Screening of Six Medicinal Plant Extracts Obtained by Two Conventional Methods and Supercritical CO2 Extraction Targeted on Coumarin Content, 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity and Total Phenols Content
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Determination of Initial Water Content
3.4. Soxhlet Extraction
3.5. Alcoholic Extracts Processing
3.6. Supercritical CO2 Extraction
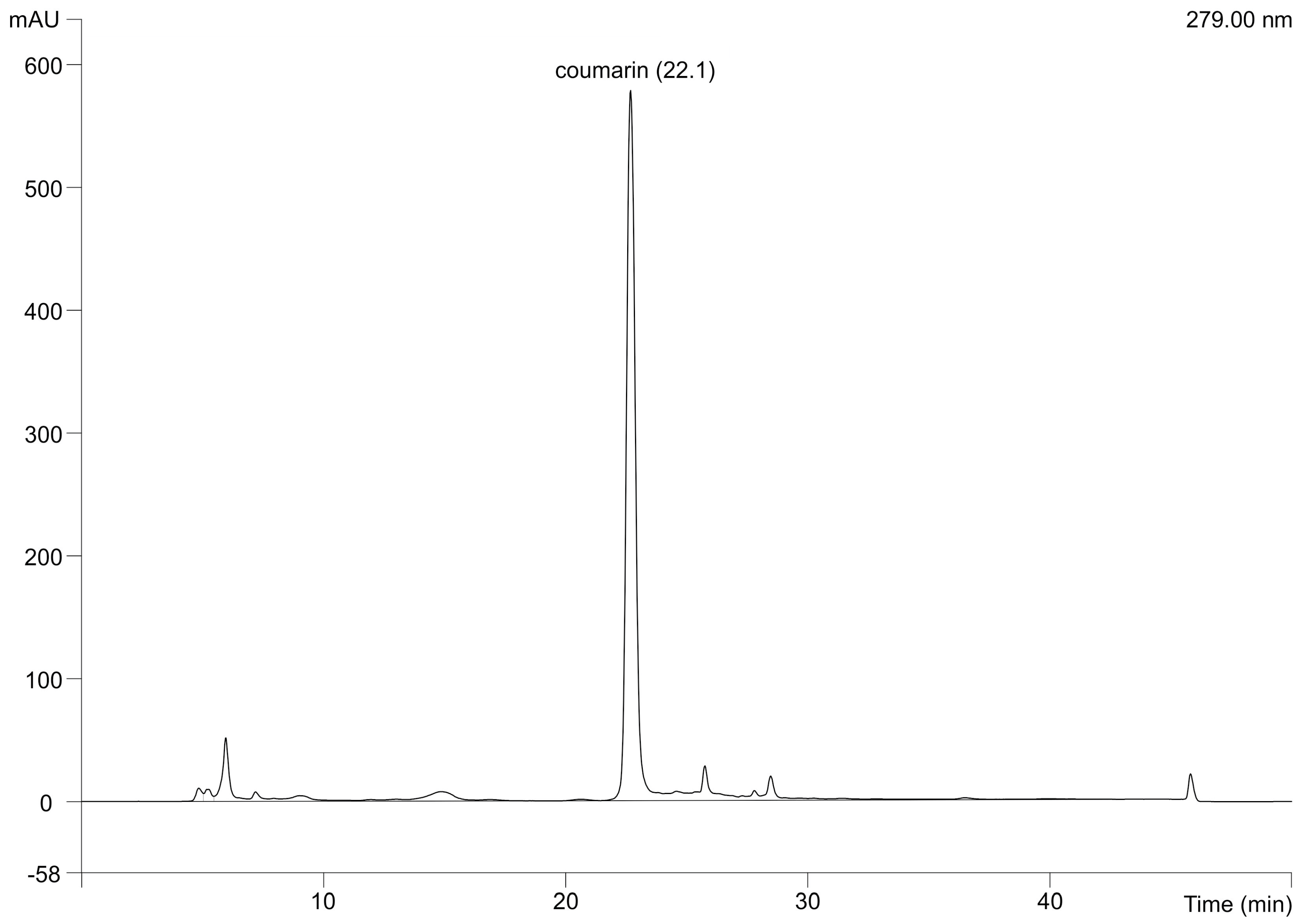
3.7. Determination of Coumarin Concentration by High Performance Liquid Chromatography
3.8. Determination of DPPH Antiradical Capacity
3.9. Determination of Total Phenolics Content
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jain, P.K.; Joshi, H. Coumarin: Chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Razavi, S.M. Plant coumarins as allelopathic agents. Int. J. Biol. Chem. 2011, 5, 86–90. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, I. History of the development and applications of coumarin and coumarin-related compounds. In Coumarins: Biology, Applications and Mode of Action; O’Kennedy, R., Thornes, R.D., Eds.; Wiley: Chichester, UK, 1997; pp. 1–22. [Google Scholar]
- Razavi, S.M.; Imanzadeh, G.; Davari, M. Coumarins from Zosima absinthifolia seeds, with allelopatic effects. J. BioSci. 2010, 4, 17–22. [Google Scholar] [CrossRef]
- Kai, K.; Shimizu, B.; Mizutani, M.; Watanabe, K.; Sakata, K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry 2006, 67, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ojala, T. Biological Screening of Plant Coumarins. Ph.D. Thesis, Faculty of Science, University of Helsinki, Helsinki, Finland, 2001. [Google Scholar]
- Sardari, S.; Nishibe, S.; Daneshtalab, U. Coumarins, the bioactive structures with antifungal property. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2000; Volume 51, pp. 335–393. [Google Scholar]
- Silvan, A.M.; Abad, M.J.; Bermejo, P.; Sollhuber, M.; Villar, A. Antiinflammatory activity of coumarins from Santolina oblongifolia. J. Nat. Prod. 1996, 59, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; El-Arab, E.E.; El-Sharkawy, K.A. The reaction of cyanoacetic acid hydrazide with 2-acetylfuran: Synthesis of coumarin, pyridine, thiophene and thiazole derivatives with potential antimicrobial activities. Sci. Pharm. 2009, 77, 355–366. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kobayashi, A.; Kajiyama, S.I.; Kawazu, K.; Kanzaki, H.; Kim, C.M. Antimicrobial constituents of Angelica dahurica roots. Phytochemistry 1997, 44, 887–889. [Google Scholar] [CrossRef]
