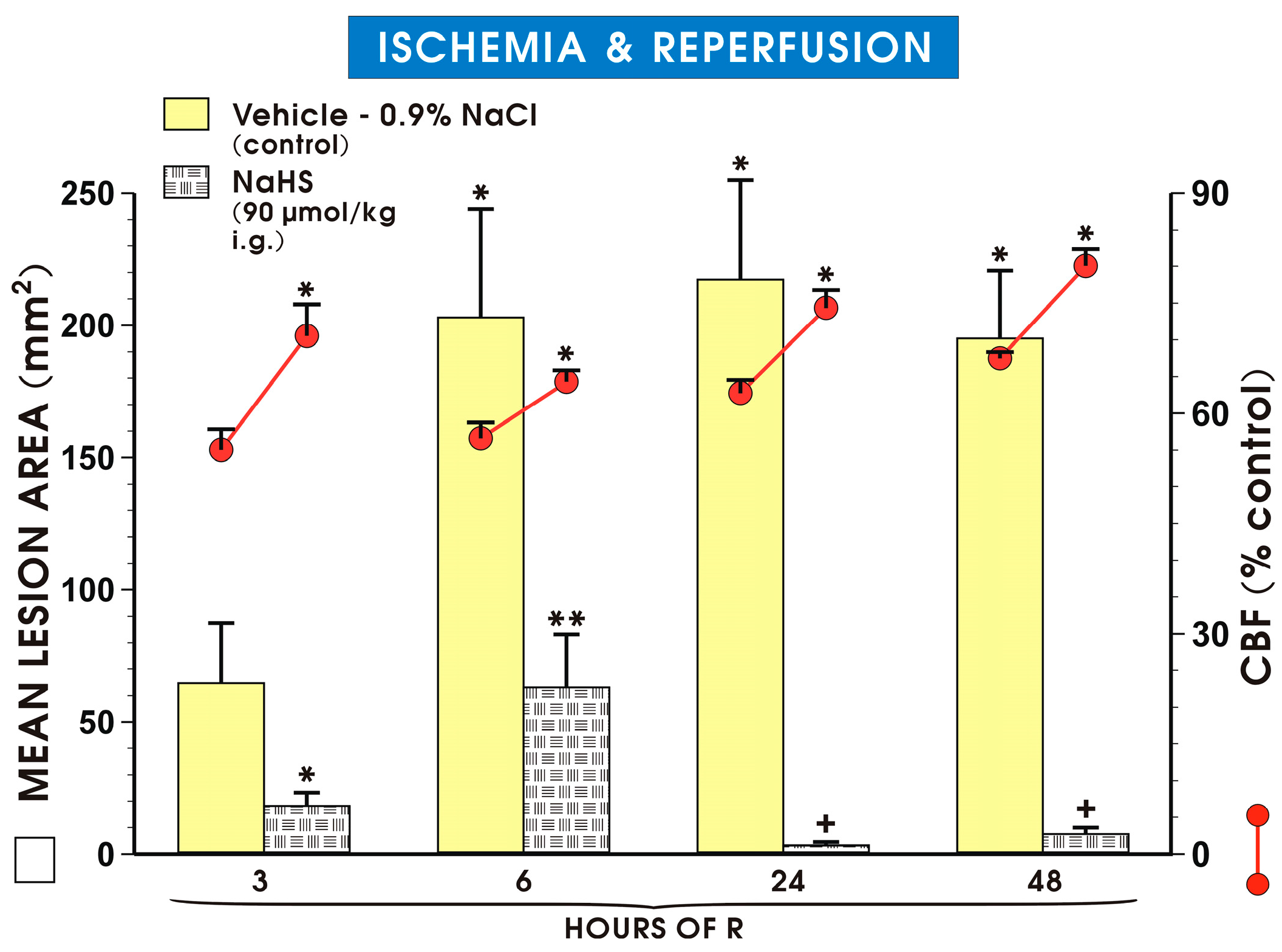
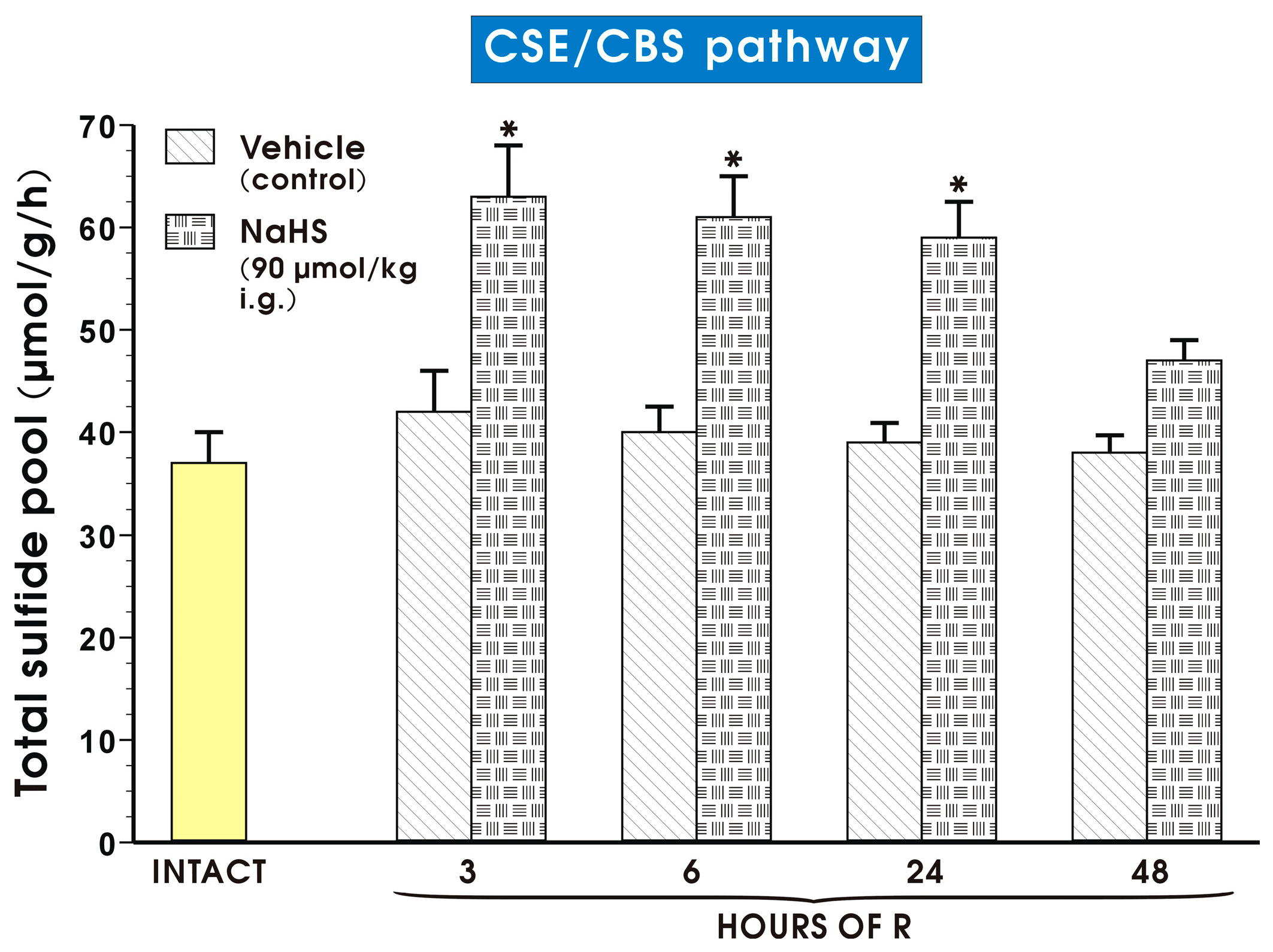
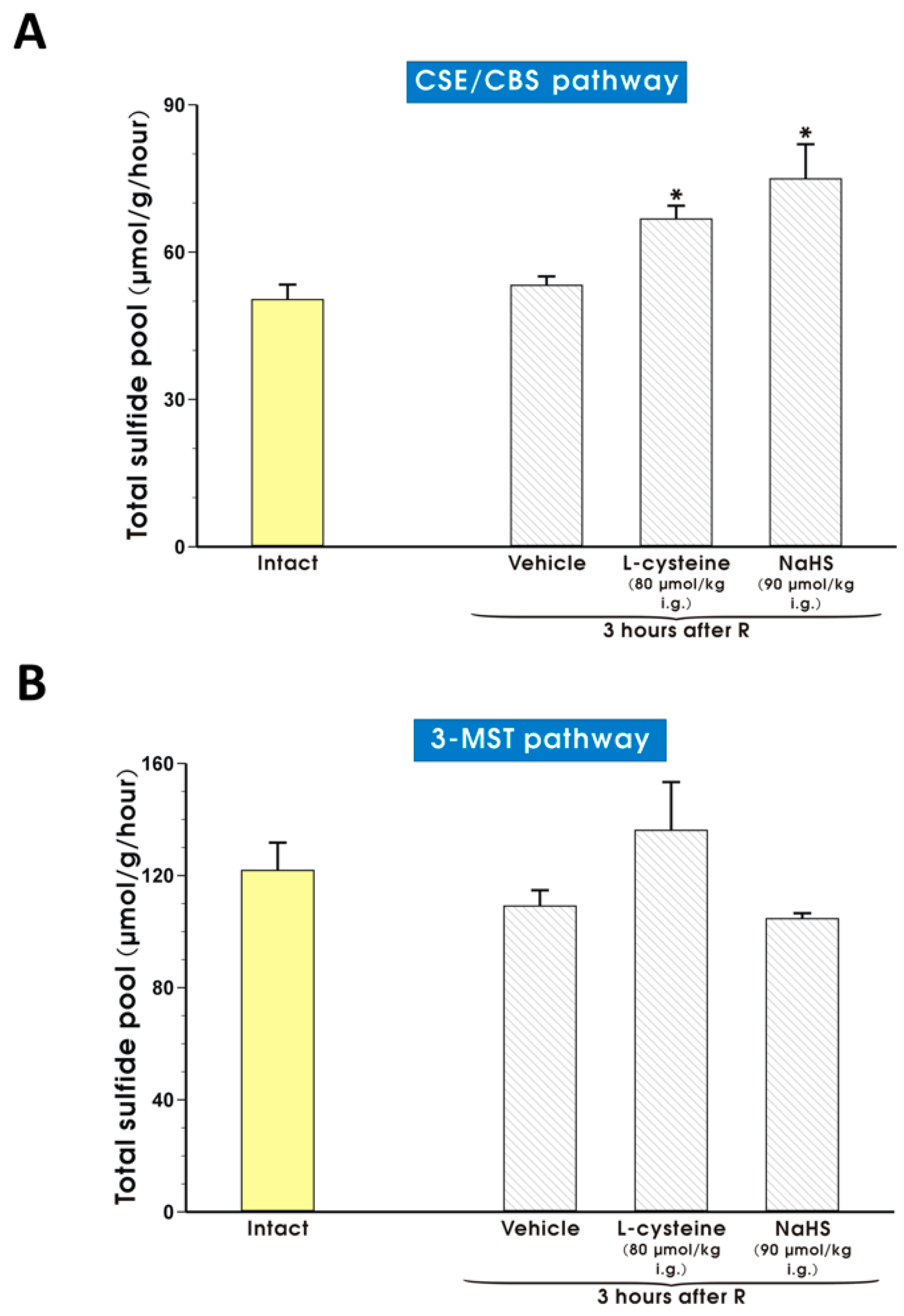
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design, I/R Injury, Afferent Sensory Nerves Ablation
4.3. Determination of I/R Lesions Area and Gastric Blood Flow Level (GBF)
4.4. Measurement of Sulfide Production in Gastric mucosa
4.5. Determination of mRNA Expression for CSE, CBS, 3-MST, HIF-1α, GPx-1, SOD-2 in Gastric Mucosa by Real Time Polymerase Chain Reaction (PCR)
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wallace, J.L.; Ferraz, J.G.; Muscara, M.N. Hydrogen sulfide: An endogenous mediator of resolution of inflammation and injury. Antioxid. Redox Signal 2012, 17, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, M.; Fukuda, R.; Bateman, R.M.; Yamamoto, T.; Suematsu, M. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal 2010, 13, 157–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal 2003, 5, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Jasnos, K.; Magierowski, M.; Kwiecien, S.; Brzozowski, T. Carbon monoxide in human physiology—Its role in the gastrointestinal tract. Postepy Hig. Med. Dosw. 2014, 68, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Magierowski, M.; Magierowska, K.; Kwiecien, S.; Brzozowski, T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules 2015, 20, 9099–9123. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, R.C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Beck, P.W. Characterization of the enzymatic capacity for cysteine desulphydration in liver and kidney of the rat. Biochem. J. 1982, 206, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, M.; Bradley, K.; Ohura, T.; Tahara, T.; Roper, M.D.; Rosenberg, L.E.; Kraus, J.P. Rat cystathionine beta-synthase. Gene organization and alternative splicing. J. Biol. Chem. 1992, 267, 11455–11461. [Google Scholar] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. Biochem. J. 2009, 146, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Magierowski, M.; Jasnos, K.; Kwiecien, S.; Brzozowski, T. Role of hydrogen sulfide in the physiology of gastrointestinal tract and in the mechanism of gastroprotection. Postepy Hig. Med. Dosw. 2013, 67, 150–156. [Google Scholar] [CrossRef]
