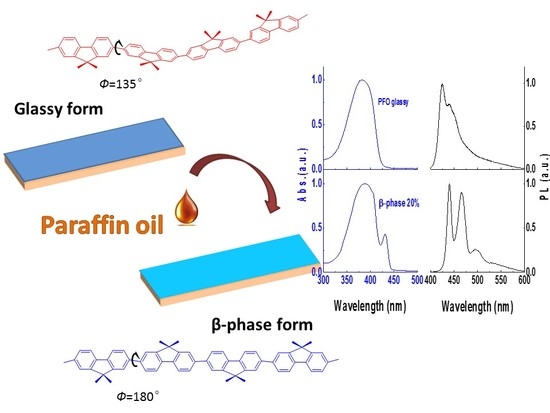
An Easy Approach to Control β-Phase Formation in PFO Films for Optimized Emission Properties
Abstract
:1. Introduction
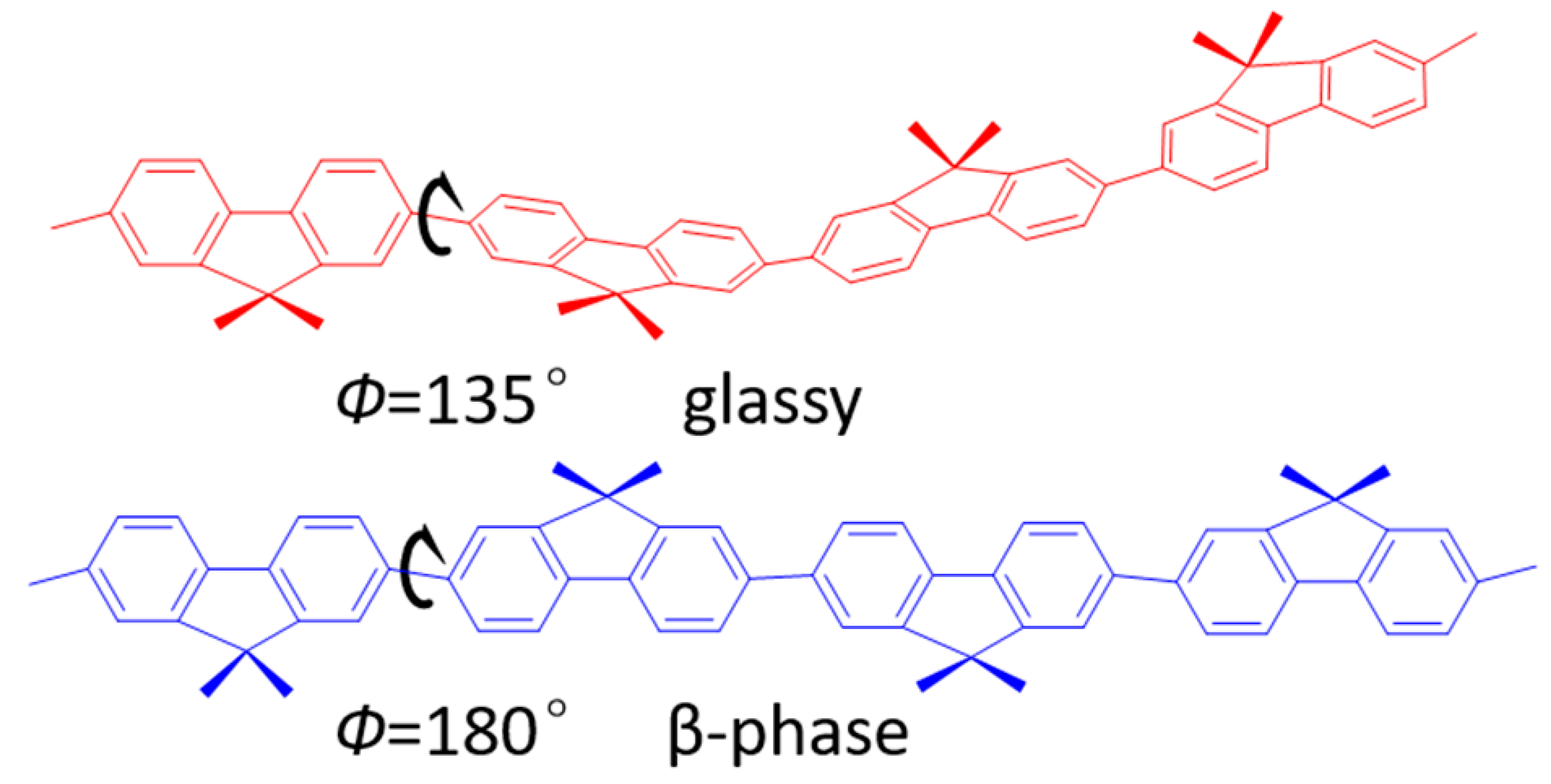
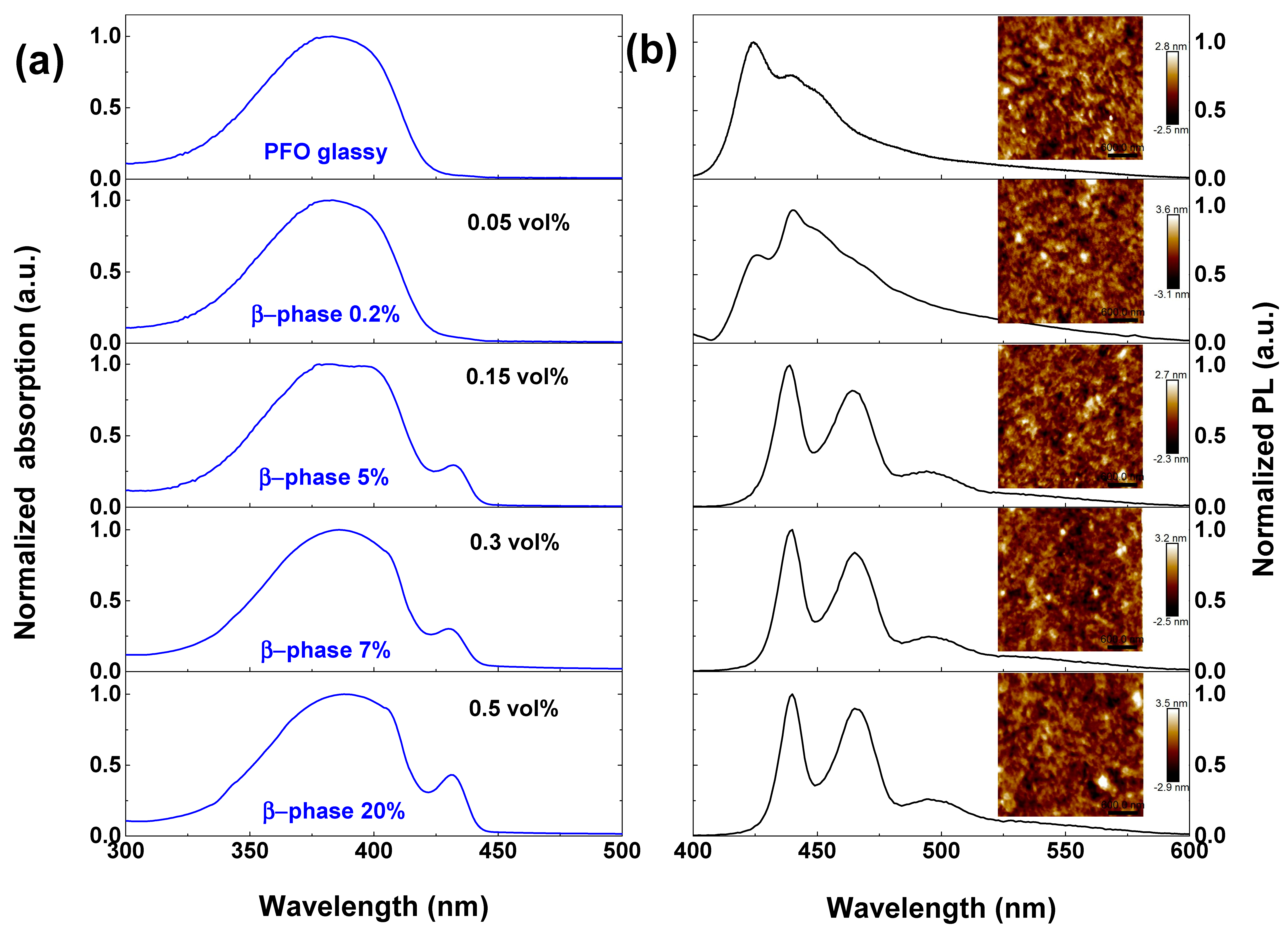
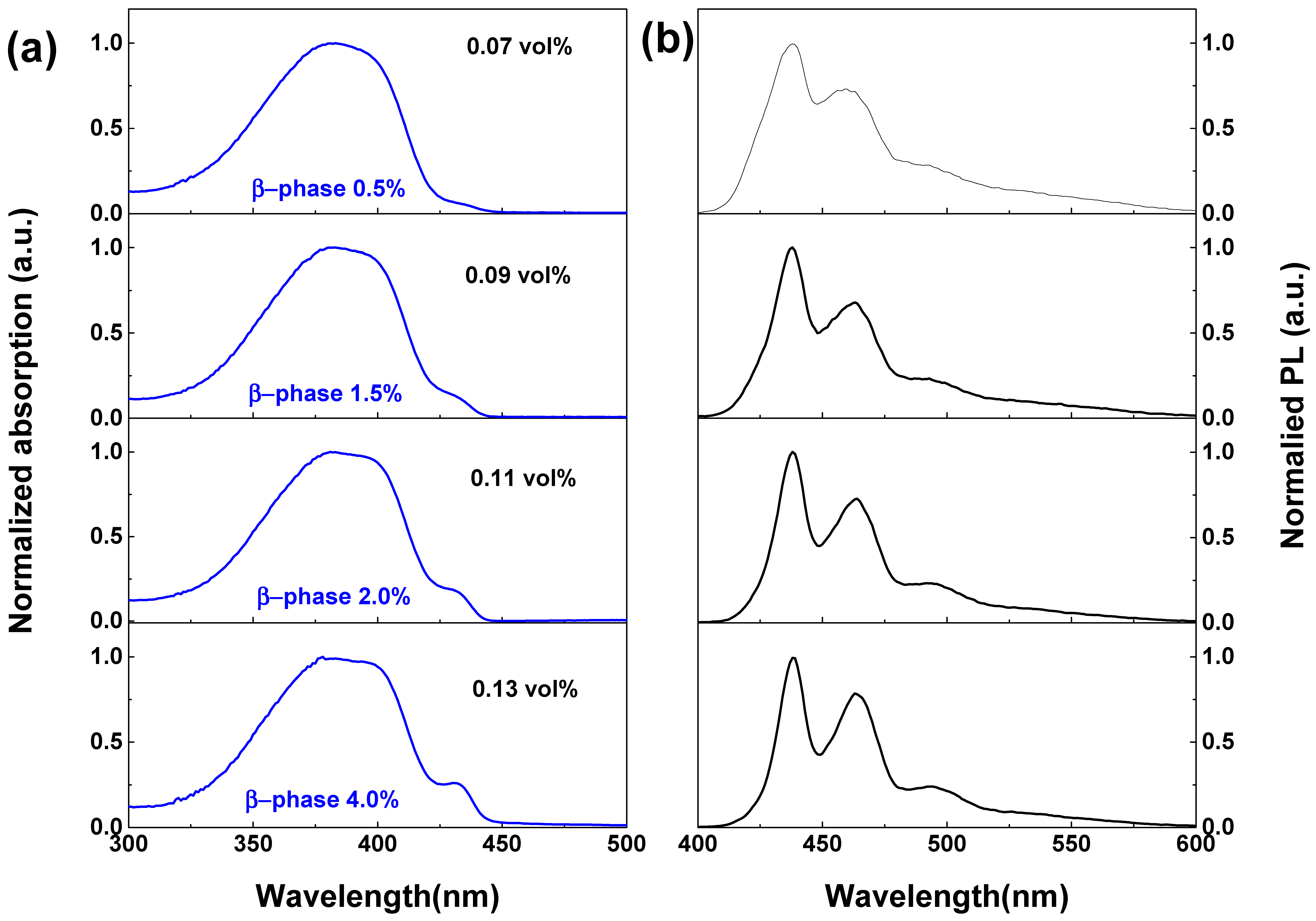
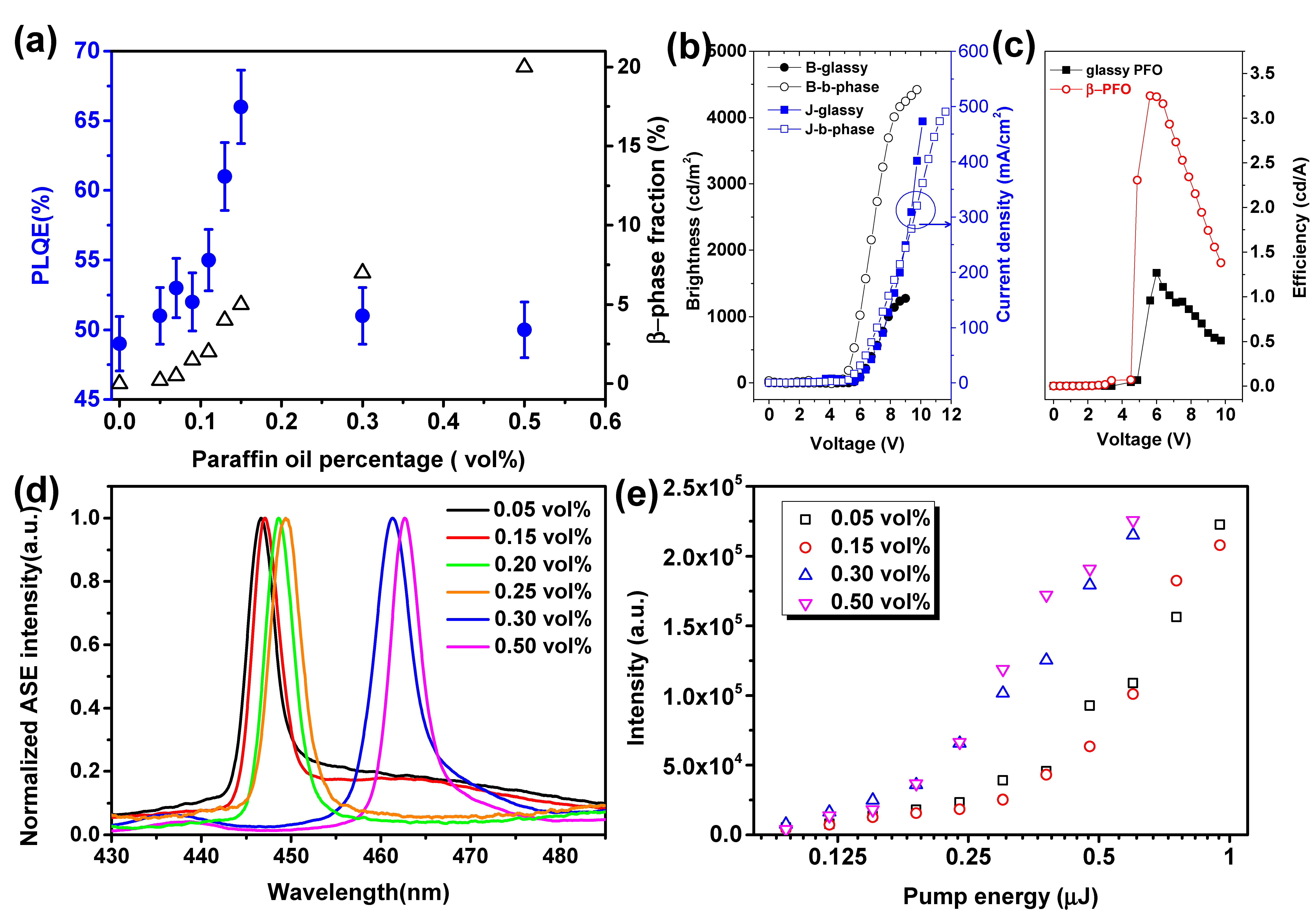
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Whitehead, K.S.; Grell, M.; Bradley, D.D.C.; Jandke, M.; Strohriegl, P. Highly polarized blue electroluminescence from homogeneously aligned films of poly(9,9-dioctylfluorene). Appl. Phys. Lett. 2000, 76, 2946–2948. [Google Scholar] [CrossRef]
- Weinfurtner, K.H.; Fujikawa, H.; Tokito, S.; Taga, Y. Highly efficient pure blue electroluminescence from polyfluorene: Influence of the molecular weight distribution on the aggregation tendency. Appl. Phys. Lett. 2000, 76, 2502–2504. [Google Scholar] [CrossRef]
- Liang, J.; Yu, L.; Zhao, S.; Ying, L.; Liu, F.; Yang, W.; Peng, J.; Cao, Y. Improving efficiency and color purity of poly(9,9-dioctylfluorene) through addition of a high boiling-point solvent of 1-chloronaphthalene. Nanotechnology 2016, 27, 284001. [Google Scholar] [CrossRef] [PubMed]
- Perevedentsev, A.; Chander, N.; Kim, J.-S.; Bradley, D.D.C. Spectroscopic properties of poly(9,9-dioctylfluorene) thin films possessing varied fractions of β-phase chain segments: Enhanced photoluminescence efficiency via conformation structuring. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1995–2006. [Google Scholar] [CrossRef]
