Determination of the Clean Air Delivery Rate (CADR) of Photocatalytic Oxidation (PCO) Purifiers for Indoor Air Pollutants Using a Closed-Loop Reactor. Part II: Experimental Results
Abstract
:1. Introduction
2. Model
3. Experimental Methods
3.1. Recirculation Closed-Loop System
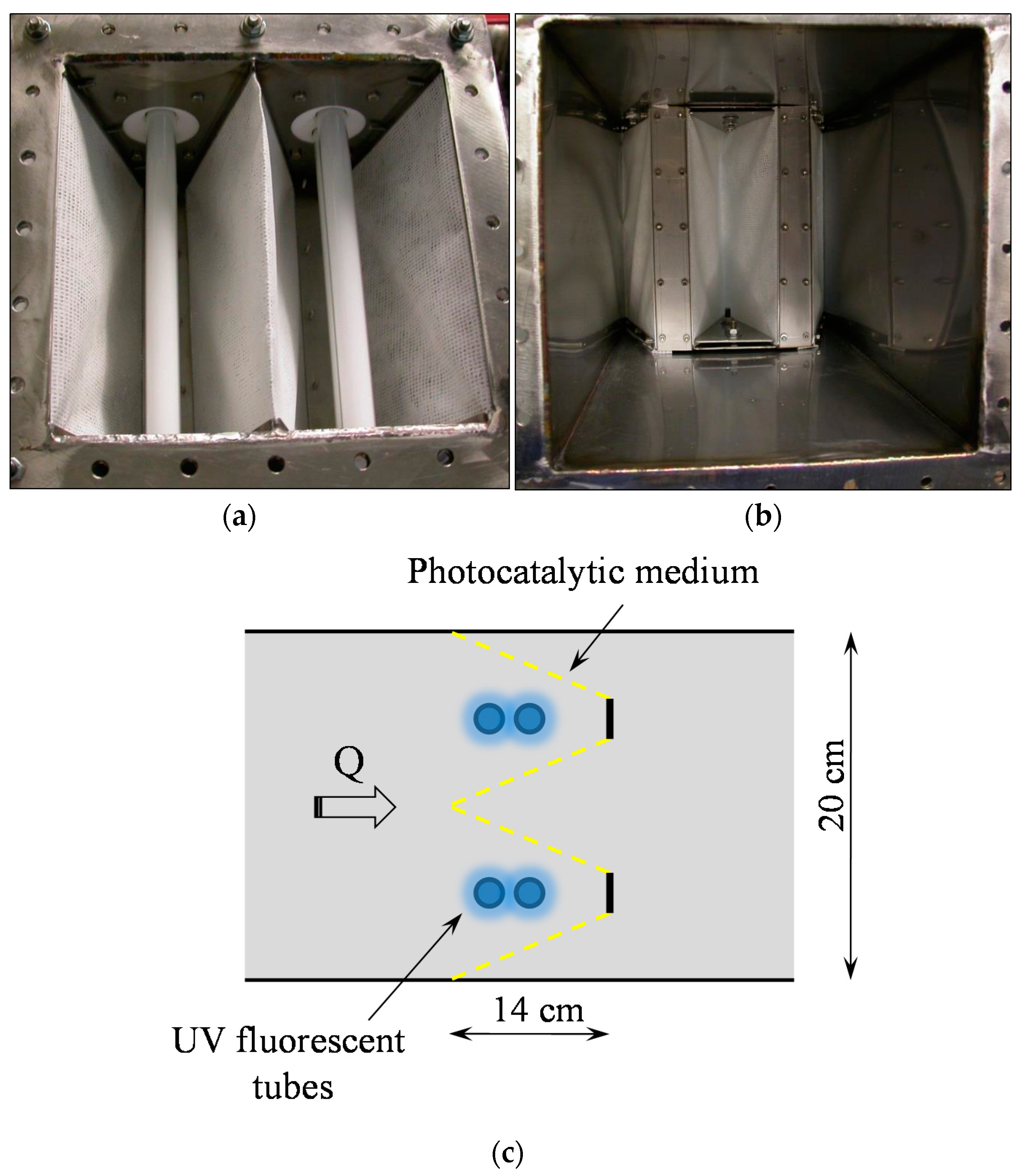
3.2. PCO Device
3.3. VOC Generation and Analytical Methods
3.4. Operating Conditions
4. Results and Discussion
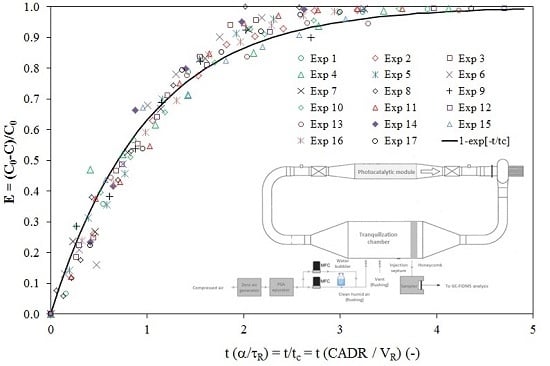
4.1. CADR Determination
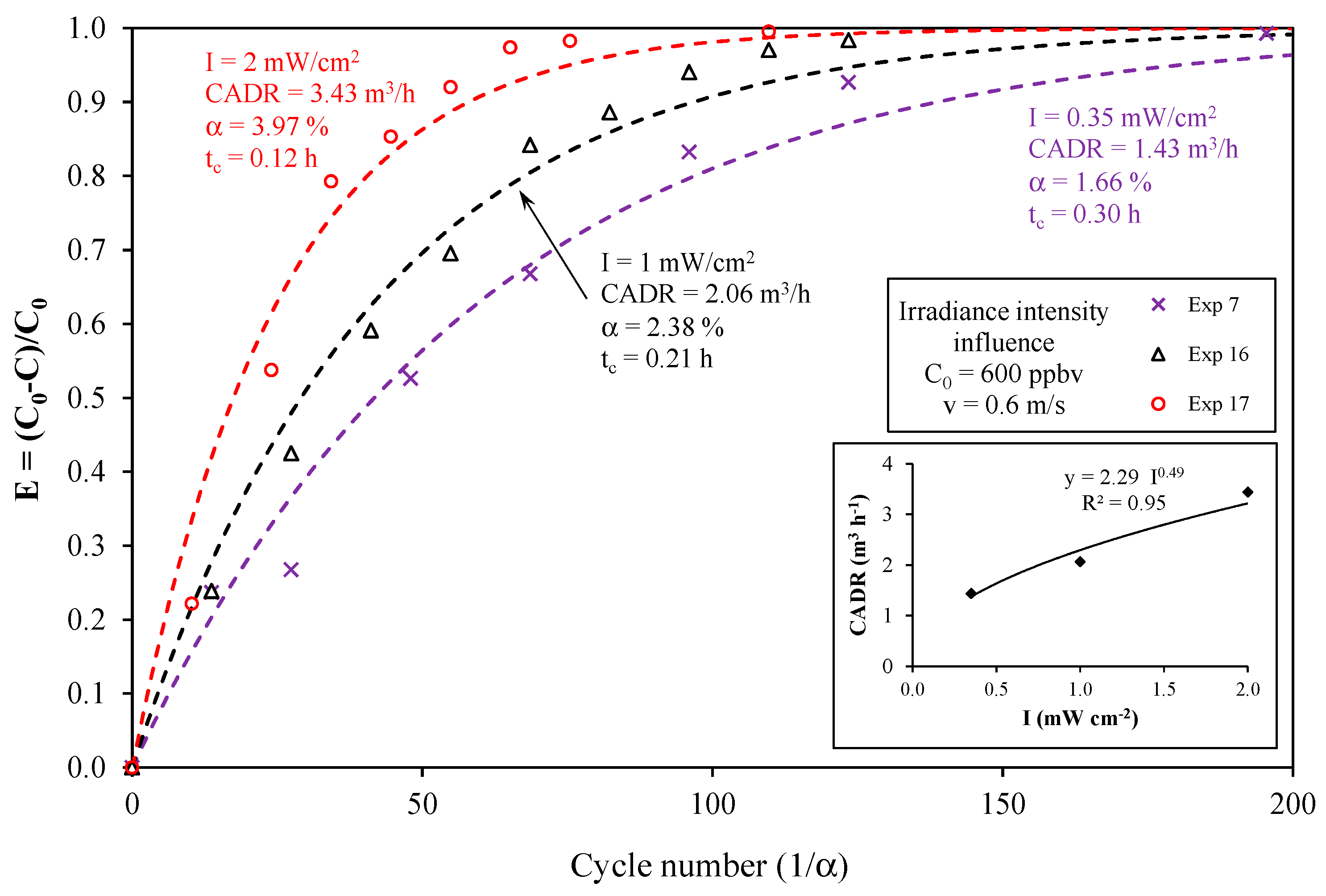
4.2. Effect of the Light Irradiance Intensity
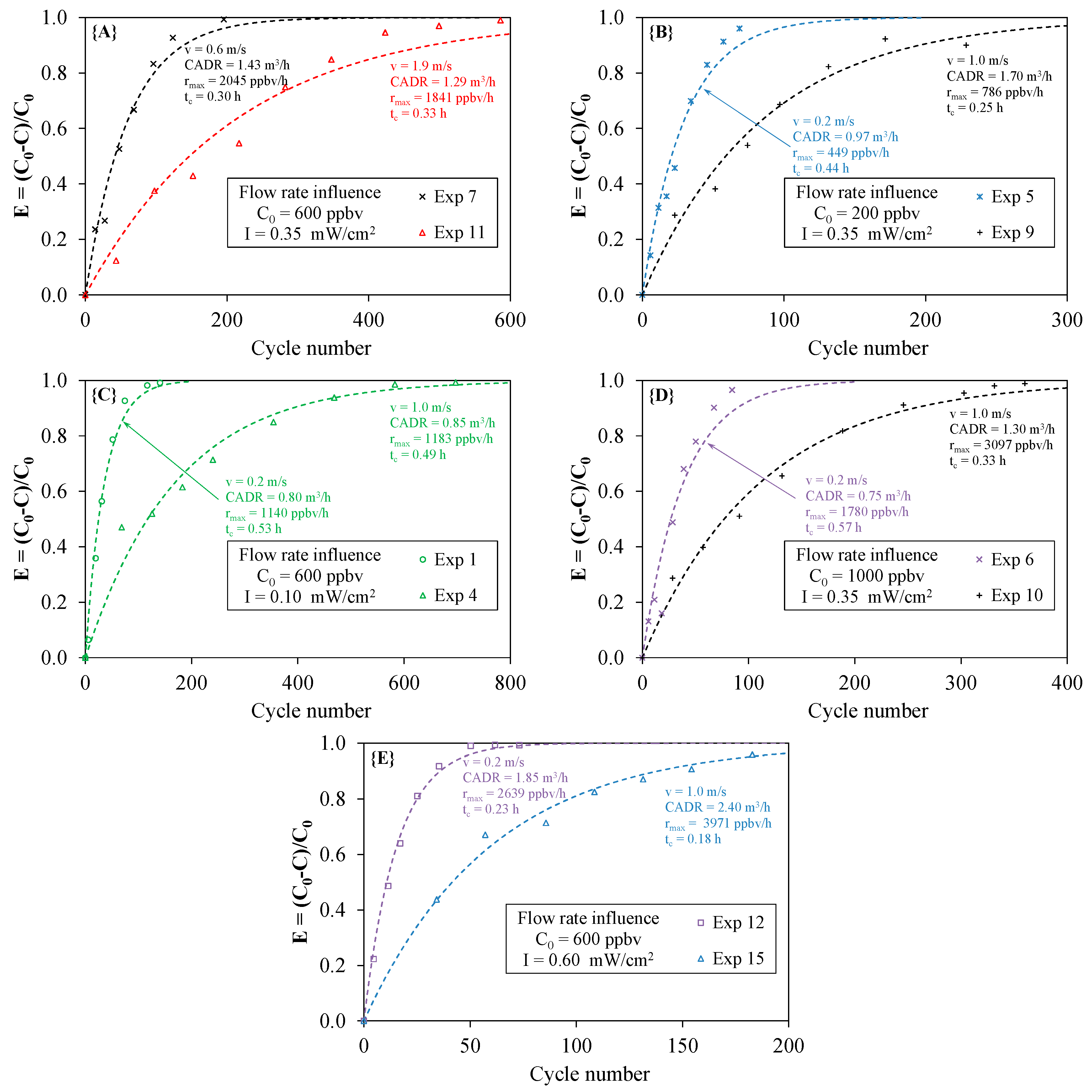
4.3. Effect of the Flow Rate
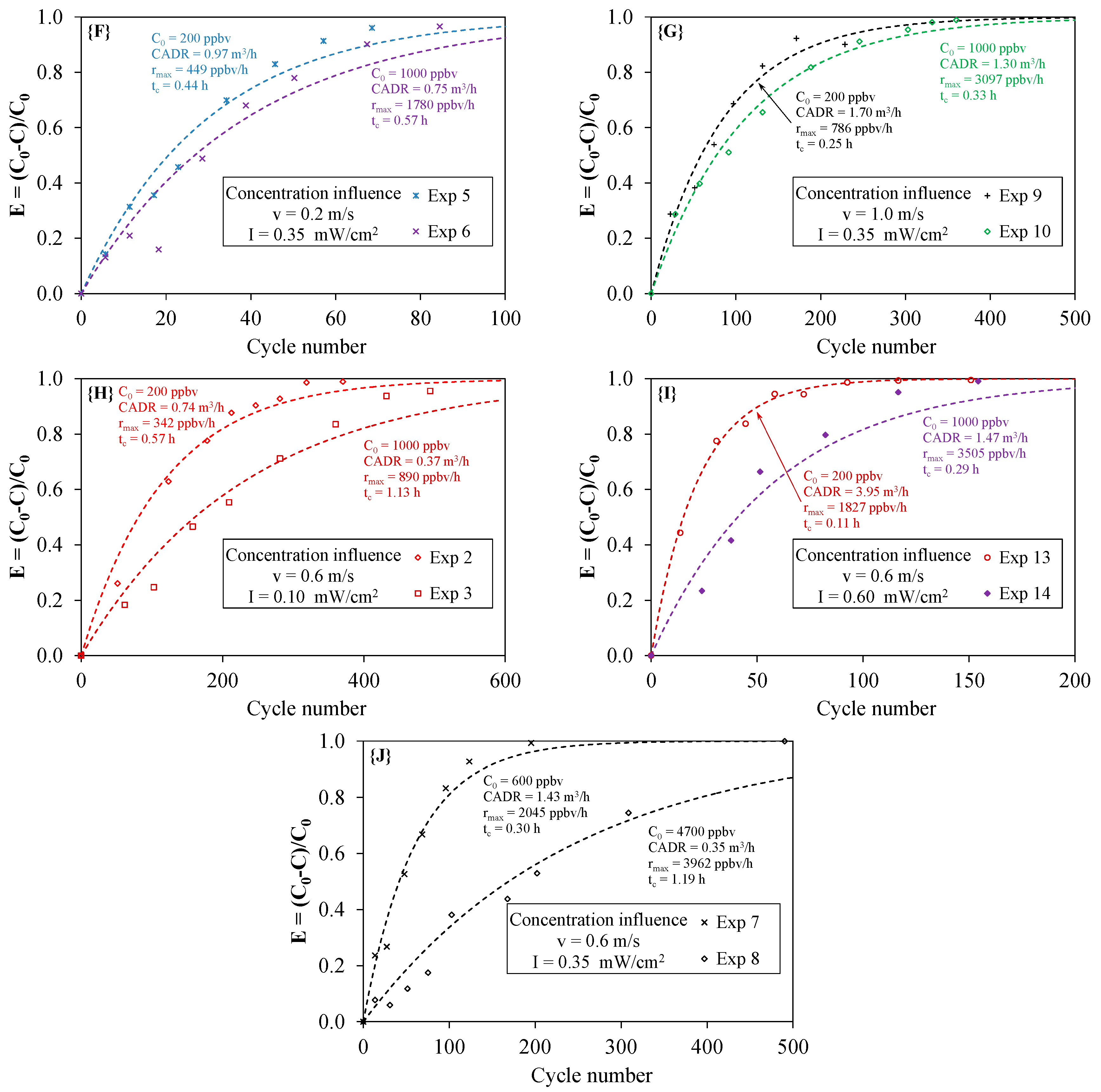
4.4. Effect of the Initial Concentration
5. Conclusions
- (i)
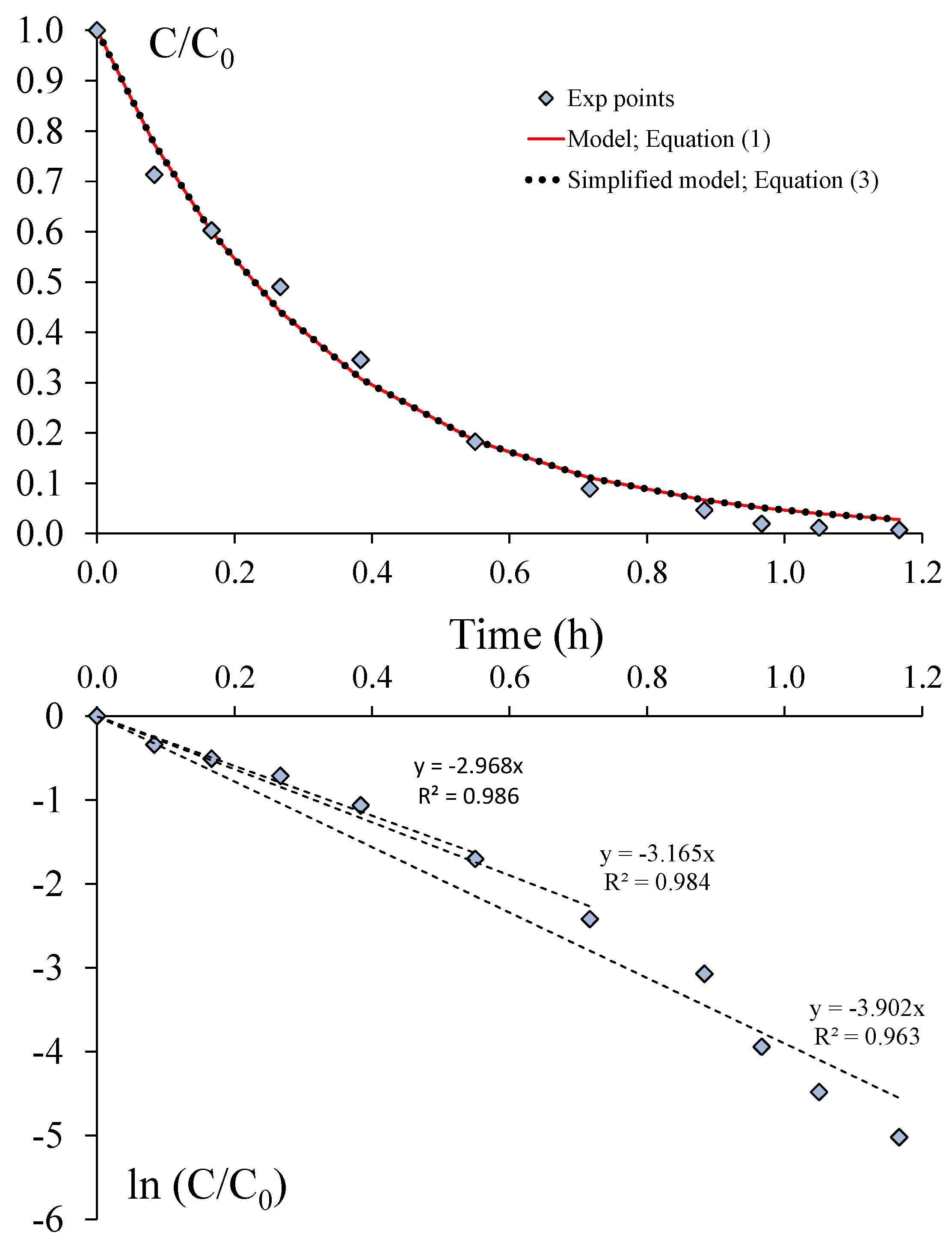