- Pouokam, E.; Steidle, J.; Diener, M. Regulation of colonic ion transport by gasotransmitters. Biol. Pharm. Bull. 2011, 34, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Ise, F.; Takasuka, H.; Hayashi, S.; Takahashi, K.; Koyama, M.; Aihara, E.; Takeuchi, K. Stimulation of duodenal HCO3− secretion by hydrogen sulfide in rats: Relation to prostaglandins, nitric oxide and sensory neurons. Acta Physiol. 2011, 201, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.J.; Wang, M.J.; Ju, L.H.; Wang, C.; Zhu, Y.C. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol. Int. 2010, 34, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Xie, N.; Su, Q.; Su, J.; Huang, C.; Liao, Q.J. Diallyl disulfide inhibits the proliferation of HT-29 human colon cancer cells by inducing differentially expressed genes. Mol. Med. Rep. 2011, 4, 553–559. [Google Scholar] [PubMed]
- Lou, L.X.; Geng, B.; Du, J.B.; Tang, C.S. Hydrogen sulfide-induced hypothermia attenuates stress-related ulceration in rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 223–228. [Google Scholar] [PubMed]
- Wallace, J.L.; Dicay, M.; McKnight, W.; Martin, G.R. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007, 21, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Magierowski, M.; Magierowska, K.; Szmyd, J.; Surmiak, M.; Sliwowski, Z.; Kwiecien, S.; Brzozowski, T. Hydrogen sulfide and carbon monoxide protect gastric mucosa compromised by mild stress against alendronate injury. Dig. Dis. Sci. 2016, 61, 3176–3189. [Google Scholar] [CrossRef] [PubMed]
- Magierowski, M.; Magierowska, K.; Hubalewska-Mazgaj, M.; Adamski, J.; Bakalarz, D.; Sliwowski, Z.; Pajdo, R.; Kwiecien, S.; Brzozowski, T. Interaction between endogenous carbon monoxide and hydrogen sulfide in the mechanism of gastroprotection against acute aspirin-induced gastric damage. Pharmacol. Res. 2016, 114, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liang, F.; Shah Masood, W.; Yan, X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-κB dependent anti-inflammation pathway. Eur. J. Pharmacol. 