- Roussaki, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Hamilakis, S.; Detsi, A. A novel synthesis of 3-aryl coumarins and evaluation of their antioxidant and lipoxygenase inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3889–3892. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.S.; Lee, I.K.; Ryoo, I.J.; Yoo, I.D. Coumarins with monoamine oxidase inhibitory activity and antioxidative coumarino-lignans from Hibiscus syriacus. J. Nat. Prod. 2001, 64, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- El-Shahawy, T.A.; Abdelhamid, M.T. Potential allelopathic effect of six phaseolus vulgaris recombinant inbred lines for weed control. Aust. J. Basic Appl. Sci. 2013, 7, 462–467. [Google Scholar]
- Wu, C.X.; Zhao, G.Q.; Liu, D.L.; Liu, S.J.; Gun, X.X.; Tang, Q. Discovery and weed inhibition effects of coumarin as the predominant allelochemical of yellow sweetclover (Melilotus officinalis). Int. J. Agric. Biol. 2016, 18, 168–175. [Google Scholar] [CrossRef]
- Vergel, N.E. Study of the Anticonvulsant Activity of Secondary Metabolites Such as Coumarin. Ph.D. Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2011. [Google Scholar]
- Thada, R.; Chockalingam, S.; Dhandapani, R.K.; Panchamoorthy, R. Extraction and Quantitation of Coumarin from Cinnamon and its Effect on Enzymatic Browning in Fresh Apple Juice: A Bioinformatics Approach to Illuminate its Antibrowning Activity. J. Agric. Food Chem. 2013, 61, 5385–5390. [Google Scholar] [CrossRef] [PubMed]
- Celeghini, R.M.S.; Vilegas, J.H.Y.; Lanças, F.M. Extraction and quantitative HPLC analysis of coumarin in hydroalcoholic extracts of Mikania glomerata Spreng. (“guaco”) leaves. J. Braz. Chem. Soc. 2001, 12, 706–709. [Google Scholar] [CrossRef]
- Leal, L.K.A.M.; Ferreira, A.A.G.; Bezerra, G.A.; Matos, F.J.A.; Viana, G.S.B. Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: A comparative study. J. Ethnopharmacol. 2000, 70, 151–159. [Google Scholar] [CrossRef]
- Bourgaud, F.; Poutaraud, A.; Guckert, A. Extraction of coumarins from plant material (Leguminosae). Phytochem. Anal. 1994, 5, 127–132. [Google Scholar] [CrossRef]
- Antunes Viegas, D.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.A.; Kumar, D.; Shah, M.Y. Angelica archangelica Linn. is an angel on earth for the treatment of diseases. Int. J. Nutr. Pharmacol. Neurol. Dis. 2011, 1, 36–50. [Google Scholar]
- Areias, F.M.; Valentao, P.; Andrade, P.B.; Moreira, M.M.; Amaral, J.; Seabra, R.M. HPLC/DAD analysis of phenolic compounds from lavender and its application to quality control. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 2563–2572. [Google Scholar] [CrossRef]
- Pastorino, G.; Marchetti, C.; Borghesi, B.; Cornara, L.; Ribulla, S.; Burlando, B. Biological activities of the legume crops Melilotus officinalis and Lespedeza capitata for skin care and pharmaceutical applications. Ind. Crops Prod. 2017, 96, 158–164. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No. 1334/2008 of the European parliament and of the council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No. 1601/91, Regulations (EC) No. 2232/96 and (EC) No. 110/2008 and Directive 2000/13/EC. Off. J. Eur. Union 2008, L354, 34–50. [Google Scholar]