- Since the volume of the PCO device is very small in relation to the volume of the reservoir, the ratio of the residence times (τP/τR) tends to zero, and consequently, the concentration-time relationship given by Equation (1) can be used to model the experimental points. Moreover, as the term α is usually determined to be lower than 5%, the simplified model characterized by a first-order decay model (Equation (3)) can be used to determine the CADR of the PCO device.
- (ii)
- Since the Excel® solver is easily usable for data analysis, such a tool should be preferred for CADR determination rather than the analysis of the curve ln(C/C0) vs. time, which involves selecting a given number of points for a correct calculation.
- (iii)
- According to the operating conditions, the CADR ranged from 0.35 to 3.95 m3·h−1, i.e., one order of magnitude for the same PCO device. The CADR was mainly dependent on the light irradiance intensity. An increase in the CADR with the square root of the light irradiance was observed.
- (iv)
- Although the CADR of the PCO device inserted in the closed-loop reactor did not theoretically depend on the flow rate (see Part I), the experimental results did not enable the confirmation of this point. The results were contrasting. Some experimental data were in agreement with the theory, whereas others disagreed. Further investigations are therefore needed to explain this ambiguity. Numerical simulations of the air stream line and velocity through the medium may be useful.
- (v)
- The maximum degradation rate rmax ranged from 342 to 4894 ppbv·h−1. As the initial concentration is one parameter influencing the degradation rate of the pollutant, tests should be performed at a given value of the initial concentration in order to compare the performances of different types of PCO apparatus.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| A | cross-sectional area of the PCO device (m2) |
| C | pollutant concentration (mol·m−3) |
| CADR | Clean Air Delivery Rate (m3·s−1) |
| E | efficiency (dimensionless) |
| k | overall degradation rate constant (s−1) |
| L | length of the PCO device (m) |
| Q | flow rate (m3·s−1) |
| r | degradation rate (mol m−3·s−1) |
| t | time (s) |
| tc | time constant of the closed-loop reactor (s) |
| VP | PCO device volume (m3) |
| VR | reservoir volume (m3) |
| Greek letters | |