2014, 725, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, L.; Zou, J.; Qiao, W.; Liu, H.; Qi, Y.; Yan, C. Protective effect of endogenous hydrogen sulfide against oxidative stress in gastric ischemia-reperfusion injury. Exp. Ther. Med. 2013, 5, 689–694. [Google Scholar] [PubMed]
- Mard, S.A.; Neisi, N.; Solgi, G.; Hassanpour, M.; Darbor, M.; Maleki, M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia-reperfusion injury in rat. Dig. Dis. Sci. 2012, 57, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Andrews, F.J.; Malcontenti-Wilson, C.; O’Brien, P.E. Polymorphonuclear leukocyte infiltration into gastric mucosa after ischemia–reperfusion. Am. J. Physiol. 1994, 266, G48–G54. [Google Scholar] [PubMed]
- Kawai, T.; Joh, T.; Iwata, F.; Itoh, M. Gastric epithelial damage induced by local ischemia–reperfusion with or without exogenous acid. Am. J. Physiol. 1994, 266, G263–G270. [Google Scholar] [PubMed]
- Yoshikawa, T.; Naito, Y.; Ueda, S.; Ichikawa, H.; Takahashi, S.; Yasuda, M.; Kondo, M. Ischemia–reperfusion injury and free radical involvement in gastric mucosal disorders. Adv. Exp. Med. Biol. 1992, 316, 231–238. [Google Scholar] [PubMed]
- Smith, G.S.; Mercer, D.W.; Cross, J.M.; Barreto, J.C.; Miller, T.A. Gastric injury induced by ethanol and ischemia–reperfusion in the rat. Differing roles for lipid peroxidation and oxygen radicals. Dig. Dis. Sci. 1996, 41, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Drozdowicz, D.; Kwiecien, S.; Pajdo, R.; Bielanski, W.; Hahn, E.G. Role of gastric acid secretion in progression of acute gastric erosions induced by ischemia reperfusion into gastric ulcers. Eur. J. Pharmacol. 2000, 398, 147–158. [Google Scholar] [CrossRef]
- Magierowski, M.; Jasnos, K.; Kwiecien, S.; Drozdowicz, D.; Surmiak, M.; Strzalka, M.; Ptak-Belowska, A.; Wallace, J.L.; Brzozowski, T. Endogenous prostaglandins and afferent sensory nerves in gastroprotective effect of hydrogen sulfide against stress-induced gastric lesions. PLoS ONE 2015, 10, e0118972. [Google Scholar] [CrossRef] [PubMed]
