DL0410 Ameliorates Memory and Cognitive Impairments Induced by Scopolamine via Increasing Cholinergic Neurotransmission in Mice
Abstract
:1. Introduction
2. Results
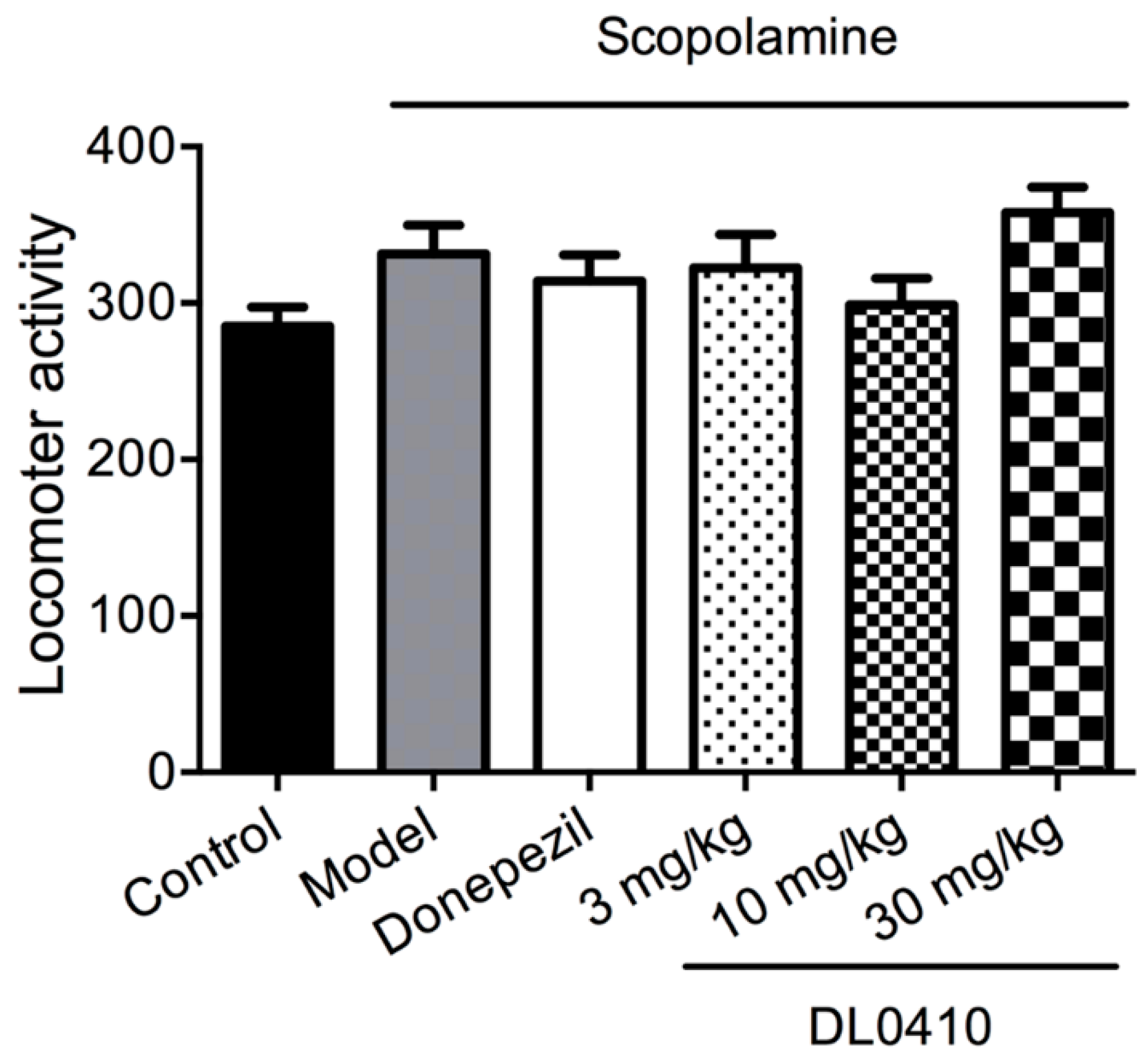
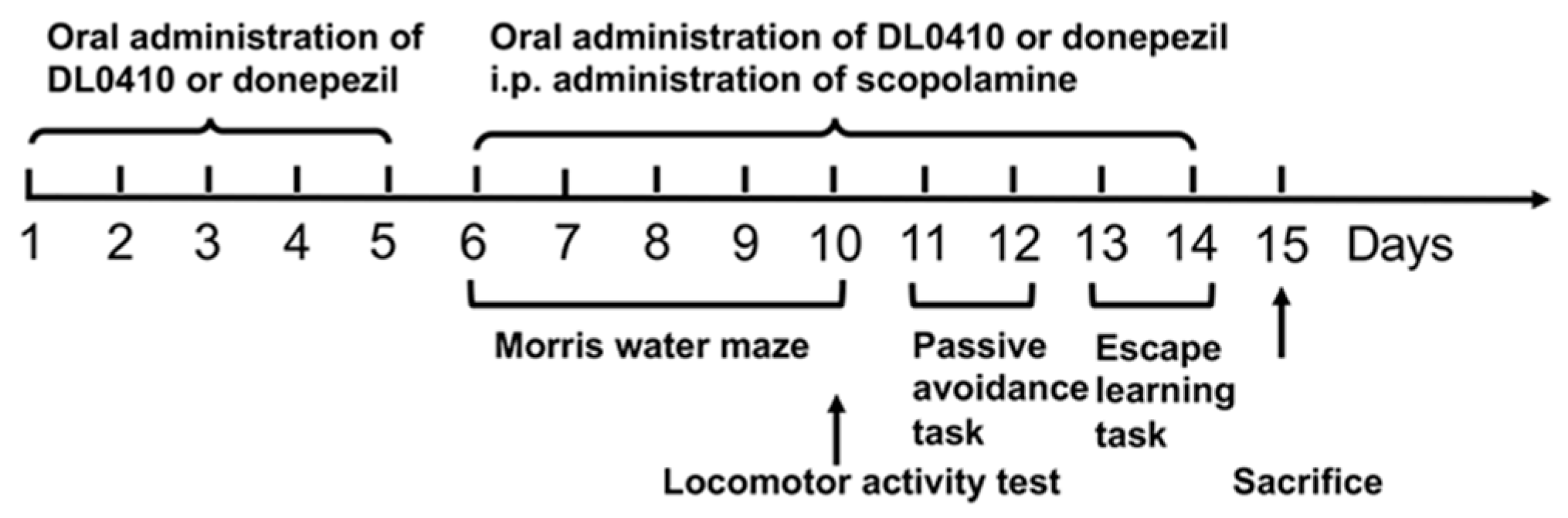
2.1. The Effect of DL0410 on the Locomotor Activity
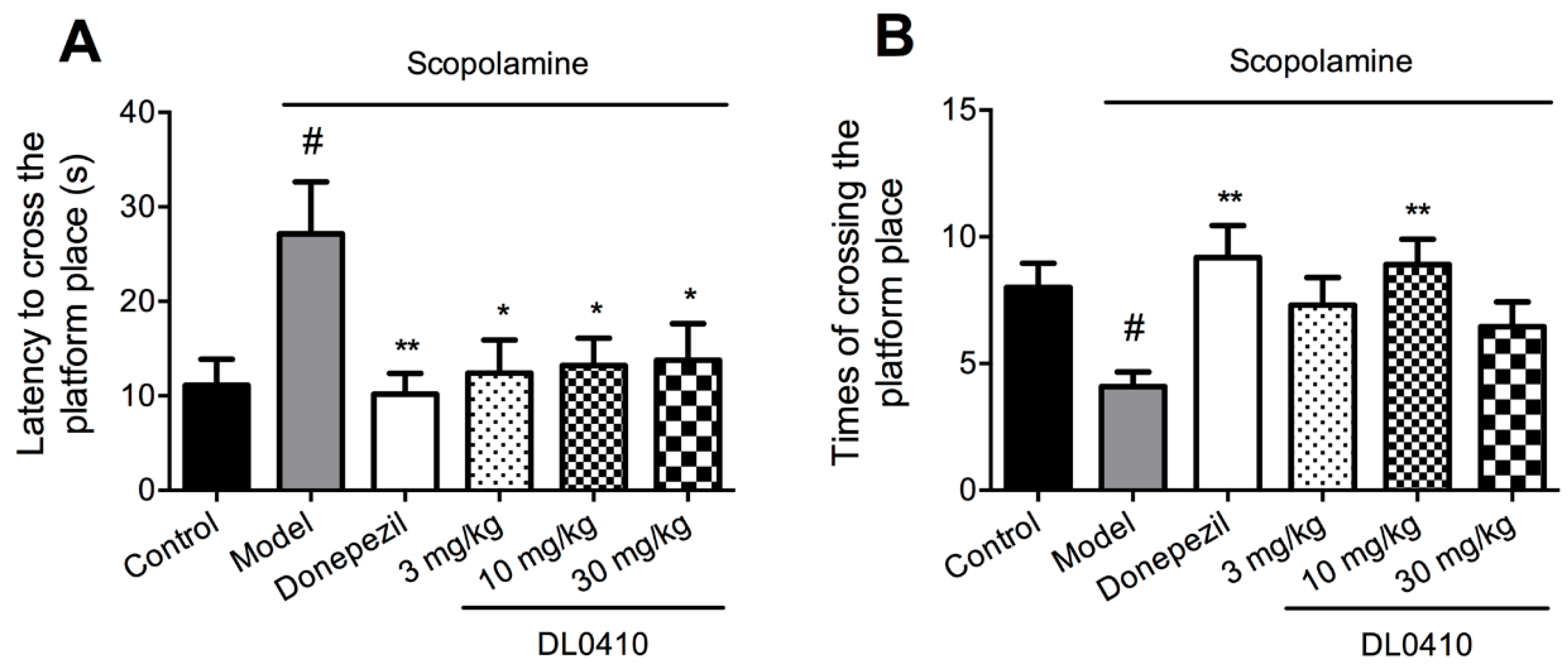
2.2. DL0410 Improved the Spatial Memory Impairments of Mice Induced by Scopolamine in the Morris Water Maze
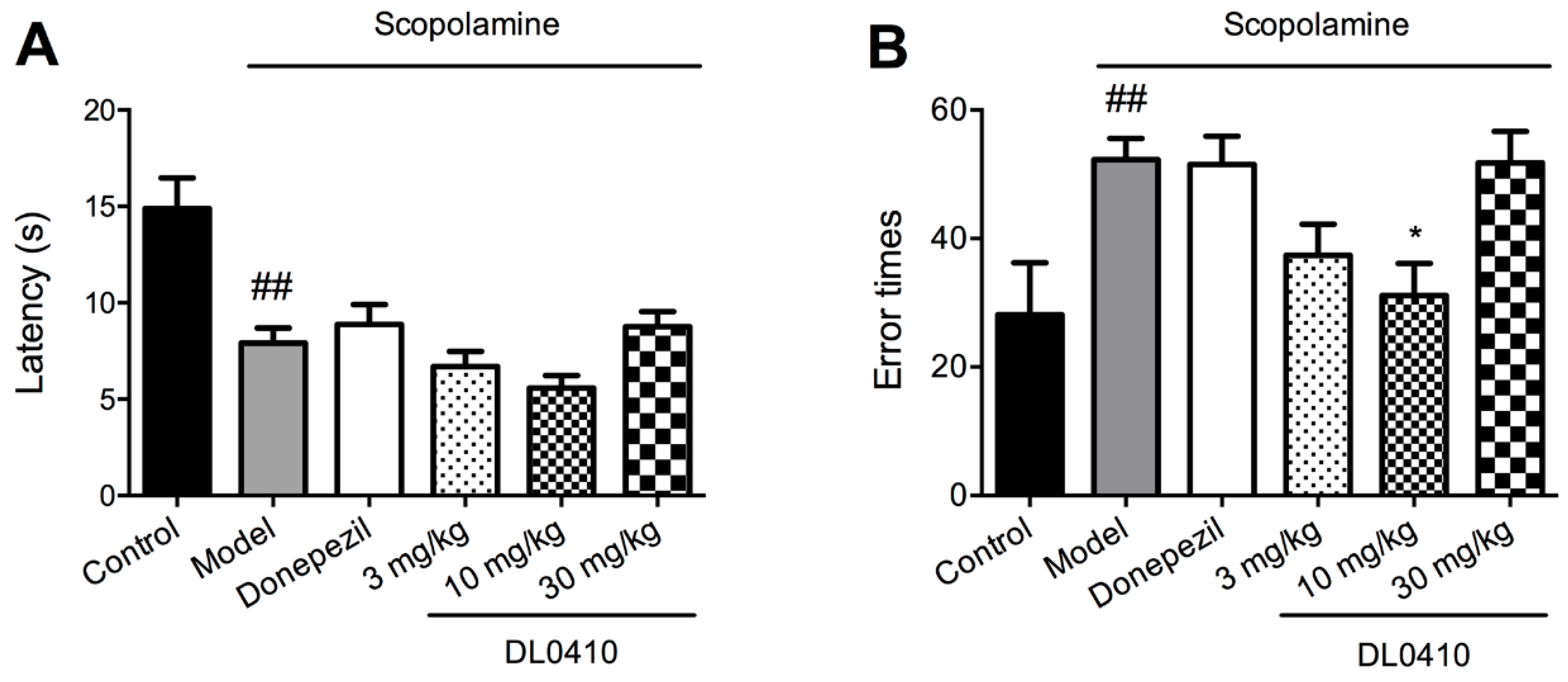
2.3. DL0410 Ameliorated Memory Impairment Induced by Scopolamine in the Passive Avoidance Task
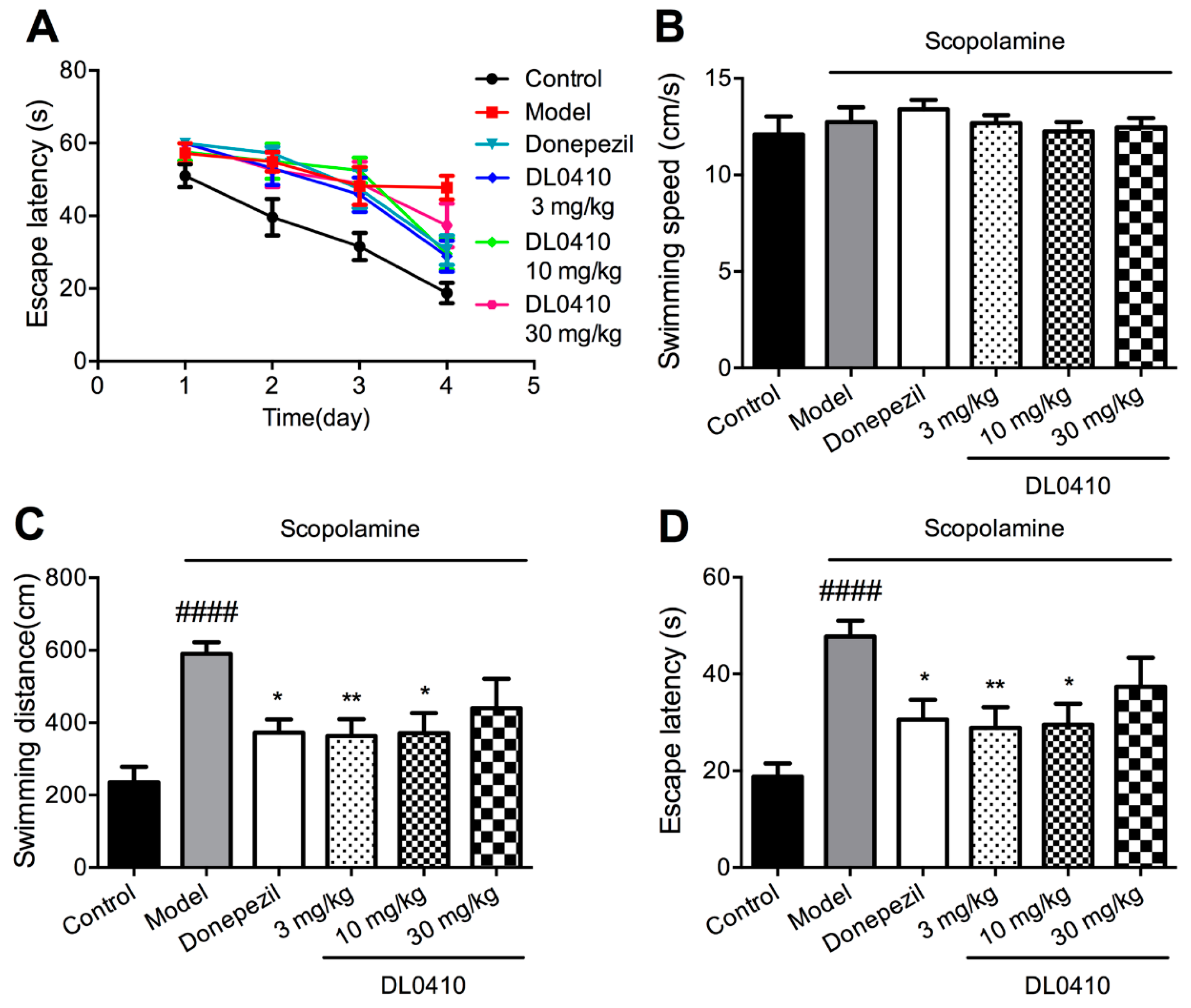
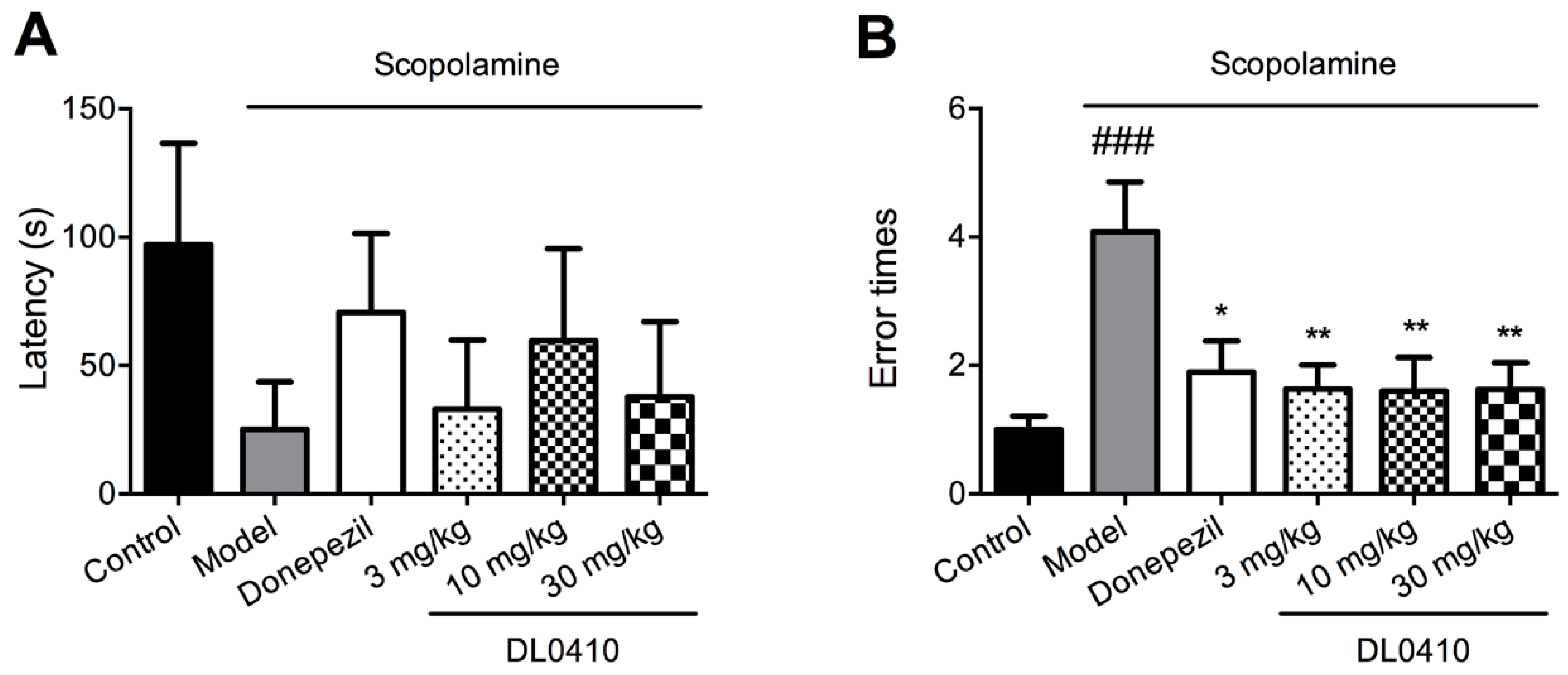
2.4. DL0410 Ameliorated Memory Deficit Induced by Scopolamine in the Escape Learning Task
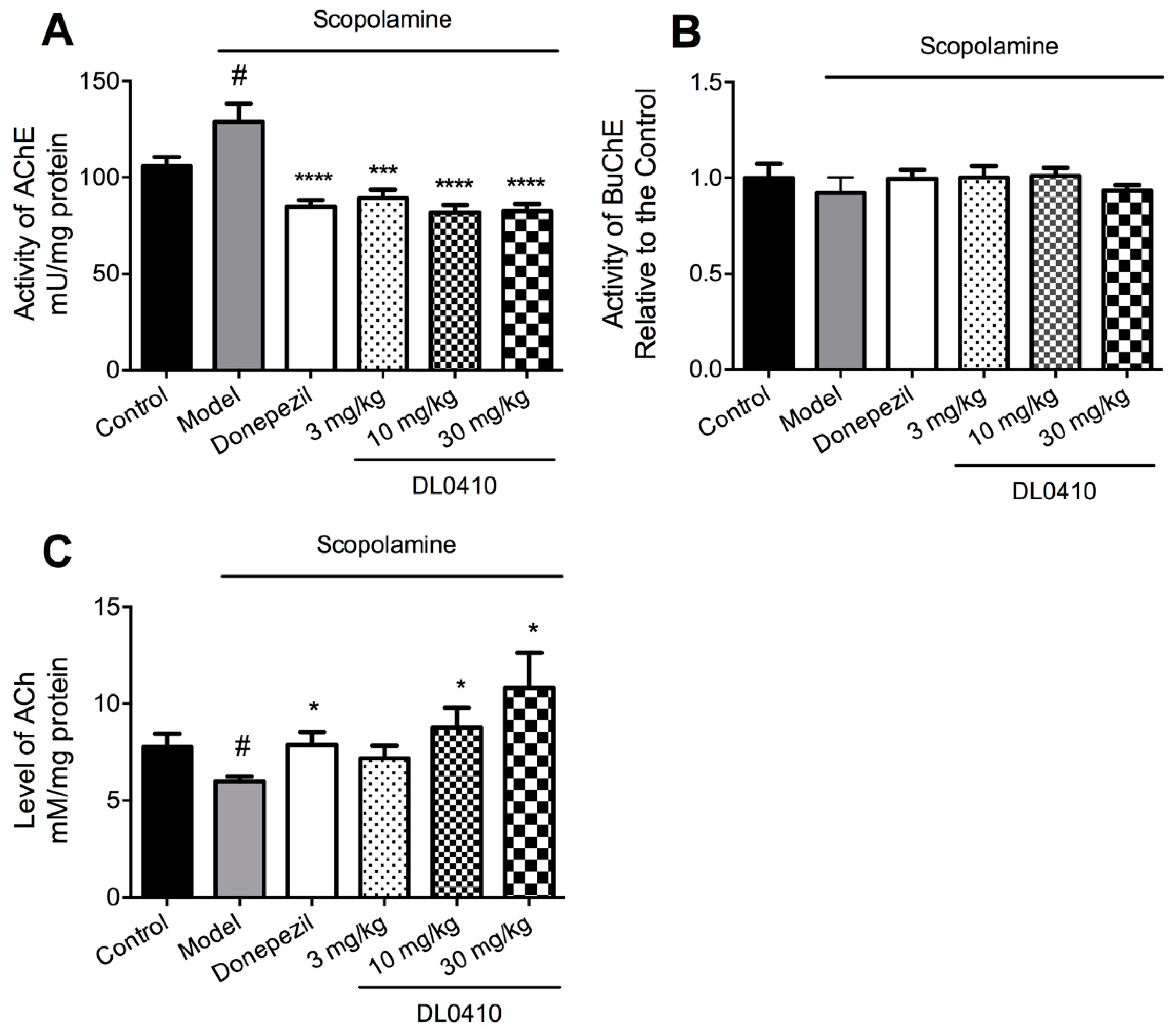
2.5. DL0410 Increased ACh Levels by the Inhibition of AChE in the Hippocampus but not BuChE
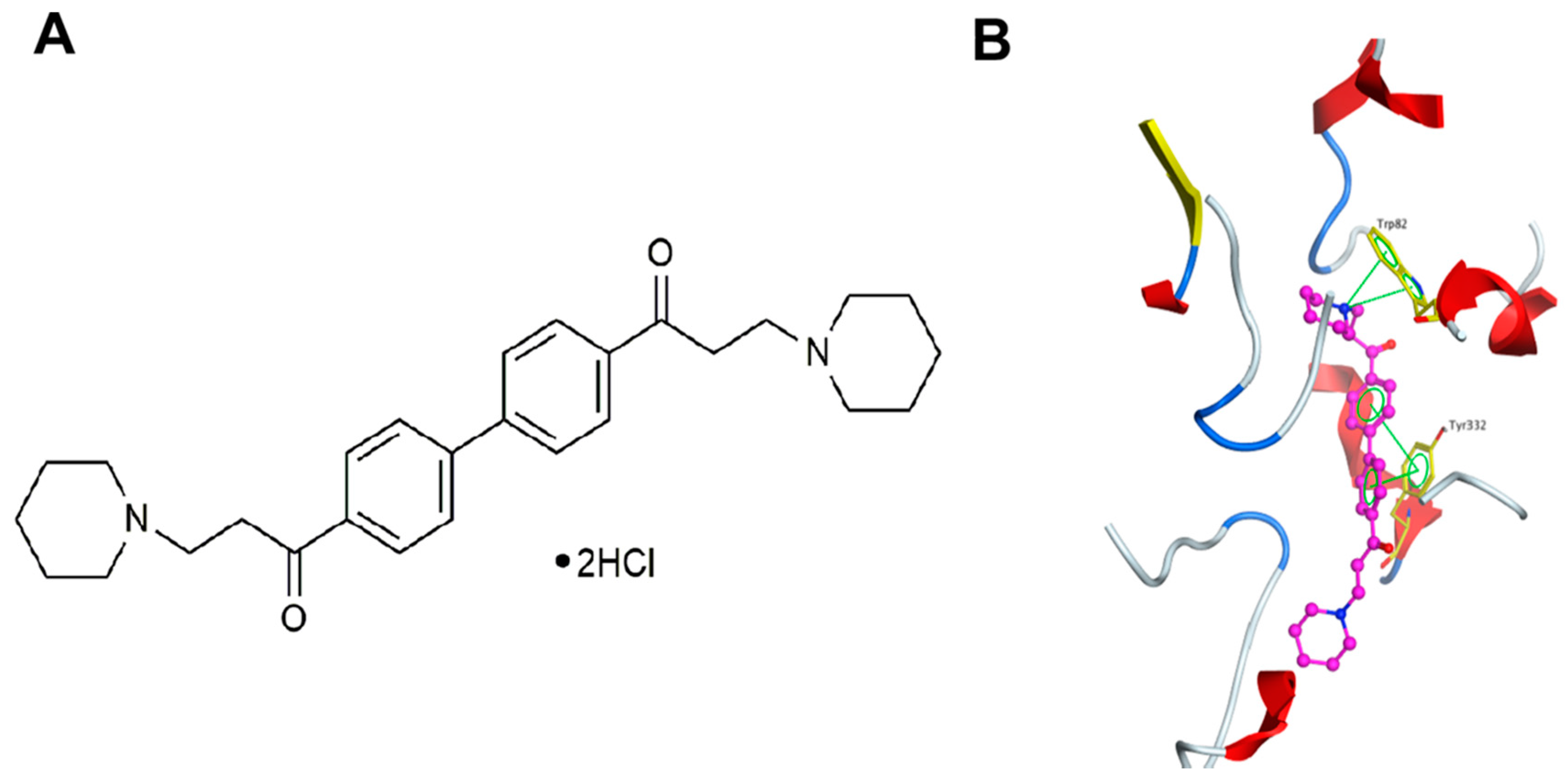
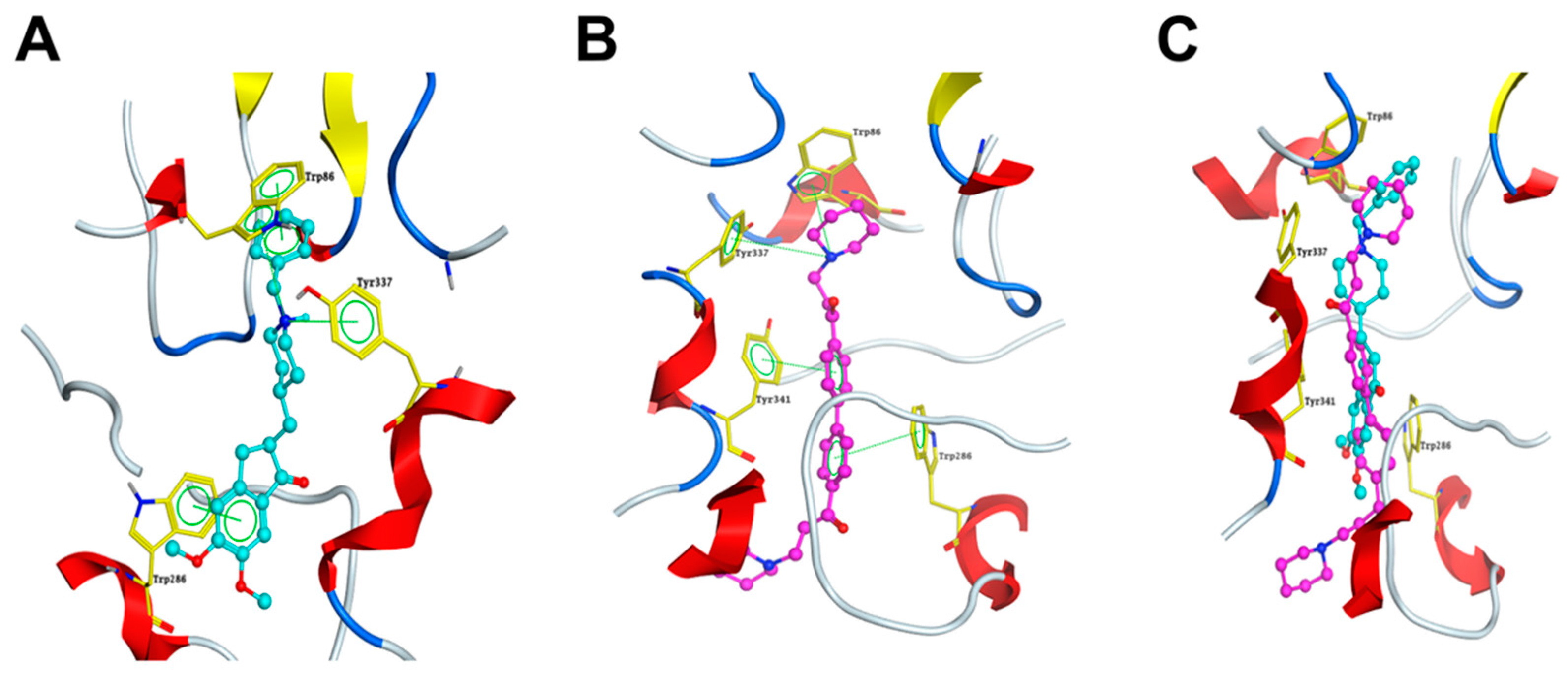
2.6. DL0410 Could Interact with the Dual Binding Site of AChE
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Animals and Treatments
4.3. Locomotor Activity Test
4.4. Morris Water Maze (MWM) Test
4.5. Passive Avoidance Task
4.6. Escape Learning Task
4.7. Preparation of Mouse Brain Tissue
4.8. Acetylcholinesterase (AChE) Activity Assay
4.9. Butyrylcholinesterase (BuChE) Activity Assay
4.10. Acetylcholine Assay
4.11. Molecular Docking
