Binding and Conversion of Selenium in Candida utilis ATCC 9950 Yeasts in Bioreactor Culture
Abstract
:1. Introduction
2. Results and Discussion
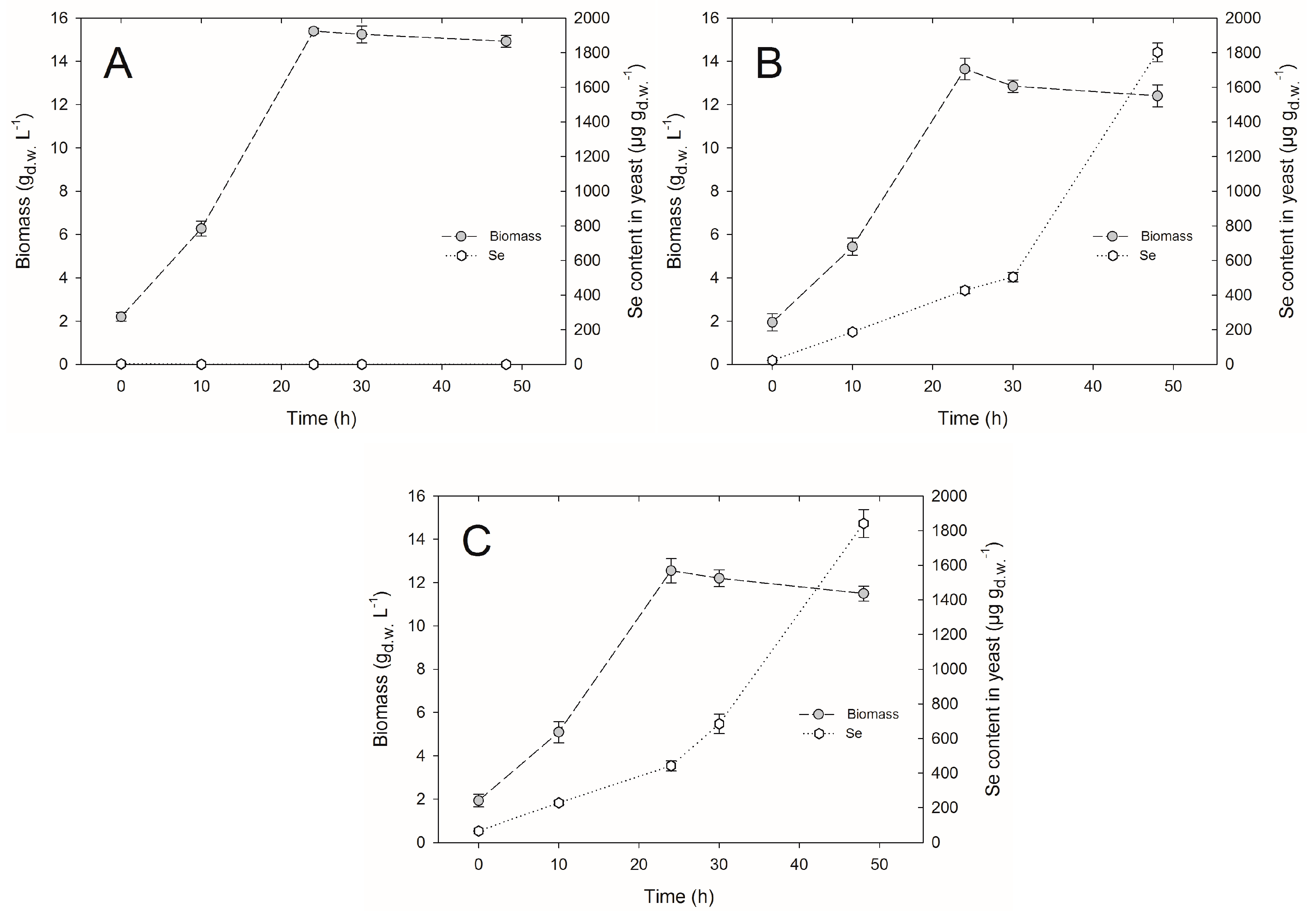
2.1. Yeast Cell Biomass Yield
2.2. Selenium Accumulation by Yeasts in Bioreactor Culture
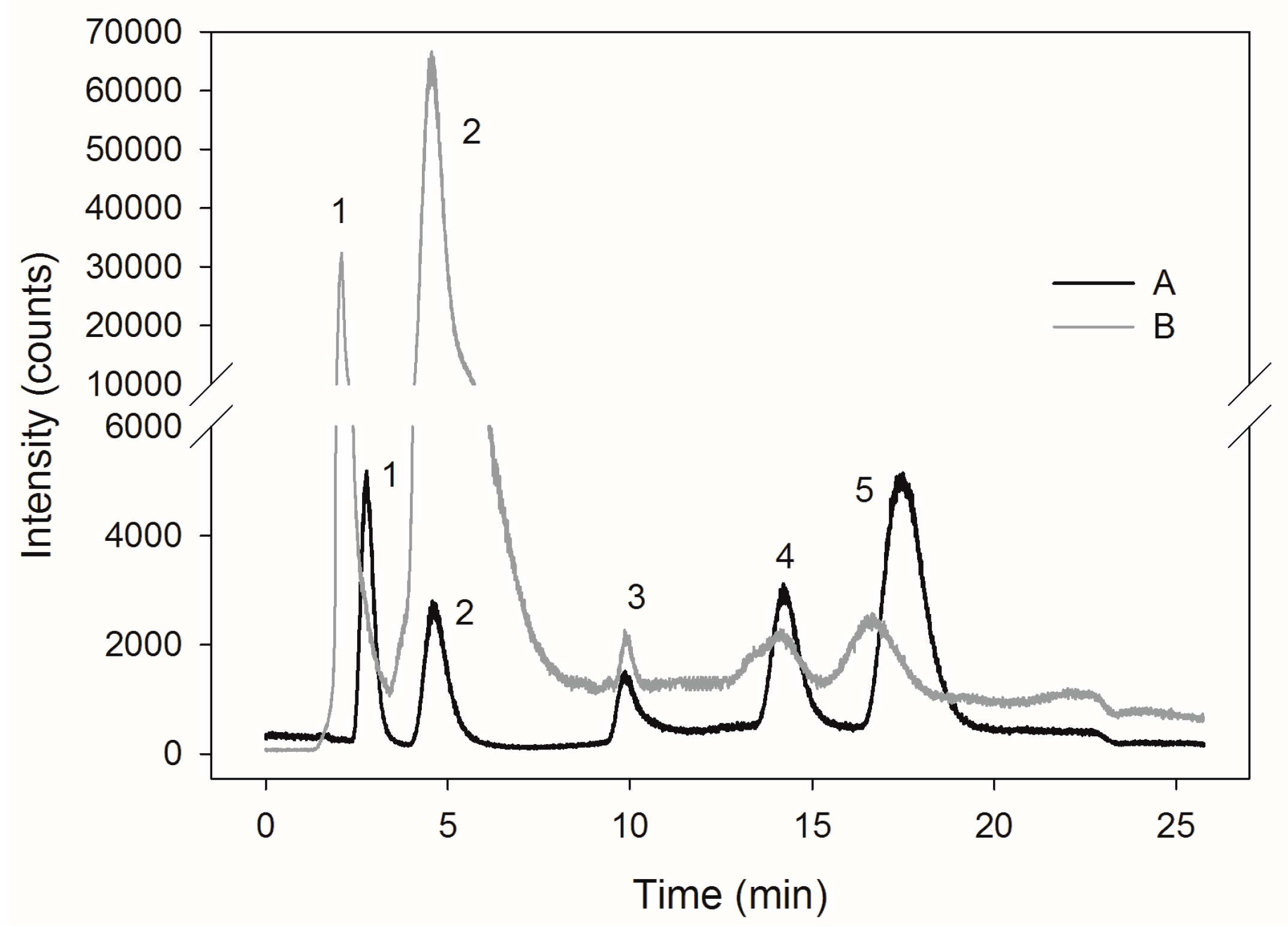
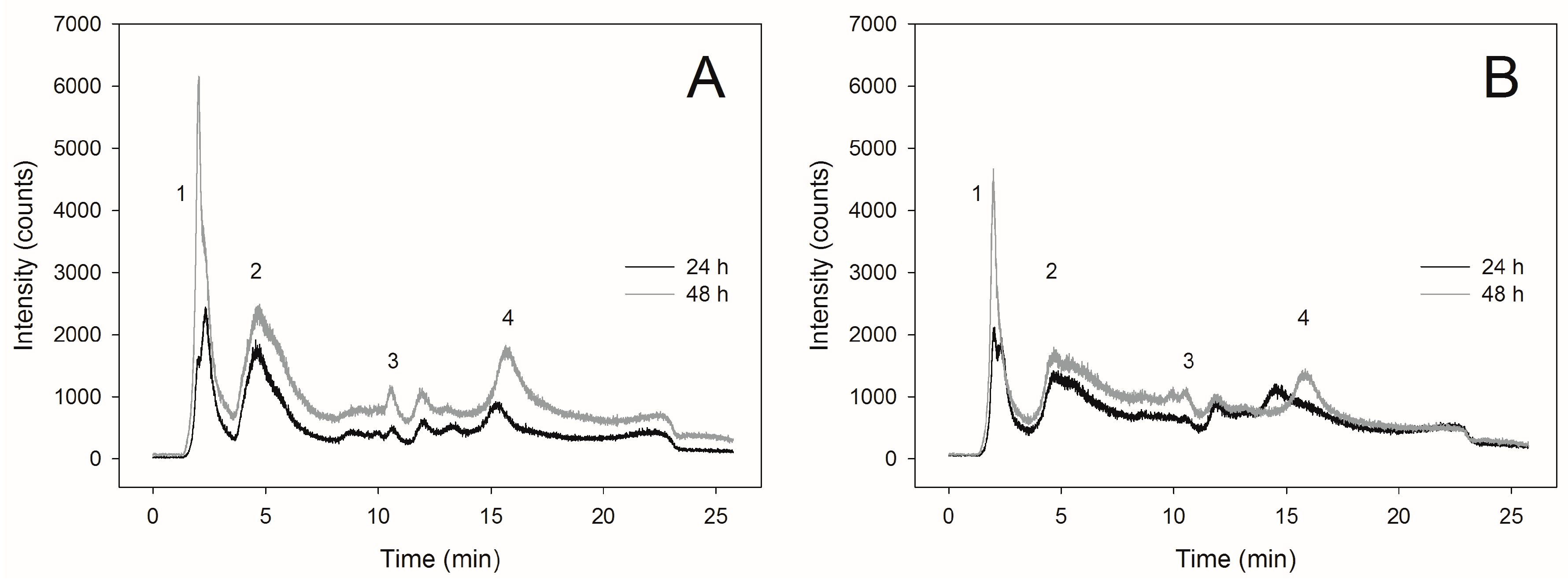
2.3. Selenite Transformation to SeMet in Yeast Cells
3. Materials and Methods
3.1. Biological Material
3.2. Microbiological Media
3.3. Yeasts Culture Using Bioreactor
3.4. Determination of Cell Biomass Yield
3.5. Determination of Selenium Content in Yeast Cell Biomass
3.6. Determination of SeMet Content
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Ruseva, B.; Himcheva, I.; Nankova, D. Importance of selenoproteins for the function of the thyroid gland. Medicine 2013, 3, 60–64. [Google Scholar]
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Egressy-Molnár, O.; Vass, A.; Németh, A.; García-Reyes, J.F.; Dernovics, M. Effect of sample preparation methods on the d,l-enantiomer ratio of extracted selenomethionine. Anal. Bioanal. Chem. 2011, 401, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Gladyshev, V.N. How selenium has altered our understanding of the genetic code. Mol. Cell Biol. 2002, 22, 3565–3576. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; Vanhaeckel, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Laffon, B.; Valdiglesias, V.; Pasaro, E.; Mendez, J. The organic selenium compound selenomethionine modulates bleomycin-induced DNA damage and repair in human leukocytes. Biol. Trace Elem. Res. 2010, 133, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Szpunar, J.; Yiannikouris, A.; Łobinski, R. Comprehensive speciation of selenium in selenium-rich yeast. TrAC Trends Anal. Chem. 2012, 41, 122–132. [Google Scholar] [CrossRef]
