Polysaccharide-Based Edible Coatings Containing Cellulase for Improved Preservation of Meat Quality during Storage
Abstract
:1. Introduction
2. Results and Discussion
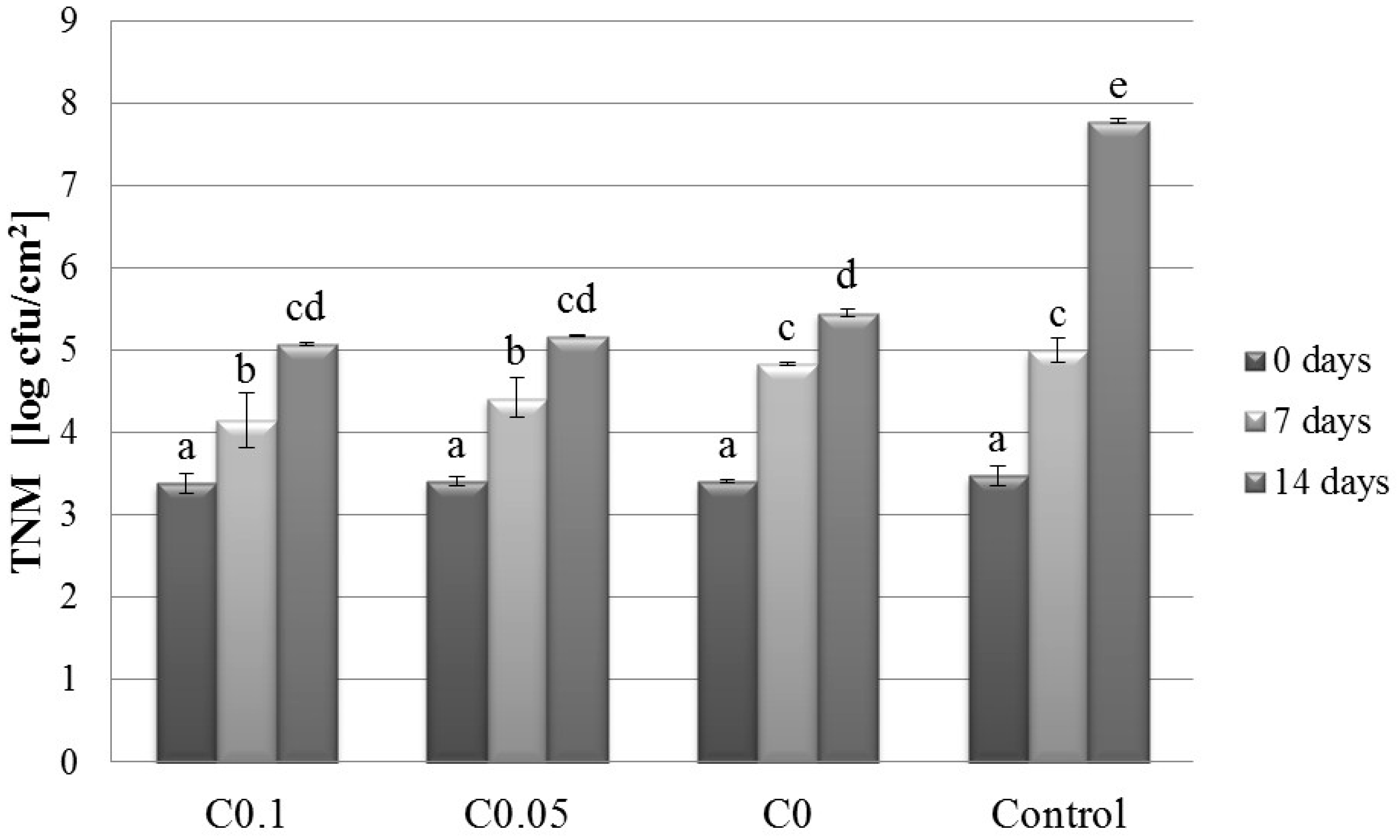
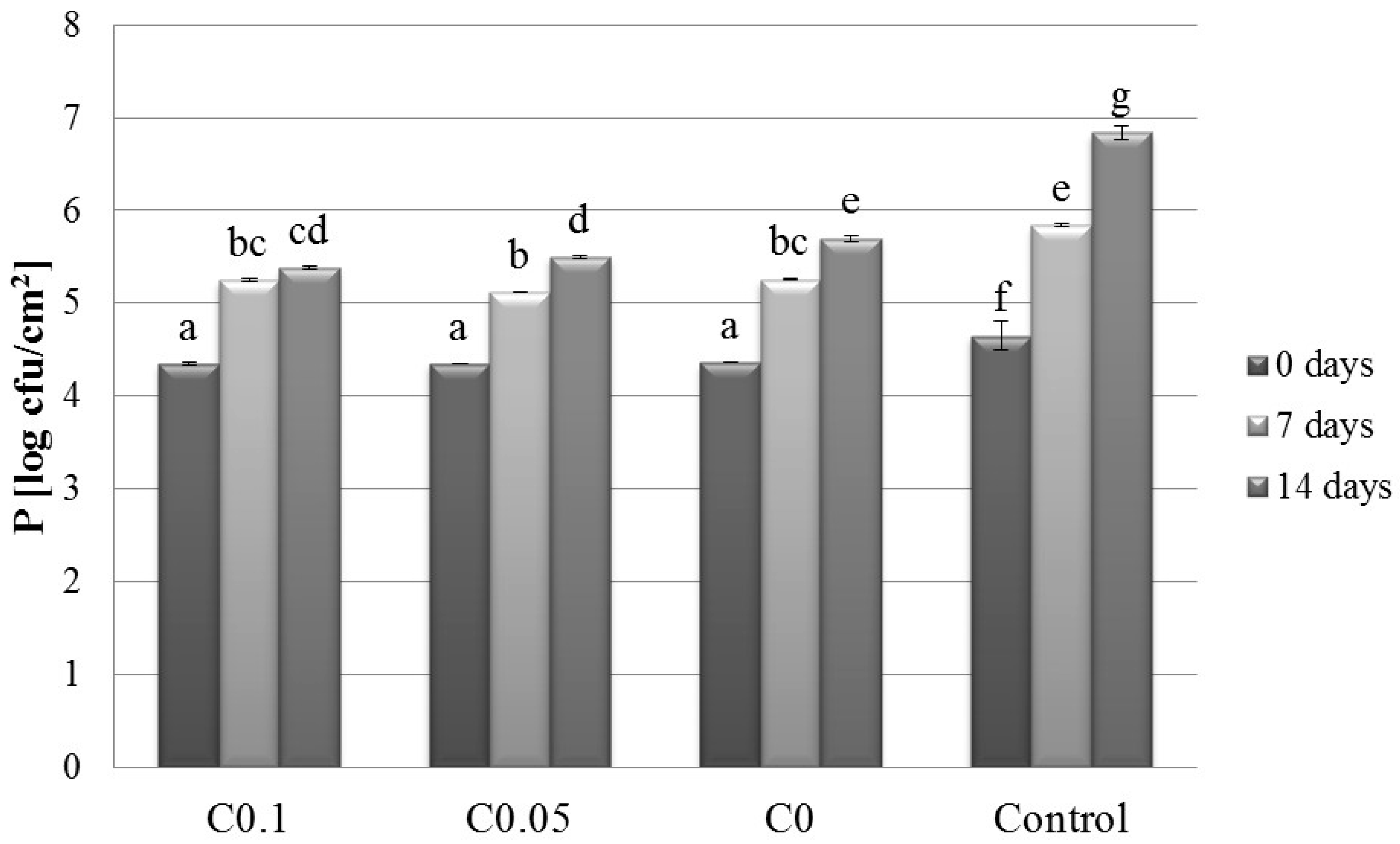
2.1. Microbiology
2.2. Physico-Chemical Parameters
3. Materials and Methods
3.1. Materials
3.2. Coat-Forming Solution
3.3. Meat Sampling
3.4. Microbial Sampling
3.4.1. Total Number of Microorganisms of Meat Sample Covered by Hydrosol
3.4.2. Psychrotrophs
3.4.3. Number of Yeast and Mold
3.5. Colour Measurement
3.6. Thiobarbituric Acid Reactive Substances (TBARS) Measurement
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khan, M.I.; Adrees, M.N.; Tariq, M.R.; Sohaib, M. Application of edible coating for improving meat quality: A review. Pakistan J. Food Sci. 2013, 23, 71–79. [Google Scholar]
- Huang, G.Q.; Sun, Y.T.; Xiao, J.X.; Yang, J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012, 135, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-F.; Kong, B.-H. Extending shelf life of chilled pork by combination of chitosan coatings with spice extracts. J. Northeast. Agric. Univ. 2008, 15, 33–37. [Google Scholar]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Effects of chitosan on shelf life of ready to cook meat products during chilled stored. LWT-Food Sci. Technol. 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Jarmoluk, A. The use of chitosan, lysozyme, and the nano-silver as antimicrobial ingredients of edible protective hydrosols applied into the surface of meat. J. Food Sci. Technol. 