- Martino, M.; Ramaiola, I.; Urbano, M.; Bracco, F.; Collina, S. Microwave-assisted extraction of coumarin and related compounds from Melilotus officinalis (L.) Pallas as an alternative to Soxhlet and ultrasound-assisted extraction. J. Chromatogr. A 2006, 1125, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Rojht, H.; Košir, I.J.; Trdan, S. Chemical analysis of three herbal extracts and observation of their activity against adults of Acanthoscelides obtectus and Leptinotarsa decemlineata using a video tracking system. J. Plant Dis. Prot. 2012, 119, 59–67. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Acosta, R.; Martınez, J.R. High-resolution gas-chromatographic analysis of the secondary metabolites obtained by subcritical-fluid extraction from Colombian rue (Ruta graveolens L.). J. Biochem. Biophys. Methods 2000, 43, 379–390. [Google Scholar] [CrossRef]
- Harmala, P.; Vuorela, H. Optimization of the high-performance liquid chromatography of coumarins in Angelica archangelica with reference to molecular structure. J. Chromatogr. 1990, 507, 367–380. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.A.; Kumar, V.; Shah, M.Y. Coumarins from Angelica archangelica Linn. and their effects on anxiety-like behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kerrola, K.; Galambosi, B.; Kallio, H. Characterization of volatile composition and odor of Angelica (Angelica archangelica Subsp. archangelica L.) root extracts. J. Agric. Food Chem. 1994, 42, 1979–1988. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; Faveri, D.M.D. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Glisic, S.; Ivanovic, J.; Ristic, M.; Skala, D. Extraction of sage (Salvia officinalis L.) by supercritical CO2: Kinetic data, chemical composition and selectivity of diterpenes. J. Supercrit. Fluids 2010, 52, 62–70. [Google Scholar] [CrossRef]
- Shabana, M.M.; El-Alfy, T.S.; El-Tantawy, M.E.; Ibrahim, A.I.; Ibrahim, G.F. Tissue culture and evaluation of some active constituents of Ruta graveolens L. II: Effect of plant growth regulators, explant type and precursor on coumarin content of Ruta graveolens L. callus cultures. Arab J. Biotechnol. 2002, 5, 45–56. [Google Scholar]
- Jokić, S.; Rajić, M.; Bilić, B.; Molnar, M. Supercritical extraction of scopoletin from Helichrysum italicum (Roth) G. Don flowers. Phytochem. Anal. 2016, 27, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Aleksovski, S.A.; Sovova, H. Supercritical CO2 extraction of Salvia officinalis L. J. Supercrit. Fluids 2007, 40, 239–245. [Google Scholar] [CrossRef]