- Bos, E.M.; Snijder, P.M.; Jekel, H.; Weij, M.; Leemans, J.C.; van Dijk, M.C.; Hillebrands, J.L.; Lisman, T.; van Goor, H.; Leuvenink, H.G. Beneficial effects of gaseous hydrogen sulfide in hepatic ischemia/reperfusion injury. Transpl. Int. 2012, 25, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.E.; Tang, Z.H.; Xie, W.; Shen, X.T.; Liu, M.H.; Peng, X.P.; Zhao, Z.Z.; Nie, D.B.; Liu, L.S.; Jiang, Z.S. Endogenous hydrogen sulfide mediates the cardioprotection induced by ischemic postconditioning in the early reperfusion phase. Exp. Ther. Med. 2012, 4, 1117–1123. [Google Scholar] [PubMed]
- Snijder, P.M.; de Boer, R.A.; Bos, E.M.; van den Born, J.C.; Ruifrok, W.P.; Vreeswijk-Baudoin, I.; van Dijk, M.C.; Hillebrands, J.L.; Leuvenink, H.G.; van Goor, H. Gaseous hydrogen sulfide protects against myocardial ischemia-reperfusion injury in mice partially independent from hypometabolism. PLoS ONE 2013, 8, e63291. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Wang, J.; Xiao, Y.; Bai, W.; Xie, L.; Shan, L.; Moore, P.K.; Ji, Y. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J. Biomed. Res. 2015, 29, 203–213. [Google Scholar] [PubMed]
- Bos, E.M.; Wang, R.; Snijder, P.M.; Boersema, M.; Damman, J.; Fu, M.; Moser, J.; Hillebrands, J.L.; Ploeg, R.J.; Yang, G.; et al. Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 2013, 24, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Vong, L.; McKnight, W.; Dicay, M.; Martin, G.R. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 2009, 137, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Walter, B.; Burnat, G.; Hess, T.; Hahn, E.G.; Konturek, S.J. Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. Eur. J. Pharmacol. 2006, 536, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Leng, Y.F.; Zhang, Y.; Xue, X.; Kang, Y.Q.; Zhang, Y. Oxidative stress and hypoxia-induced factor 1α expression in gastric ischemia. World J. Gastroenterol. 2011, 17, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Biernat, J.; Pawlik, W.W.; Sendur, R.; Dembinski, A.; Brzozowski, T.; Konturek, S.J. Role of afferent nerves and sensory peptides in the mediation of hepatic artery buffer response. J. Physiol. Pharmacol. 2005, 56, 133–145. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Engel, M.; Burnat, G.; Gaca, P.; Kwiecien, S.; Pajdo, R.; Konturek, S.J. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J. Physiol. Pharmacol. 2009, 60, 41–47. [Google Scholar] [PubMed]