4.12. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2007, 3, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Mensah-Nyagan, A.G.; Eckert, A. Alzheimer, mitochondria and gender. Neurosci. Biobehav. Rev. 2016, 67, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Thal, L.J.; Gage, F.H.; Fisher, L.J. Cholinergic strategies for alzheimer’s disease. J. Mol. Med. 1998, 76, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Schliebs, R.; Arendt, T. The significance of the cholinergic system in the brain during aging and in alzheimer’s disease. J. Neural Transm. 2006, 113, 1625–1644. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Parsons, C.G.; Danysz, W.; Dekundy, A.; Pulte, I. Memantine and cholinesterase inhibitors: Complementary mechanisms in the treatment of alzheimer’s disease. Neurotox. Res. 2013, 24, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yang, R.; Gao, L.; Zhou, D.; Yang, S.; Liu, A.-L.; Du, G.-H. Predictions of buche inhibitors using support vector machine and naive bayesian classification techniques in drug discovery. J. Chem. Inf. Model. 2013, 53, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, W.; Song, J.K.; Feng, Z.Y.; Yang, R.Y.; Wu, S.; Wang, L.; Liu, A.L.; Du, G.H. DL0410, a novel dual cholinesterase inhibitor, protects mouse brains against abeta-induced neuronal damage via the Akt/JNK signaling pathway. Acta Pharmacol. Sin. 2016, 37, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef] [PubMed]