- Pedrero, Z.; Madrid, Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: A review. Anal. Chim. Acta 2009, 634, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Selenium yeast: Composition, quality, analysis and safety. Pure Appl. Chem. 2006, 78, 105–109. [Google Scholar] [CrossRef]
- Gharieb, M.M.; Gadd, G.M. Role of glutathione in detoxification of metal(loid)s by Saccharomyces cerevisiae. BioMetals 2004, 17, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, V.; Hillestrøm, P.R.; Kapolna, E.; Larsen, E.H.; Olsson, L. Metabolic and bioprocess engineering for production of selenized yeast with increased content of seleno-methylselenocysteine. Metab. Eng. 2011, 13, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Płaczek, M. Spectrophotometric evaluation of selenium binding by Saccharomyces cerevisiae ATCC MYA-2200 and Candida utilis ATCC 9950 yeast. J. Trace Elem. Med. Biol. 2016, 35, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, D.; Wei, G.; Liu, Z.; Ge, X. Selenium-enriched Candida utilis: Efficient preparation with l-methionine and antioxidant capacity in rats. J. Trace Elem. Med. Biol. 2013, 27, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A. Influence of selenium content inthe culture medium on protein profile of yeast cells Candida utilis ATCC 9950. Oxid. Med. Cell. Longev. 2015, 2015, 659750. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.; Wellinger, R.E. Yeast as a model system to study metabolic impact of selenium compounds. Microbial. Cell 2015, 2, 139–149. [Google Scholar] [CrossRef]