- Heliotis, G.; Xia, R.D.; Turnbull, G.A.; Andrew, P.; Barnes, W.L.; Samuel, I.D.W.; Bradley, D.D.C. Emission characteristics and performance comparison of polyfluorene lasers with one- and two-dimensional distributed feedback. Adv. Funct. Mater. 2004, 14, 91–97. [Google Scholar] [CrossRef]
- Xia, R.D.; Heliotis, G.; Hou, Y.B.; Bradley, D.D.C. Fluorene-based conjugated polymer optical gain media. Org. Electron. 2003, 4, 165–177. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, X.; Zhang, Q.; Chi, L.; Chen, T.; Xia, R.; Wu, L.; Luer, L.; Cabanillas-Gonzalez, J. Novel fluorene-based copolymers containing branched 2-methyl-butyl-substituted fluorene-co-benzothiadiazole units for remarkable optical gain enhancement in green-yellow emission range. J. Phys. Chem. C 2016, 120, 11350–11358. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Xu, W.; Li, X.; Liu, J.; Guo, X.; Xia, R.; Huang, W. Efficient amplified spontaneous emission from oligofluorene-pyrene starbursts with improved electron affinity property. Opt. Express 2015, 23, A465–A470. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Zhang, Q.; Xu, W.; Jiang, Y.; Xia, R.; Bradley, D.D.C.; Li, D.; Wen, X. Solution-processed anthracene-based molecular glasses as stable blue-light-emission laser gain media. Org. Electron. 2015, 18, 95–100. [Google Scholar] [CrossRef]