- Lu, W.; Li, J.; Gong, L.; Xu, X.; Han, T.; Ye, Y.; Che, T.; Luo, Y.; Li, J.; Zhan, R.; et al. H2S modulates duodenal motility in male rats via activating TRPV1 and K(ATP) channels. Br. J. Pharmacol. 2014, 171, 1534–1550. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Kamisaki, Y.; Kitano, M.; Kishimoto, Y.; Nakamoto, K.; Itoh, T. A new gastric ulcer model induced by ischemia-reperfusion in the rat: Role of leukocytes on ulceration in rat stomach. Life Sci. 1996, 59, 295–301. [Google Scholar] [CrossRef]
- Magierowski, M.; Katarzyna, J.; Sliwowski, Z.; Surmiak, M.; Krzysiek-Maczka, G.; Ptak-Belowska, A.; Kwiecien, S.; Brzozowski, T. Exogenous Asymmetric Dimethylarginine (ADMA) in Pathogenesis of Ischemia-Reperfusion-Induced Gastric Lesions: Interaction with Protective Nitric Oxide (NO) and Calcitonin Gene-Related Peptide (CGRP). Int. J. Mol. Sci. 2014, 15, 4946–4964. [Google Scholar] [CrossRef] [PubMed]
- Czekaj, R.; Majka, J.; Ptak-Belowska, A.; Szlachcic, A.; Targosz, A.; Magierowska, K.; Strzalka, M.; Magierowski, M.; Brzozowski, T. Role of curcumin in protection of gastric mucosa against stress-induced gastric mucosal damage. Involvement of hypoacidity, vasoactive mediators and sensory neuropeptides. J. Physiol. Pharmacol. 2016, 67, 261–275. [Google Scholar] [PubMed]
- Kwiecien, S.; Magierowska, K.; Magierowski, M.; Surmiak, M.; Hubalewska-Mazgaj, M.; Pajdo, R.; Sliwowski, Z.; Chmura, A.; Wojcik, D.; Brzozowski, T. Role of sensory afferent nerves, lipid peroxidation and antioxidative enzymes in the carbon monoxide-induced gastroprotection against stress ulcerogenesis. J. Physiol. Pharmacol. 2016, 67, 717–729. [Google Scholar] [PubMed]
- Konturek, S.J.; Brzozowski, T.; Pytko-Polonczyk, J.; Drozdowicz, D. Exogenous and endogenous cholecystokinin protects gastric mucosa against the damage caused by ethanol in rats. Eur. J. Pharmacol. 1995, 273, 57–62. [Google Scholar] [CrossRef]