2015, 52, 5996–6002. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jia, Z.; Huang, J.; Zhang, C. Preparation of Low Molecular Weight Chitosan by Complex Enzymes Hydrolysis. Int. J. Chem. 2011, 3, 180–186. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Tavaria, F.K.; Soares, J.C.; Ramos, O.S.; Monteiro, M.J.; Pintado, M.E.; Xavier Malcata, F. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol. 2008, 25, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurnaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Ambrozik-Haba, J.; Kulig, D.; Jarmoluk, A. Modification Effect of Cellulase on the Physicochemical Characteristic of Polysaccharides Edible Films. Int. J. Polym. Sci. 2015, 184616. [Google Scholar] [CrossRef]
- Malinowska-Pańczyk, E.; Kołodziejska, I. The influence of moderate pressure and subzero temperature on the shelf life of minced cod, salmon, pork and beef meat. Food Technol. Biotechnol. 2013, 51, 570–576. [Google Scholar]
- Zhao, F.; Zhou, G.; Ye, K.; Wang, S.; Xu, X.; Li, C. Microbial changes in vacuum-packed chilled pork during storage. Meat Sci. 2015, 100, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, S.; Board, R.; Roller, S. Chitosan inhibits growth of spoilage microorganisms in chilled pork products. Food Microbiol. 2002, 19, 175–182. [Google Scholar] [CrossRef]
- Tsai, G.J.; Su, W.H.; Chen, H.C.; Pan, C.L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Gardrat, C.; Castellan, A.; Coma, V.; Jarmoluk, A. The use of lysozyme to prepare biologically active chitooligomers. Polimeros 2015, 25, 35–41. [Google Scholar] [CrossRef]