- Yi, J.; Niu, Q.; Xu, W.; Hao, L.; Yang, L.; Chi, L.; Fang, Y.; Huang, J.; Xia, R. Significant lowering optical loss of electrodes via using conjugated polyelectrolytes interlayer for organic laser in electrically driven device configuration. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Yap, B.K.; Xia, R.D.; Campoy-Quiles, M.; Stavrinou, P.N.; Bradley, D.D.C. Simultaneous optimization of charge carrier mobility and optical gain in semiconducting polymer films. Nat. Mater. 2008, 7, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Cadby, A.J.; Lane, P.A.; Mellor, H.; Martin, S.J.; Grell, M.; Giebeler, C.; Bradley, D.D.C.; Wohlgenannt, M.; An, C.; Vardeny, Z.V. Film morphology and photophysics of polyfluorene. Phys. Rev. B 2000, 62, 15604–15609. [Google Scholar] [CrossRef]
- Ariu, M.; Sims, M.; Rahn, M.D.; Hill, J.; Fox, A.M.; Lidzey, D.G.; Oda, M.; Cabanillas-Gonzalez, J.; Bradley, D.D.C. Exciton migration in beta-phase poly(9,9-dioctylfluorene). Phys. Rev. B Condens. Matter 2003, 67, 195333. [Google Scholar] [CrossRef]
- Grell, M.; Bradley, D.D.C.; Long, X.; Chamberlain, T.; Inbasekaran, M.; Woo, E.P.; Soliman, M. Chain geometry, solution aggregation and enhanced dichroism in the liquid-crystalline conjugated polymer poly(9,9-dioctylfluorene). Acta Polym. 1998, 49, 439–444. [Google Scholar] [CrossRef]