- Magierowska, K.; Magierowski, M.; Hubalewska-Mazgaj, M.; Adamski, J.; Surmiak, M.; Sliwowski, Z.; Kwiecien, S.; Brzozowski, T. Carbon monoxide (CO) released from tricarbonyldichlororuthenium(II) dimer (CORM-2) in gastroprotection against experimental ethanol–induced gastric damage. PLoS ONE 2015, 10, e0140493. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Magierowska, K.; Magierowski, M.; Surmiak, M.; Adamski, J.; Mazur-Bialy, A.I.; Pajdo, R.; Sliwowski, Z.; Kwiecien, S.; Brzozowski, T. Protective role of carbon monoxide (CO) produced by heme oxygenase and derived from CO–releasing molecule CORM-2 in pathogenesis of stress–induced gastric lesions. Lack of involvement of nitric oxide (NO). Int. J. Mol. Sci. 2016, 17, 442. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magierowski, M.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Pajdo, R.; Ginter, G.; Kwiecien, S.; Brzozowski, T. Exogenous and Endogenous Hydrogen Sulfide Protects Gastric Mucosa against the Formation and Time-Dependent Development of Ischemia/Reperfusion-Induced Acute Lesions Progressing into Deeper Ulcerations. Molecules 2017, 22, 295. https://doi.org/10.3390/molecules22020295
Magierowski M, Magierowska K, Hubalewska-Mazgaj M, Sliwowski Z, Pajdo R, Ginter G, Kwiecien S, Brzozowski T. Exogenous and Endogenous Hydrogen Sulfide Protects Gastric Mucosa against the Formation and Time-Dependent Development of Ischemia/Reperfusion-Induced Acute Lesions Progressing into Deeper Ulcerations. Molecules. 2017; 22(2):295. https://doi.org/10.3390/molecules22020295
Chicago/Turabian StyleMagierowski, Marcin, Katarzyna Magierowska, Magdalena Hubalewska-Mazgaj, Zbigniew Sliwowski, Robert Pajdo, Grzegorz Ginter, Slawomir Kwiecien, and Tomasz Brzozowski. 2017. "Exogenous and Endogenous Hydrogen Sulfide Protects Gastric Mucosa against the Formation and Time-Dependent Development of Ischemia/Reperfusion-Induced Acute Lesions Progressing into Deeper Ulcerations" Molecules 22, no. 2: 295. https://doi.org/10.3390/molecules22020295