- Pérez-Corona, M.T.; Sánchez-Martínez, M.; Valderrama, M.J.; Rodríguez, M.E.; Cámara, C.; Madrid, Y. Selenium biotransformation by Saccharomyces cerevisiae and Saccharomyces bayanus during white wine manufacture: Laboratory-scale experiments. Food Chem. 2011, 124, 1050–1055. [Google Scholar]
- Yin, H.; Fan, G.; Gu, Z. Optimization of culture parameters of selenium-enriched yeast (Saccharomyces cerevisiae) by response surface methodology (RSM). LWT Food Sci. Technol. 2010, 43, 666–669. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kurcz, A. Effects selenium on morphological changes in Candida utilis ATCC 9950 yeast cells. Biol. Trace Elem. Res. 2016, 169, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Revanasiddappa, H.D.; Kiran Kumar, T.N. A facile spectrophotometric method for the determination of selenium. Anal. Sci. 2001, 17, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- El-Nasser Khattab, A.; Ihab, A.; Mohamed, K. Molecular analysis of genetically improved therapeutic Saccharomyces cerevisiae strains with high selenium uptake. J. Am. Sci. 2010, 6, 326–337. [Google Scholar]
- Kitajima, T.; Chiba, Y. Selenomethionine metabolism and its toxicity in yeast. Biomol. Concepts 2013, 4, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Qie, Y.; Wang, M.; Li, X.; Zhao, R. Selenomethionine: An effective selenium source for sow to improve Se distribution, antioxidant status, and growth performance of pig offspring. Biol. Trace Elem. Res. 2011, 142, 481–491. [Google Scholar] [CrossRef] [PubMed]