- Grell, M.; Bradley, D.D.C.; Inbasekaran, M.; Woo, E.P. A glass-forming conjugated main-chain liquid crystal polymer for polarized electroluminescence applications. Adv. Mater. 1997, 9, 798–802. [Google Scholar] [CrossRef]
- Grell, M.; Knoll, W.; Lupo, D.; Meisel, A.; Miteva, T.; Neher, D.; Nothofer, H.G.; Scherf, U.; Yasuda, A. Blue polarized electroluminescence from a liquid crystalline polyfluorene. Adv. Mater. 1999, 11, 671–675. [Google Scholar] [CrossRef]
- Ariu, M.; Lidzey, D.G.; Sims, M.; Cadby, A.J.; Lane, P.A.; Bradley, D.D.C. The effect of morphology on the temperature-dependent photoluminescence quantum efficiency of the conjugated polymer poly(9, 9-dioctylfluorene). J. Phys. Condens. Matter 2002, 14, 9975–9986. [Google Scholar] [CrossRef]
- Lu, H.-H.; Liu, C.-Y.; Chang, C.-H.; Chen, S.-A. Self-dopant formation in poly(9,9-di-N-octylfluorene) via a dipping method for efficient and stable pure-blue electrolumineseence. Adv. Mater. 2007, 19, 2574–2579. [Google Scholar] [CrossRef]
- Rothe, C.; Galbrecht, F.; Scherf, U.; Monkman, A. The beta-phase of poly(9,9-dioctylfluorene) as a potential system for electrically pumped organic lasing. Adv. Mater. 2006, 18, 2137–2140. [Google Scholar] [CrossRef]
- Ryu, G.; Xia, R.; Bradley, D.D.C. Optical gain characteristics of beta-phase poly(9,9-dioctylfluorene). J. Phys. Condens. Matter 2007, 19, 056205. [Google Scholar] [CrossRef]