- Roman, G.P.; Neagu, E.; Radu, G.L. Antiradical activities of Salvia officinalis and Viscum album L. extracts concentrated by ultrafiltration process. Acta Sci. Pol. Technol. Aliment. 2009, 8, 47–58. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 7th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Jokić, S.; Horvat, G.; Aladić, K. Design of SFE System Using a Holistic Approach-Problems and Challenges. In Supercritical Fluid Extraction: Technology, Applications and Limitations; Lindy, J., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2015; pp. 95–122. [Google Scholar]
- Jokić, S.; Bijuk, M.; Aladić, K.; Bilić, M.; Molnar, M. Optimization of supercritical CO2 extraction of grape seed oil using response surface methodology. Int. J. Food Sci. Technol. 2016, 51, 403–410. [Google Scholar] [CrossRef]
- Šarkanj, B.; Molnar, M.; Čačić, M.; Gille, L. 4-Methyl-7-hydroxycoumarin antifungal and antioxidant activity enhancement by substitution with thiosemicarbazide and thiazolidinone moieties. Food chem. 2013, 139, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Šeruga, M.; Novak, I.; Medvidović-Kosanović, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebens.-Rundsch. 2007, 103, 369–378. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
| Properties | H. italicum | A. archangelica | L. officinalis | S. officinalis | M. officinalis | R. graveolens |
|---|---|---|---|---|---|---|
| Moisture content (%) | 12.88 ± 0.01 | 12.02 ± 0.04 | 11.93 ± 0.01 | 12.42 ± 0.06 | 13.66 ± 0.04 | 12.19 ± 0.08 |
| Soxhlet extraction | 4.95 ± 0.24 | 2.39 ± 0.29 | 4.13 ± 0.18 | 5.33 ± 0.38 | 1.29 ± 0.04 | 2.03 ± 0.27 |
| 96% EtOH | 6.70 ± 0.28 | 6.15 ± 0.41 | 9.75 ± 0.33 | 9.50 ± 0.22 | 4.40 ± 0.04 | 9.95 ± 0.34 |
| 50% EtOH | 10.1 ± 0.36 | 11.75 ± 0.48 | 12.3 ± 0.29 | 10.35 ± 0.48 | 10.00 ± 0.49 | 14.95 ± 0.44 |
| SC-CO2 (300 bar) | 4.85 ± 0.20 | 0.35 ± 0.11 | 2.19 ± 0.31 | 4.28 ± 0.31 | 0.05 ± 0.03 | 0.60 ± 0.11 |
| SC-CO2 (150 bar) | 2.86 ± 0.56 | <0.01 | 2.65 ± 0.51 | 3.77 ± 0.19 | <0.01 | <0.01 |
| Extraction Method | H. italicum | A. archangelica | L. officinalis | S. officinalis | M. officinalis | R. graveolens |
|---|---|---|---|---|---|---|
| Hexane extraction | 0.00 | 0.00 | 0.00 | 0.00 | 8.86 ± 0.67 | 0.47 ± 0.11 |
| Extraction with 96% EtOH | 0.00 | 0.00 | 3.77 ± 0.61 | 0.00 | 316.37 ± 8.10 | 0.00 |
| Extraction with 50% EtOH | 0.00 | 0.00 | 0.00 | 0.00 | 146.43 ± 9.15 | 0.00 |
| SC-CO2 extraction (300 bar) | 0.00 | 0.91 ± 0.09 | 2.92 ± 0.17 | 1.45 ± 0.18 | n.d. | 0.53 ± 0.00 |
| SC-CO2 extraction (150 bar) | 0.00 | n.d. | 3.13 ± 0.13 | 2.62 ± 0.00 | n.d | n.d. |
| Extraction Method | % DPPH Scavenging Activity | |||||
|---|---|---|---|---|---|---|
| H. italicum | A. archangelica | L. officinalis | S. officinalis | M. officinalis | R. graveolens | |