| τP | residence time in the PCO device (s) |
| τR | residence time in the reservoir (s) |
| α | fractional yield of the treated flow rate (dimensionless) |
References
- Destaillats, H.; Sleiman, M.; Sullivan, D.P.; Jacquiod, C.; Sablayrolles, J.; Molins, L. Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions. Appl. Catal. B Environ. 2012, 128, 159–170. [Google Scholar] [CrossRef]
- Pichat, P. Some views about indoor air photocatalytic treatment using TiO2: Conceptualization of humidity effects, active oxygen species, problem of C1–C3 carbonyl pollutants. Appl. Catal. B Environ. 2010, 99, 428–434. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Photocatalytic air cleaners and materials technologies—Abilities and limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Costarramone, N.; Kartheuser, B.; Pecheyran, C.; Pigot, T.; Lacombe, S. Efficiency and harmfulness of air-purifying photocatalytic commercial devices: From standardized chamber tests to nanoparticles release. Catal. Today 2015, 252, 35–40. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Walker, A.T.S.; Wragg, A.A. The modelling of concentration—time relationships in recirculating electrochemical reactor systems. Electrochim. Acta 1977, 22, 1129–1134. [Google Scholar] [CrossRef]
- Ginestet, A. Development and Evaluation of a New Test Method for Portable air cleaners—CR15_New Test Method for Portable Air Cleaners. Avaiable Online: http://www.aivc.org/sites/default/files/members_area/medias/pdf/CR/CR15_New%20test%20method%20for%20portable%20air%20cleaners.pdf (accessed on 17 September 2016).