- Khan, A.L.T.; Sreearunothai, P.; Herz, L.M.; Banach, M.J.; Kohler, A. Morphology-dependent energy transfer within polyfluorene thin films. Phys. Rev. B Condens. Matter 2004, 69, 085201. [Google Scholar] [CrossRef]
- Shaw, P.E.; Ruseckas, A.; Peet, J.; Bazan, G.C.; Samuel, I.D.W. Exciton-exciton annihilation in mixed-phase polyfluorene films. Adv. Funct. Mater. 2010, 20, 155–161. [Google Scholar] [CrossRef]
- Bansal, A.K.; Ruseckas, A.; Shaw, P.E.; Samuel, I.D.W. Fluorescence quenchers in mixed phase polyfluorene films. J. Phys. Chem. C 2010, 114, 17864–17867. [Google Scholar] [CrossRef]
- Peet, J.; Brocker, E.; Xu, Y.; Bazan, G.C. Controlled beta-phase formation in poly(9,9-di-N-octylfluorene) by processing with alkyl additives. Adv. Mater. 2008, 20, 1882–1885. [Google Scholar] [CrossRef]
- Sirtonski, M.R.; McFarlane, S.L.; Veinot, J.G.C. Stabilizing the optical properties of pfo through addition of a non-volatile low molecular weight aromatic ether. J. Mater. Chem. 2010, 20, 8147–8152. [Google Scholar] [CrossRef]
- Ryu, G.; Stavrinou, P.N.; Bradley, D.D.C. Spatial patterning of the beta-phase in poly (9,9-dioctylfluorene): A metamaterials-inspired molecular conformation approach to the fabrication of polymer semiconductor optical structures. Adv. Funct. Mater. 2009, 19, 3237–3242. [Google Scholar] [CrossRef]