- McSheehy, S.; Pannier, F.; Szpunar, J.; Potin-Gautier, M.; Lobinski, R. Speciation of seleno compounds in yeast aqueous extracts by three-dimensional liquid chromatography with inductively coupled plasma mass spectrometric and electrospray mass spectrometric detection. Analyst 2002, 127, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.; Connolly, C.; Murphy, R. Accelerated determination of selenomethionine in selenized yeast: Validation of analytical method. Biol. Trace Elem. Res, 2013, 151, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, Z.; Encinar, J.R.; Madrid, Y.; Camará, C. Application of species-specific isotope dilution analysis to the correction for selenomethionine oxidation in Se-enriched yeast sample extracts during storage. J. Anal. At. Spectrom. 2007, 22, 1061–1066. [Google Scholar] [CrossRef]
- Arnaudguilhem, C.; Bierla, K.; Ouerdane, L.; Preud’homme, H.; Yiannikouris, A.; Łobiński, R. Selenium metabolomics in yeast using complementary reversed-phase/hydrophilic ion interaction (HILIC) liquid chromatography-electro-spray hybrid quadrupole trap/Orbitrap mass spectrometry. Anal. Chim. Acta 2012, 757, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ponce de León, C.A.; Bayoán, M.M.; Paquin, C. Selenium incorporation into Saccharomyces cerevisiae cells: A study of different incorporation methods. J. Appl. Microbiol. 2002, 92, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Uden, P.C.; Boakye, H.T.; Kahakachchi, C.; Tyson, J.F. Selective detection and identification of Se containing compounds—Review and recent development. J. Chromatogr. A 2004, 24, 85–93. [Google Scholar] [CrossRef]