- Zhang, Y.; Mo, J.; Li, Y.; Sundell, J.; Wargocki, P.; Zhang, J.; Little, J.C.; Corsi, R.; Deng, Q.; Leung, M.H.K.; et al. Can commonly-used fan-driven air cleaning technologies improve indoor air quality? A literature review. Atmos. Environ. 2011, 45, 4329–4343. [Google Scholar] [CrossRef]
- Batault, F.; Héquet, V.; Raillard, C.; Thévenet, F.; Locoge, N.; le Coq, L. How chemical and physical mechanisms enable the influence of the operating conditions in a photocatalytic indoor air treatment device to be modeled. Chem. Eng. J. 2017, 307, 766–775. [Google Scholar] [CrossRef]
- Obee, T.N. Photooxidation of Sub-Parts-per-Million Toluene and Formaldehyde Levels on Titania Using a Glass-Plate Reactor. Environ. Sci. Technol. 1996, 30, 3578–3584. [Google Scholar] [CrossRef]
- Moulis, F.; Krýsa, J. Photocatalytic degradation of several VOCs (n-hexane, n-butyl acetate and toluene) on TiO2 layer in a closed-loop reactor. Catal. Today 2013, 209, 153–158. [Google Scholar] [CrossRef]
- Peral, J.; Ollis, D.F. Heterogeneous photocatalytic oxidation of gas-phase organics for air purification: Acetone, 1-butanol, butyraldehyde, formaldehyde, and m-xylene oxidation. J. Catal. 1992, 136, 554–565. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Photocatalysis fundamentals revisited to avoid several misconceptions. Appl. Catal. B Environ. 2010, 99, 461–468. [Google Scholar] [CrossRef]
- Puma, G.L.; Salvadó-Estivill, I.; Obee, T.N.; Hay, S.O. Kinetics rate model of the photocatalytic oxidation of trichloroethylene in air over TiO2 thin films. Separ. Purif. Technol. 2009, 67, 226–232. [Google Scholar] [CrossRef]
- Raillard, C.; Maudhuit, A.; Héquet, V.; le Coq, L.; Sablayrolles, J.; Molins, L. Use of Experimental Designs to Establish a Kinetic Law for a Gas Phase Photocatalytic Process. Int. J. Chem. React. Eng. 2014, 12, 113–122. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Modeling and validation of a photocatalytic oxidation reactor for indoor environment applications. Chem. Eng. Sci. 2011, 66, 5945–5954. [Google Scholar] [CrossRef]
- Sample Availability: Not available.