- Stavrinou, P.N.; Ryu, G.; Campoy-Quiles, M.; Bradley, D.D.C. The change in refractive index of poly(9,9-dioctylfluorene) due to the adoption of the beta-phase chain conformation. J. Phys. Condens. Matter 2007, 19, 466107. [Google Scholar] [CrossRef]
- Nassyrov, D.; Mueller, C.; Roige, A.; Burgues-Ceballos, I.; Oriol Osso, J.; Amabilino, D.B.; Garriga, M.; Isabel Alonso, M.; Goni, A.R.; Campoy-Quiles, M. Vapour printing: Patterning of the optical and electrical properties of organic semiconductors in one simple step. J. Mater. Chem. 2012, 22, 4519–4526. [Google Scholar] [CrossRef]
- Grell, M.; Bradley, D.D.C.; Ungar, G.; Hill, J.; Whitehead, K.S. Interplay of physical structure and photophysics for a liquid crystalline polyfluorene. Macromolecules 1999, 32, 5810–5817. [Google Scholar] [CrossRef]
- Caruso, M.E.; Anni, M. Real-time investigation of solvent swelling induced beta-phase formation in poly(9,9-dioctylfluorene). Phys. Rev. B Condens. Matter 2007, 76, 054207. [Google Scholar] [CrossRef]
- Chen, S.H.; Su, A.C.; Su, C.H.; Chen, S.A. Crystalline forms and emission behavior of poly(9,9-di-N-octyl-2,7-fluorene). Macromolecules 2005, 38, 379–385. [Google Scholar] [CrossRef]