| Hexane extraction | 94.3 ± 0.06 | 9.5 ± 0.52 | 4.0 ± 1.99 | 100 ± 0.00 | 9.0 ± 0.28 | 16.8 ± 1.46 |
| Extraction with 96% EtOH | 93.5 ± 0.12 | 8.8 ± 0.31 | 33.2 ± 0.45 | 95.2 ± 0.05 | 35.6 ± 0.65 | 59.3 ± 0.61 |
| Extraction with 50% EtOH | 93.0 ± 0.17 | 9.0 ± 0.12 | 24.2 ± 0.32 | 93.2 ± 0.09 | 30.2 ± 0.98 | 60.3 ± 0.14 |
| SC-CO2 (300 bar) | 79.12 ± 0.45 | 1.7 ± 0.8 | 3.2 ± 0.65 | 95.7 ± 0.44 | n.d. | 16.8 ± 0.84 |
| SC-CO2 (150 bar) | n.d. | n.d. | 10.8 ± 0.92 | 95.3 ± 0.51 | n.d. | n.d. |
| Extraction Method | Total Phenols (TPs) | |||||
|---|---|---|---|---|---|---|
| H. italicum | A. archangelica | L. officinalis | S. officinalis | M. officinalis | R. graveolens | |
| Hexane extraction | 67.4 ± 3.1 | 17.3 ± 1.6 | 7.2 ± 0.0 | 82.3 ± 7.4 | 10.5 ± 0.6 | 11.9 ± 0.7 |
| Extraction with 96% EtOH | 132.1 ± 3.8 | 14.3 ± 0.4 | 63.4 ± 1.2 | 88.2 ± 7.6 | 47.1 ± 3.6 | 89.5 ± 7.0 |
| Extraction with 50% EtOH | 104.8 ± 6.0 | 11.8 ± 0.5 | 61.9 ± 2.8 | 90.6 ± 5.9 | 46.3 ± 7.4 | 56.6 ± 4.3 |
| SC-CO2 (300 bar) | 65.3 ± 0.5 | 8.7 ± 0.6 | 6.4 ± 3.9 | 61.8 ± 4.4 | n.d. | 13.3 ± 1.3 |
| SC-CO2 (150 bar) | n.d. | n.d. | 4.8 ± 0.3 | 53.8 ± 2.7 | n.d | n.d. |
| Common Name of Plant | Latin Name of Plant | Part of Plant |
|---|---|---|
| Immortelle | Helichrysum italicum (Roth) G. Don | flowers |
| Angelica | Angelica archangelica L. | root |
| Lavender | Lavandula officinalis L. | flowers |
| Sage | Salvia officinalis L. | leaves |
| Yellow melilot | Melilotus officinalis L. | herb |
| Rue | Ruta graveolens L. | leaves |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnar, M.; Jerković, I.; Suknović, D.; Bilić Rajs, B.; Aladić, K.; Šubarić, D.; Jokić, S. Screening of Six Medicinal Plant Extracts Obtained by Two Conventional Methods and Supercritical CO2 Extraction Targeted on Coumarin Content, 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity and Total Phenols Content. Molecules 2017, 22, 348. https://doi.org/10.3390/molecules22030348
Molnar M, Jerković I, Suknović D, Bilić Rajs B, Aladić K, Šubarić D, Jokić S. Screening of Six Medicinal Plant Extracts Obtained by Two Conventional Methods and Supercritical CO2 Extraction Targeted on Coumarin Content, 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity and Total Phenols Content. Molecules. 2017; 22(3):348. https://doi.org/10.3390/molecules22030348
Chicago/Turabian StyleMolnar, Maja, Igor Jerković, Dragica Suknović, Blanka Bilić Rajs, Krunoslav Aladić, Drago Šubarić, and Stela Jokić. 2017. "Screening of Six Medicinal Plant Extracts Obtained by Two Conventional Methods and Supercritical CO2 Extraction Targeted on Coumarin Content, 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity and Total Phenols Content" Molecules 22, no. 3: 348. https://doi.org/10.3390/molecules22030348