- Ayouni, L.; Barbier, F.; Imbert, J.L.; Gauvrit, J.Y.; Lantéri, P.; Grenier-Loustalot, M.F. New separation method for organic and inorganic selenium compounds based on anion exchange chromatography followed by inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2006, 385, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Połatajko, A.; Banaś, B.; Ruiz Encinar, J.; Szpunar, J. Investigation of the recovery of selenomethionine from selenized yeast by two-dimensional LC–ICP MS. Anal. Bioanal. Chem. 2005, 381, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
| Selenium Concentration in the Medium | Culvitation Time (h) | |
|---|---|---|
| 24 h | 48 h | |
| 20 mg∙L−1 | 109.9 ± 5.62 *,c | 238.8 ± 9.52 d |
| 30 mg∙L−1 | 42.8 ± 4.43 a | 61.3 ± 5.07 b |
| HPLC ICP-MS | |
|---|---|
| Column | Hamilton PRP–X100, 10 µm × 250 mm × 4.1 mm |
| Column temperature | 23 °C |
| Flow | 1 mL·min−1 |
| Mobile phase | phase A: 5 mmol/mL ammonium acetate, pH 4.7 phase B: 150 mmol/mL ammonium acetate, pH 4.7 |
| Elution program | 0–4 min: 100% phase A 4–7 min: 0–100% phase B 7–25 min: 100% phase B |
| Split | 100 μL |
| Detector | ICP-MS |
| Determined isotope | Se 82 |
| Time of analysis | 25 min |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kieliszek, M.; Błażejak, S.; Kurek, E. Binding and Conversion of Selenium in Candida utilis ATCC 9950 Yeasts in Bioreactor Culture. Molecules 2017, 22, 352. https://doi.org/10.3390/molecules22030352
Kieliszek M, Błażejak S, Kurek E. Binding and Conversion of Selenium in Candida utilis ATCC 9950 Yeasts in Bioreactor Culture. Molecules. 2017; 22(3):352. https://doi.org/10.3390/molecules22030352
Chicago/Turabian StyleKieliszek, Marek, Stanisław Błażejak, and Eliza Kurek. 2017. "Binding and Conversion of Selenium in Candida utilis ATCC 9950 Yeasts in Bioreactor Culture" Molecules 22, no. 3: 352. https://doi.org/10.3390/molecules22030352