- Chen, M.-C.; Hung, W.-C.; Su, A.-C.; Chen, S.-H.; Chen, S.-A. Nanoscale ordered structure distribution in thin solid film of conjugated polymers: Its significance in charge transport across the film and in performance of electroluminescent device. J. Phys. Chem. B 2009, 113, 11124–11133. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huang, X.; Sun, G.; Gu, C.; Lu, D.; Ma, Y. Study of beta phase and chains aggregation degrees in poly(9,9-dioctylfluorene) (PFO) solution. J. Phys. Chem. C 2012, 116, 7993–7999. [Google Scholar] [CrossRef]
- Sample Availability: “Not available.” All compounds we used are commercial products whose manufacturer information is provided in the “Materials and methods” section in the paper above.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Chi, L.; Hai, G.; Fang, Y.; Li, X.; Xia, R.; Huang, W.; Gu, E. An Easy Approach to Control β-Phase Formation in PFO Films for Optimized Emission Properties. Molecules 2017, 22, 315. https://doi.org/10.3390/molecules22020315
Zhang Q, Chi L, Hai G, Fang Y, Li X, Xia R, Huang W, Gu E. An Easy Approach to Control β-Phase Formation in PFO Films for Optimized Emission Properties. Molecules. 2017; 22(2):315. https://doi.org/10.3390/molecules22020315
Chicago/Turabian StyleZhang, Qi, Lang Chi, Gang Hai, Yueting Fang, Xiangchun Li, Ruidong Xia, Wei Huang, and Erdan Gu. 2017. "An Easy Approach to Control β-Phase Formation in PFO Films for Optimized Emission Properties" Molecules 22, no. 2: 315. https://doi.org/10.3390/molecules22020315