- Dighe, S.N.; Deora, G.S.; De la Mora, E.; Nachon, F.; Chan, S.; Parat, M.O.; Brazzolotto, X.; Ross, B.P. Discovery and structure-activity relationships of a highly selective butyrylcholinesterase inhibitor by structure-based virtual screening. J. Med. Chem. 2016, 59, 7683–7689. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Tian, Y.; Shang, J.; Sun, X.; Chen, H.; Wang, H.; Tan, W. Design, synthesis, biological evaluation, and molecular modeling studies of chalcone-rivastigmine hybrids as cholinesterase inhibitors. Bioorg. Med. Chem. 2017, 25, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Zhao, G.; Wang, D.-M.; Pang, X.-C.; Wang, S.-B.; Fang, J.-S.; Li, C.; Liu, A.-L.; Wu, S.; Du, G.-H. DL0410 can reverse cognitive impairment, synaptic loss and reduce plaque load in APP/PS1 transgenic mice. Pharmacol. Biochem. Behav. 2015, 139, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef]
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M. Axon degeneration mechanisms: Commonality amid diversity. Nat. Rev. Neurosci. 2005, 6, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.; Schliemann, T.; Sorensen, J.C.; Zimmer, J.; West, M.J. Memory impaired aged rats: No loss of principal hippocampal and subicular neurons. Neurobiol. Aging 1996, 17, 143–147. [Google Scholar] [CrossRef]
- Rapp, P.R.; Gallagher, M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. USA 1996, 93, 9926–9930. [Google Scholar] [CrossRef] [PubMed]