- Caldara, F.R.; Santos, L.S.; Nieto, V.M.O.; Foppa, L.; Santos, R.K.S.; Paz, I.C.L.A.; Garcia, R.G.; Nääs, I.A. Microbiological growth in normal and PSE pork stored under refrigeration. Rev. Bras. Saúde Prod. Anim. 2014, 15, 459–469. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. Antimicrobial and antioxidative strategies to reduce pathogens and extend the shelf life of fresh red meats. Comp. Rev. Food Sci. Food Saf. 2012, 11, 340–354. [Google Scholar] [CrossRef]
- Chantarasataporn, P.; Tepkasikul, P.; Kingcha, Y.; Yoksan, R.; Pichyangkura, R.; Visessanguan, W.; Chirachanchai, S. Water-based oligochitosan and nanowhisker chitosan as potential food preservatives for shelf-life extension of minced pork. Food Chem. 2014, 159, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Leistner, L.; Gould, G.W. Hurdle Technologies: Combination Treatment for Food Stability, Safety and Quality; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Gill, A.O.; Gill, C.O. Preservative packaging for fresh meats, poultry, and fin fish. In Innovations in Food Packaging; Han, J.H., Ed.; Elsevier Academic: New York, NY, USA, 2005; pp. 204–226. [Google Scholar]
- Egan, A.F.; Eustace, I.J.; Shay, B.J. Meat packaging—Maintaining the quality and prolonging the storage life of chilled beef, pork and lamb. In Proceedings of the 34th International Congress of Meat Science and Technology, Brisbane, Australia, 1998; Volume 34, pp. 68–75.