| Experimental Conditions | Experimental Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I (mW·cm−²) | v (m·s−1) | τR (s) | C0 (ppbv) | α (−) | R2 | CADR (m3·h−1) | 1/α (−) | tc (h) | tc (s) | rmax (ppbv·h−1) | |
| Exp 1 | 0.10 | 0.2 | 52.5 | 600 | 0.0278 | 0.988 | 0.80 | 36 | 0.53 | 1917 | 1140 |
| Exp 2 | 0.10 | 0.6 | 17.5 | 200 | 0.0086 | 0.973 | 0.74 | 117 | 0.57 | 2052 | 342 |
| Exp 3 | 0.10 | 0.6 | 17.5 | 1000 | 0.0043 | 0.956 | 0.37 | 232 | 1.13 | 4065 | 890 |
| Exp 4 | 0.10 | 1.0 | 10.5 | 600 | 0.0059 | 0.937 | 0.85 | 169 | 0.49 | 1781 | 1183 |
| Exp 5 | 0.35 | 0.2 | 52.5 | 200 | 0.0337 | 0.969 | 0.97 | 30 | 0.44 | 1584 | 449 |
| Exp 6 | 0.35 | 0.2 | 52.5 | 1000 | 0.0259 | 0.927 | 0.75 | 39 | 0.57 | 2055 | 1780 |
| Exp 7 | 0.35 | 0.6 | 17.5 | 600 | 0.0166 | 0.971 | 1.43 | 60 | 0.30 | 1062 | 2045 |
| Exp 8 | 0.35 | 0.6 | 17.5 | 4700 | 0.0041 | 0.964 | 0.35 | 244 | 1.19 | 4273 | 3962 |
| Exp 9 | 0.35 | 1.0 | 10.5 | 200 | 0.0118 | 0.961 | 1.70 | 85 | 0.25 | 901 | 786 |
| Exp 10 | 0.35 | 1.0 | 10.5 | 1000 | 0.0090 | 0.984 | 1.30 | 111 | 0.33 | 1171 | 3097 |
| Exp 11 | 0.35 | 1.9 | 5.5 | 600 | 0.0047 | 0.966 | 1.29 | 212 | 0.33 | 1173 | 1841 |
| Exp 12 | 0.60 | 0.2 | 52.5 | 600 | 0.0643 | 0.994 | 1.85 | 16 | 0.23 | 843 | 2639 |
| Exp 13 | 0.60 | 0.6 | 17.5 | 200 | 0.0457 | 0.991 | 3.95 | 22 | 0.11 | 392 | 1827 |
| Exp 14 | 0.60 | 0.6 | 17.5 | 1000 | 0.0170 | 0.929 | 1.47 | 59 | 0.29 | 1039 | 3505 |
| Exp 15 | 0.60 | 1.0 | 10.5 | 600 | 0.0167 | 0.969 | 2.40 | 60 | 0.18 | 635 | 3971 |
| Exp 16 | 1.00 | 0.6 | 17.5 | 600 | 0.0238 | 0.979 | 2.06 | 42 | 0.21 | 743 | 2934 |
| Exp 17 | 2.00 | 0.6 | 17.5 | 600 | 0.0397 | 0.962 | 3.43 | 25 | 0.12 | 449 | 4894 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Héquet, V.; Batault, F.; Raillard, C.; Thévenet, F.; Le Coq, L.; Dumont, É. Determination of the Clean Air Delivery Rate (CADR) of Photocatalytic Oxidation (PCO) Purifiers for Indoor Air Pollutants Using a Closed-Loop Reactor. Part II: Experimental Results. Molecules 2017, 22, 408. https://doi.org/10.3390/molecules22030408
Héquet V, Batault F, Raillard C, Thévenet F, Le Coq L, Dumont É. Determination of the Clean Air Delivery Rate (CADR) of Photocatalytic Oxidation (PCO) Purifiers for Indoor Air Pollutants Using a Closed-Loop Reactor. Part II: Experimental Results. Molecules. 2017; 22(3):408. https://doi.org/10.3390/molecules22030408
Chicago/Turabian StyleHéquet, Valérie, Frédéric Batault, Cécile Raillard, Frédéric Thévenet, Laurence Le Coq, and Éric Dumont. 2017. "Determination of the Clean Air Delivery Rate (CADR) of Photocatalytic Oxidation (PCO) Purifiers for Indoor Air Pollutants Using a Closed-Loop Reactor. Part II: Experimental Results" Molecules 22, no. 3: 408. https://doi.org/10.3390/molecules22030408