- Pepeu, G.; Giovannini, M.G. Cholinesterase inhibitors and beyond. Curr. Alzheimer Res. 2009, 6, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Lahiri, D.K.; Giacobini, E.; Greig, N.H. Advances in alzheimer therapy: Understanding pharmacological approaches to the disease. Curr. Alzheimer Res. 2009, 6, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Valentine, E.R.; Wilding, J.M.; Kapur, N. Routes to remembering: The brains behind superior memory. Nat. Neurosci. 2003, 6, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Pallas, M.; Camins, A.; Smith, M.A.; Perry, G.; Lee, H.G.; Casadesus, G. From aging to alzheimer’s disease: Unveiling “the switch” with the senescence-accelerated mouse model (samp8). J. Alzheimer’s Dis. JAD 2008, 15, 615–624. [Google Scholar] [PubMed]
- Guo, C.; Shen, J.; Meng, Z.; Yang, X.; Li, F. Neuroprotective effects of polygalacic acid on scopolamine-induced memory deficits in mice. Phytomedicine 2016, 23, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shin, E.J.; Lee, B.H.; Choi, S.H.; Jung, S.W.; Cho, I.H.; Hwang, S.H.; Kim, J.Y.; Han, J.S.; Chung, C.; et al. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-beta protein, and mouse model of alzheimer’s disease. Mol. Cells 2015, 38, 796–805. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.P. Muscarinic receptors—Characterization, coupling and function. Pharmacol. Ther. 1993, 58, 319–379. [Google Scholar] [CrossRef]
- Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Wess, J. Use of m1-m5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem. Res. 2003, 28, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ko, H.J.; Jeon, S.J.; Lee, S.; Lee, H.E.; Kim, H.N.; Woo, E.R.; Ryu, J.H. The memory-enhancing effect of erucic acid on scopolamine-induced cognitive impairment in mice. Pharmacol. Biochem. Behav. 2016, 142, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Iimura, Y.; Yamanishi, Y.; Yamatsu, K. Synthesis and structure-activity relationships of acetylcholinesterase inhibitors: 1-benzyl-4-[(5,6-dimethoxy-1-oxoindan-2-yl)methyl]piperidine hydrochloride and related compounds. J. Med. Chem. 1995, 38, 4821–4829. [Google Scholar] [CrossRef] [PubMed]
- Catto, M.; Pisani, L.; Leonetti, F.; Nicolotti, O.; Pesce, P.; Stefanachi, A.; Cellamare, S.; Carotti, A. Design, synthesis and biological evaluation of coumarin alkylamines as potent and selective dual binding site inhibitors of acetylcholinesterase. Bioorg. Med. Chem. 2013, 21, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Alvarez, A.; Perez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into alzheimer's fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef]
- Szutowicz, A.; Bielarczyk, H.; Gul, S.; Ronowska, A.; Pawelczyk, T.; Jankowska-Kulawy, A. Phenotype-dependent susceptibility of cholinergic neuroblastoma cells to neurotoxic inputs. Metab. Brain Dis. 2006, 21, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Joerchel, S.; Raap, M.; Bigl, M.; Eschrich, K.; Schliebs, R. Oligomeric beta-amyloid(1–42) induces the expression of alzheimer disease-relevant proteins in cholinergic sn56.B5.G4 cells as revealed by proteomic analysis. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2008, 26, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Fodero, L.R.; Mok, S.S.; Losic, D.; Martin, L.L.; Aguilar, M.I.; Barrow, C.J.; Livett, B.G.; Small, D.H. Alpha7-nicotinic acetylcholine receptors mediate an abeta(1–42)-induced increase in the level of acetylcholinesterase in primary cortical neurones. J. Neurochem. 2004, 88, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Pettit, D.L.; Shao, Z.; Yakel, J.L. Beta-amyloid(1–42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, Rc120. [Google Scholar]
- Laursen, B.; Mork, A.; Plath, N.; Kristiansen, U.; Bastlund, J.F. Cholinergic degeneration is associated with increased plaque deposition and cognitive impairment in APPswe/PS1dE9 mice. Behav. Brain Res. 2013, 240, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Del Monte-Millan, M.; Garcia-Palomero, E.; Valenzuela, R.; Usan, P.; de Austria, C.; Munoz-Ruiz, P.; Rubio, L.; Dorronsoro, I.; Martinez, A.; Medina, M. Dual binding site acetylcholinesterase inhibitors: Potential new disease-modifying agents for AD. J. Mol. Neurosci. MN 2006, 30, 85–88. [Google Scholar] [CrossRef]
- Alvarez, A.; Opazo, C.; Alarcon, R.; Garrido, J.; Inestrosa, N.C. Acetylcholinesterase promotes the aggregation of amyloid-beta-peptide fragments by forming a complex with the growing fibrils. J. Mol. Biol. 1997, 272, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wehle, S.; Kuzmanovic, N.; Merget, B.; Holzgrabe, U.; Konig, B.; Sotriffer, C.A.; Decker, M. Acetylcholinesterase inhibitors with photoswitchable inhibition of beta-amyloid aggregation. ACS Chem. Neurosci. 2014, 5, 377–389. [Google Scholar] [CrossRef]
- Galdeano, C.; Viayna, E.; Arroyo, P.; Bidon-Chanal, A.; Blas, J.R.; Munoz-Torrero, D.; Luque, F.J. Structural determinants of the multifunctional profile of dual binding site acetylcholinesterase inhibitors as anti-alzheimer agents. Curr. Pharm. Des. 2010, 16, 2818–2836. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhao, H.T.; Zhong, S.M.; Wang, Z.Y.; Chen, Z.F.; Liang, H. Novel oxoisoaporphine-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. Bioorg. Med. Chem. Lett. 2012, 22, 2257–2261. [Google Scholar] [CrossRef]
- Duan, S.; Guan, X.; Lin, R.; Liu, X.; Yan, Y.; Lin, R.; Zhang, T.; Chen, X.; Huang, J.; Sun, X.; et al. Silibinin inhibits acetylcholinesterase activity and amyloid beta peptide aggregation: A dual-target drug for the treatment of alzheimer’s disease. Neurobiol. Aging 2015, 36, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protocols 2006, 1, 848–858. [Google Scholar] [CrossRef]
- D’Hooge, R.; De Deyn, P.P. Applications of the morris water maze in the study of learning and memory. Brain Res. Rev. 2001, 36, 60–90. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, H.; Park, H.; Cho, W.K.; Ma, J.Y. Fermented sipjeondaebo-tang alleviates memory deficits and loss of hippocampal neurogenesis in scopolamine-induced amnesia in mice. Sci. Rep. 2016, 6, 22405. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE) (2010) Version 2010.10; Chemical Computing Group Inc.: Montreal, QC, Canada, 2010.
- Lian, W.; Fang, J.; Li, C.; Pang, X.; Liu, A.-L.; Du, G.-H. Discovery of influenza a virus neuraminidase inhibitors using support vector machine and naïve bayesian models. Mol. Divers. 2015, 20, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds DL0410 are available from the authors.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, W.; Fang, J.; Xu, L.; Zhou, W.; Kang, D.; Xiong, W.; Jia, H.; Liu, A.-L.; Du, G.-H. DL0410 Ameliorates Memory and Cognitive Impairments Induced by Scopolamine via Increasing Cholinergic Neurotransmission in Mice. Molecules 2017, 22, 410. https://doi.org/10.3390/molecules22030410
Lian W, Fang J, Xu L, Zhou W, Kang D, Xiong W, Jia H, Liu A-L, Du G-H. DL0410 Ameliorates Memory and Cognitive Impairments Induced by Scopolamine via Increasing Cholinergic Neurotransmission in Mice. Molecules. 2017; 22(3):410. https://doi.org/10.3390/molecules22030410
Chicago/Turabian StyleLian, Wenwen, Jiansong Fang, Lvjie Xu, Wei Zhou, De Kang, Wandi Xiong, Hao Jia, Ai-Lin Liu, and Guan-Hua Du. 2017. "DL0410 Ameliorates Memory and Cognitive Impairments Induced by Scopolamine via Increasing Cholinergic Neurotransmission in Mice" Molecules 22, no. 3: 410. https://doi.org/10.3390/molecules22030410