- Garcia-Lopez, M.L.; Prieto, M.; Otero, A. The physiological attributes of Gram-negative bacteria associated with spoilage of meat and meat products. In The microbiology of Meat and Poultry; Davies, A., Board, R., Eds.; Blackie Academic and Professional: London, UK, 1998; pp. 1–34. [Google Scholar]
- Araújo, L.F.; Oliveira, L.S.C.; Perazzo Neto, A.; Alsina, O.L.S.; Silva, F.L.H. Equilíbriohigroscópico da palma forrageira: Relação com a umidade ótima parafermentação sólida. Rev. Bras. Eng. Agríc. Ambient 2005, 9, 379–384. [Google Scholar] [CrossRef]
- Leistner, L. Microbiology of ready-to-serve foods. Die Fleischwirtsch. 1978, 58, 2008–2011. [Google Scholar]
- Grajales-Lagunes, A.; Rivera-Bautista, C.; Ruiz-Cabrera, M.; Gonzalez-Garcia, R.; Ramirez-Telles, J.; Abud-Archila, M. Effect of lactic acid on the meat quality properties and the taste of pork Serratus ventralis muscle. Agric. Food Sci. 2012, 21, 171–181. [Google Scholar]
- Nerín, C.; Tovar, L.; Djenane, D.; Camo, J.; Salafranca, J.; Beltrán, J.A.; Roncalés, P. Stabilization of beef meat by a new active packaging containing natural antioxidants. J. Agric. Food Chem. 2006, 52, 5598–5605. [Google Scholar]
- Jo, C.; Lee, J.W.; Lee, K.H.; Byun, M.W. Quality properties of pork sausage prepared with water-soluble chitosan oligomer. Meat Sci. 2001, 59, 369–375. [Google Scholar] [CrossRef]
- Zahran, D.A. Effectiveness of Gamma Irradiated Chitosan for Fresh Meat Preservation. Arab J. Nucl. Sci. Appl. 2015, 48, 143–152. [Google Scholar]
- Mokhtar, S.M.; Youssef, K.M.; Morsy, N.E. The effects of natural antioxidants on colour, lipid stability and sensory evaluation of fresh beef patties stored at 4 °C. J. Agroaliment. Process. Technol. 2014, 20, 282–292. [Google Scholar]
- Park, P.J.; Je, J.Y.; Kim, S.K. Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr. Polym. 2004, 55, 17–22. [Google Scholar] [CrossRef]
- ISO 18593:2004. Microbiology of Food and Animal Feeding Stuffs; Horizontal Methods for Sampling Techniques from Surfaces Using Contact Plates and Swabs; ISO: London, UK, 2004. [Google Scholar]
- ISO 17604:2003. Microbiology of Food and Animal Feeding Stuffs; Carcass Sampling for Microbiological Analysis; ISO: London, UK, 2003. [Google Scholar]
- ISO 2293:1988. Meat and Meat Products. Enumeration of Microorganisms; Colony Count Technique at 30 °C; ISO: London, UK, 1988. [Google Scholar]
- ISO 17410:2001. Microbiology of Food and Animal Feeding Stuffs; Horizontal Method for the Enumeration of Psychrotrophic Microorganisms; ISO: London, UK, 2001. [Google Scholar]
- ISO 215271:2008. Microbiology of Food and Animal Feeding Stuffs; Horizontal Method for the Enumeration of Yeasts and Molds. Part 1: Colony count Technique in Products with Water Activity Greater than 0.95; ISO: London, UK, 2008. [Google Scholar]
- Sample Availability: Not available.

| Interaction Effects C × T * (% × Days) | Parameters | |||||
|---|---|---|---|---|---|---|
| pH | aw | L* | a* | b* | TBARS # (mg MDA/kg) | |
| C0T0 | 5.47 ± 0.01 ab | 0.925 ± 0.001 b | 53.91 ± 0.34 ac | 6.89 ± 0.62 abc | 4.82 ± 0.20 ab | 0.26 ± 0.05 a |
| C0.05T0 | 5.42 ± 0.02 e | 0.926 ± 0.001 b | 52.78 ± 0.40 a | 5.76 ± 0.65 ab | 3.71 ± 0.55 af | 0.25 ± 0.01 a |
| C0.1T0 | 5.43 ± 0.01 e | 0.925 ± 0.001 b | 51.89 ± 1.82 a | 6.20 ± 0.09 ab | 2.77 ± 1.01 f | 0.26 ± 0.01 a |
| C0T7 | 5.48 ± 0.01 d | 0.921 ± 0.001 a | 59.45 ± 0.30 bd | 5.47 ± 0.33 a | 8.23 ± 0.40 de | 0.68 ± 0.02 d |
| C0.05T7 | 5.51 ± 0.01 ac | 0.921 ± 0.000 a | 55.13 ± 1.43 ac | 6.22 ± 0.21 ab | 4.86 ± 0.80 ab | 0.66 ± 0.01 c |
| C0.1T7 | 5.54 ± 0.01 b | 0.919 ± 0.000 d | 52.61 ± 0.56 a | 8.28 ± 0.54 c | 7.13 ± 0.29 cde | 0.53 ± 0.01 b |
| C0T14 | 5.47 ± 0.01 ac | 0.923 ± 0.000 c | 60.61 ± 1.04 d | 7.37 ± 0.30 bc | 8.85 ± 0.17 e | 1.28 ± 0.01 h |
| C0.05T14 | 5.50 ± 0.02 c | 0.922 ± 0.000 a | 56.90 ± 0.76 bc | 6.85 ± 0.62 ab | 5.95 ± 0.92 bc | 1.08 ± 0.21 g |
| C0.1T14 | 5.52 ± 0.01 abc | 0.921 ± 0.001 a | 59.63 ± 2.02 bd | 6.30 ± 0.49 ab | 6.83 ± 0.63 cd | 1.05 ± 0.02 f |
| Control (meat) T0 | 5.52 ± 0.00 ab | 0.926 ± 0.000 b | 58.62 ± 0.06 bd | 8.57 ± 1.21 c | 0.95 ± 0.32 g | 0.26 ± 0.01 a |
| Control (meat) T7 | 5.52 ± 0.01 abc | 0.922 ± 0.001 ac | 56.31 ± 0.77 bc | 5.69 ± 0.11 ab | 5.02 ± 0.24 ab | 0.97 ± 0.01 e |
| Control (meat) T14 | 5.54 ± 0.00 b | 0.921 ± 0.000 a | 38.01 ± 1.14 e | 3.68 ± 0.20 d | 7.46 ± 0.27 cde | 1.54 ± 0.01 i |
| Variants Coding * | Variables | Constant Factors | |||||
|---|---|---|---|---|---|---|---|
| Cellulase (%) | Time (Days) | Chitosan (%) | HPMC (%) | Glycerol (%) | Lactic Acid (%) | Sample | |
| C0T0 | 0 | 0 | 1 | 1 | 25 | 0.25 | Meat slice |
| C0.05T0 | 0.05 | 0 | 1 | 1 | 25 | 0.25 | Meat slice |
| C0.1T0 | 0.1 | 0 | 1 | 1 | 25 | 0.25 | Meat slice |
| C0T7 | 0 | 7 | 1 | 1 | 25 | 0.25 | Meat slice |
| C0.05T7 | 0.05 | 7 | 1 | 1 | 25 | 0.25 | Meat slice |
| C0.1T7 | 0.1 | 7 | 1 | 1 | 25 | 0.25 | Meat slice |
| C0T14 | 0 | 14 | 1 | 1 | 25 | 0.25 | Meat slice |
| C0.05T14 | 0.05 | 14 | 1 | 1 | 25 | 0.25 | Meat slice |
| C0.1T14 | 0.1 | 14 | 1 | 1 | 25 | 0.25 | Meat slice |
| Control (meat) T0 | - | 0 | - | - | - | - | Meat slice |
| Control (meat) T7 | - | 7 | - | - | - | - | Meat slice |
| Control (meat) T14 | - | 14 | - | - | - | - | Meat slice |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimoch-Korzycka, A.; Jarmoluk, A. Polysaccharide-Based Edible Coatings Containing Cellulase for Improved Preservation of Meat Quality during Storage. Molecules 2017, 22, 390. https://doi.org/10.3390/molecules22030390
Zimoch-Korzycka A, Jarmoluk A. Polysaccharide-Based Edible Coatings Containing Cellulase for Improved Preservation of Meat Quality during Storage. Molecules. 2017; 22(3):390. https://doi.org/10.3390/molecules22030390
Chicago/Turabian StyleZimoch-Korzycka, Anna, and Andrzej Jarmoluk. 2017. "Polysaccharide-Based Edible Coatings Containing Cellulase for Improved Preservation of Meat Quality during Storage" Molecules 22, no. 3: 390. https://doi.org/10.3390/molecules22030390






